Abstract
Schizophrenia is a devastating neuropsychiatric syndrome associated with distributed brain dysconnectivity that may involve large-scale thalamo-cortical systems. Incomplete characterization of thalamic connectivity in schizophrenia limits our understanding of its relationship to symptoms and to diagnoses with shared clinical presentation, such as bipolar illness, which may exist on a spectrum. Using resting-state functional magnetic resonance imaging, we characterized thalamic connectivity in 90 schizophrenia patients versus 90 matched controls via: (1) Subject-specific anatomically defined thalamic seeds; (2) anatomical and data-driven clustering to assay within-thalamus dysconnectivity; and (3) machine learning to classify diagnostic membership via thalamic connectivity for schizophrenia and for 47 bipolar patients and 47 matched controls. Schizophrenia analyses revealed functionally related disturbances: Thalamic over-connectivity with bilateral sensory–motor cortices, which predicted symptoms, but thalamic under-connectivity with prefrontal–striatal–cerebellar regions relative to controls, possibly reflective of sensory gating and top-down control disturbances. Clustering revealed that this dysconnectivity was prominent for thalamic nuclei densely connected with the prefrontal cortex. Classification and cross-diagnostic results suggest that thalamic dysconnectivity may be a neural marker for disturbances across diagnoses. Present findings, using one of the largest schizophrenia and bipolar neuroimaging samples to date, inform basic understanding of large-scale thalamo-cortical systems and provide vital clues about the complex nature of its disturbances in severe mental illness.
Keywords: bipolar illness, connectivity, resting state, schizophrenia, thalamus
Introduction
Schizophrenia is a common, multifaceted, and heterogeneous neuropsychiatric syndrome (Walker et al. 2004) associated with disturbances in perception (Yoon et al. 2008), belief (Corlett, Honey, et al. 2007), emotion (Holt et al. 2011), and cognition (Barch and Braver 2007). Limited understanding of schizophrenia neurobiology has constrained development of effective treatments for its broad range of symptoms and impairments (Krystal et al. 2003), making it one of the most profoundly disabling medical conditions worldwide (Murray et al. 1996). This illness has been conceptualized as a disorder of distributed brain connectivity (Stephan et al. 2006), with hypothesized wide-spread disruptions in neuronal communication at the level of large-scale neural systems (Lynall et al. 2010; van den Heuvel et al. 2010; Cole, Anticevic, et al. 2011; Fornito et al. 2011; Salomon et al. 2011).
Growing evidence implicates significant thalamo-cortical communication disturbances in schizophrenia (Carlsson and Carlsson 1990a, 1990b; Andreasen et al. 1994; Andreasen 1997; Carlsson et al. 2001; Lewis et al. 2001). Indeed, a fundamental aspect of large-scale brain organization across mammalian species is recurrent thalamo-cortico-striatal circuits (Alexander et al. 1986). Its complex multinuclear structure (Haber and McFarland 2001) enables the thalamus to serve as a nexus for parallel circuits through which diverse cortical and subcortical functions are integrated. These parallel distributed circuits have been implicated in the schizophrenia pathophysiology on the basis of neuropathology studies (Lewis 2000; Cronenwett and Csernansky 2010; Lisman 2012), preclinical lesion models (Volk and Lewis 2003; Selemon et al. 2009), structural imaging studies (Csernansky et al. 2004; Harms et al. 2007), and computational models (Lisman et al. 2010). Moreover, thalamic abnormalities are implicated in sensory gating (Geyer et al. 2001; Turetsky et al. 2007) and filtering disruptions (Oltmanns and Neale 1975; Anticevic et al. 2011) associated with this disorder (Andreasen 1997).
One emerging strategy for characterizing thalamic disturbances in schizophrenia is to study low-frequency fluctuations present in the blood oxygenation level-dependent (BOLD) signal—resting-state functional connectivity (Raichle and Snyder 2007; Biswal et al. 2010). These low-frequency fluctuations are temporally correlated within spatially distinct but functionally related networks (Fox et al. 2005), establishing an intrinsic functional architecture (Smith et al. 2009) across species (Vincent et al. 2007). Identified networks agree with other measures of structural and functional connectivities in healthy populations (Greicius et al. 2009) and allow characterizing distributed circuit abnormalities in neuropsychiatric illness (Fox and Greicius 2010; Anticevic, Brumbaugh, et al. 2012). While resting-state approaches have been used to investigate thalamo-cortical systems in healthy adults (Zhang et al. 2010), less is known about cortex-wide and within-thalamic information flow disturbances in schizophrenia. Studies of thalamic connectivity in schizophrenia can build on 2 key properties of this region: (1) The thalamus is widely connected to the entire cortical mantle in a topographically organized fashion and may represent a node particularly sensitive to network-level disturbances in this illness; (2) the thalamus is organized into anatomically segregated nuclei, which can be readily defined by neuroimaging methodology (Fischl et al. 2004), providing a lens for examining parallel yet distributed cortical connectivity alterations in schizophrenia.
To date, thalamic connectivity studies in schizophrenia focused exclusively on the medio-dorsal nucleus (Welsh et al. 2010) or employed restricted cortical regions-of-interest (ROI) approaches (Guller et al. 2012; Woodward et al. 2012). While these studies provided clues about thalamic connectivity alterations in schizophrenia, their small sample sizes or ROI-focused approaches limit inferences about the precise location of thalamo-cortical or within-thalamic connectivity abnormalities. That is, thalamus is likely not uniformly disconnected with large portions of the cortex. Rather, it might show specific patterns of connectivity disturbance in each cortical territory (or thalamic subdivision), which may include reduced and/or increased connectivity in patients. The methods used in prior studies could not address this issue, which is critical to understand the nature and localization of thalamo-cortical perturbation in schizophrenia. Using data-driven approaches with the thalamus as a starting point, we are able to identify the specific pattern of thalamo-cortical connectivity disturbance and—by extension—to test whether disturbances are uniform across thalamo-cortical loops, or whether there are specific dysconnectivity patterns that shed light on both the “typical” clinical profile and clinical heterogeneity within schizophrenia (Insel 2010).
Besides improving the understanding of schizophrenia, characterization of cortico-thalamic information flow could yield biomarkers that might better guide the psychiatric diagnostic process—a key challenge in clinical neuroscience (Meyer-Lindenberg 2010). For example, bipolar disorder and schizophrenia share putative genetic risk mechanisms, and there are reports of similarities in the circuit disturbances associated with these disorders (Van Snellenberg and de Candia 2009; Anticevic, Brumbaugh, et al. 2012; Meda et al. 2012). Therefore, we extended analyses to bipolar illness and hypothesized that bipolar patients may also exhibit thalamo-cortical disruptions. We also hypothesized that thalamic connectivity may provide a sensitive marker across diagnoses, whereby it would scale as a function of illness severity.
Here, we employ rigorous clinical criteria to investigate thalamo-cortical dysconnectivity in one of the largest schizophrenia (N = 90) and bipolar (N = 67) neuroimaging samples by: (1) Examining thalamic coupling via individual-specific anatomically derived thalamic seeds to obtain a comprehensive cortex-wide assay of disturbances. Based on the previous reports (Woodward et al. 2012), we hypothesized alterations in connectivity across sensory and prefrontal regions, but also complex patterns of over- to under-connectivity that may not cleanly follow ROI-based boundaries; (2) testing if observed thalamo-cortical coupling disturbances scale with symptoms; (3) replicating results via an independently diagnosed schizophrenia sample and computing formal effect sizes to guide future clinical neuroimaging studies; (4) testing whether identified thalamo-cortical connectivity increases and reductions constitute shared versus independent disturbances; (5) examining the hypothesis of differential dysconnectivity across thalamic nuclei in schizophrenia, to provide evidence for the “locus” of disturbed thalamo-cortical information flow; and (6) extending findings to bipolar illness to inform current NIMH Research Domain Criteria (RDoC) initiatives to develop biomarker-driven diagnostic systems (Cuthbert and Insel 2010). Here, we explicitly tested whether individuals with bipolar illness show a “graded” pattern of disturbances, evident relative to matched controls, but not as severe as those found in schizophrenia. Finally, we employ multivariate pattern analysis (MVPA) to formally test whether thalamo-cortical connectivity is sensitive enough for the diagnostic classification of both schizophrenia and bipolar illness. This final analysis serves as a key validation to show that, in principle, observed disrupted thalamic connectivity might aid diagnostic decisions.
Materials and Methods
Participants
Ninety schizophrenia patients and 90 carefully demographically matched healthy controls participated in the study (Table 1). Sixty-seven additional patients diagnosed with bipolar illness and 47 matched controls were selected and carefully characterized in a prior study (Anticevic, Brumbaugh, et al. 2012). We additionally selected 23 schizophrenia patients and 23 carefully matched controls, characterized independently of the discovery sample (Anticevic et al. 2011). All subjects met identical methodological stringency criteria. Supplementary Tables 3 and 4 show complete demographics for bipolar and schizophrenia replication samples; comprehensive clinical details can be found in our prior work (Anticevic et al. 2011; Anticevic, Brumbaugh, et al. 2012). For complete details regarding inclusion/exclusion criteria, group matching and dealing with the missing data please see Supplementary Materials and Methods. Of note, all attempted analyses on these data were fully orthogonal to any previously published effects using the bipolar and schizophrenia replication samples, ensuring independence of reported effects.
Table 1.
Clinical and demographic characteristics
| Characteristic | Controls (N = 90) |
Patients (N = 90) |
Significance |
|||
|---|---|---|---|---|---|---|
| M | SD | M | SD | T-value/χ2 | P-value (2-tailed) | |
| Age (in years) | 30.71 | 11.99 | 32.93 | 11.25 | 1.28 | 0.20 |
| Gender (% male) | 66 | 73 | 1.13 | 0.26 | ||
| Father's education (in years) | 14.37 | 3.21 | 13.67 | 3.47 | 1.42 | 0.16 |
| Mother's education (in years) | 13.99 | 2.81 | 13.50 | 2.92 | 1.15 | 0.25 |
| Participant's education (in years) | 15.24 | 2.22 | 13.18 | 2.21 | 6.26* | <0.001 |
| Handedness (% right) | 84.21 | 80.00 | 0.85 | 0.40 | ||
| Signal-to-noise | 215.37 | 45.25 | 206.81 | 62.05 | 1.06 | 0.30 |
| IQ estimate | 106.77 | 8.92 | 97.78 | 15.71 | 4.55* | <0.001 |
| Medication (CPZ equivalents) | — | — | 229.00 | 195.81 | — | — |
| PANSS positive symptoms | — | — | 15.80 | 4.73 | — | — |
| PANSS negative symptoms | — | — | 14.34 | 5.53 | — | — |
| PANSS general psychopathology | — | — | 30.48 | 7.18 | — | — |
| PANSS total psychopathology | — | — | 60.51 | 14.25 | — | — |
Notes: Age, education levels, parental education, age at diagnosis, and duration of illness are expressed in years.
PANSS: Positive and Negative Syndrome Scale; M: mean; SD: standard deviation; IQ: intelligence quotient.
*A significant T-statistic for the between-group t-test. For bipolar and schizophrenia replication sample demographics, see Supplementary Tables 3 and 4.
Symptoms and Medication
Schizophrenia symptom severity was determined using the Positive and Negative Syndrome Scale (PANSS), a widely used symptom instrument, which captures positive, negative, and general psychopathology symptom dimensions (Kay et al. 1987) (Table 1). Seventy-five of the 90 schizophrenia patients were receiving antipsychotic treatment. All medication were converted to chlorpromazine equivalents (Andreasen et al. 2010) and verified by trained raters (A.A., M.S.B., and A.S.). Reported effects were not altered when covaried for medication. Bipolar patients were in remission at the time of the scan (Anticevic, Brumbaugh, et al. 2012).
Neuroimaging Data Acquisition
Images sensitive to the BOLD signal were acquired at the Olin Neuropsychiatry Research Center using a Siemens-Allegra 3-T scanner, with axial slices parallel to the anterior–posterior commissure (AC–PC) using a T2*-weighted gradient-echo, echo-planar sequence [time repetition (TR)/time echo (TE) = 1500/27 ms, flip angle = 60°, field of view = 24 × 24 cm, acquisition matrix = 64 × 64, voxel size = 3.43 × 3.43 × 4 mm] covering the whole brain. The acquisition lasted 5.25 min and produced 210 volumetric images per subject (29 slices/volume, interslice gap = 1 mm). Subjects were instructed to lay awake in the scanner and keep their eyes open. Subjects were monitored on a video camera to ensure that they stayed awake and were removed from the analyses if they fell asleep during the scan, or if their head movement >1 mm along any axis. Structural images were acquired using a T1-weighted, 3-dimensional magnetization-prepared rapid gradient-echo sequence (TR/TE/time to inversion = 2200/4.13/766 ms, flip angle = 13°, voxel size [isotropic] = 0.8 mm, image size = 240 × 320 × 208 voxels), with axial slices parallel to the AC–PC line. Bipolar patients (N = 67) and their respective matched healthy controls (N = 47) underwent data collection with identical acquisition parameters at the Olin Neuropsychiatry Research Center using a Siemens-Allegra 3-T scanner (Anticevic, Brumbaugh, et al. 2012). Schizophrenia replication subjects (N = 23) and their respective matched healthy controls (N = 23) underwent data collection at Washington University in St. Louis using a Siemens Tim-Trio 3-T scanner. Full acquisition details for the schizophrenia replication sample and their respective matched controls can be found in our prior work (Anticevic et al. 2011; Cole, Anticevic, et al. 2011; Anticevic, Brumbaugh, et al. 2012).
Data Preprocessing and Analysis
All preprocessing followed our published work (Repovs et al. 2011; Anticevic, Gancsos, et al. 2012; Anticevic, Repovs, et al. 2012; Repovs and Barch 2012) and included: (1) Slice-time correction, (2) first 5 images removed from each run, (3) elimination of odd/even slice intensity differences, (4) rigid-body motion correction, (5) correction for magnetic field inhomogeneity, (6) 12-parameter affine transform of the structural image to the Talairach coordinate system, and (7) coregistration of fMRI volumes to the structural image with 3 × 3 × 3 mm resampling. As noted in Supplementary Materials and Methods, single-to-noise (SNR) was a key criterion for group matching and was determined by obtaining the mean signal and standard deviation for a given slice across the BOLD run, while excluding all nonbrain voxels across all frames (Anticevic, Repovs, et al. 2012). After removal, there were no significant between-group SNR differences for the discovery schizophrenia versus control sample (SCZ-mean = 215.37; CON-mean = 206.81; P = 0.29, NS). This same stringent SNR criteria were applied across the bipolar and schizophrenia replication samples as detailed previously (Cole, Anticevic, et al. 2011; Anticevic, Brumbaugh, et al. 2012).
To remove the sources of spurious correlations present in resting-state BOLD data, all fMRI time-series underwent high-pass temporal filtering (0.009 Hz), nuisance signal removal from ventricles, deep white matter, global mean signal (GMS), 6 rigid-body motion correction parameters, and their first derivatives, followed by low-pass temporal filtering (0.08 Hz). In addition, given the growing concerns that excessive moment can impact between-group differences (especially those involving clinical group comparisons), we implemented additional careful volume censoring (“scrubbing”) movement correction as reported by Power et al. (2012a, 2012b) to ensure that head-motion artifacts are not driving observed effects (Satterthwaite et al. 2012; van Dijk et al. 2012). Image frames with possible artifactual fluctuations in intensity were identified using 2 criteria with a procedure suggested by Power et al. (2012b). First, frames in which sum of the displacement across all 6 rigid-body movement correction parameters >0.5 mm (assuming 50 mm cortical sphere radius) were identified. Secondly, root mean square (RMS) of differences in intensity between the current and preceding frame was computed across all voxels and divided by mean intensity. Frames in which normalized RMS exceeded the value of 3 were identified. The frames flagged by either criterion were marked for exclusion, as well as the one preceding and 2 frames following the flagged frame. Subjects with >50% frames flagged were completely excluded from analyses. Of note, the proportion of flagged frames in the discovery sample was 10% for controls and 17% for patients. This proportion was significantly higher for patients [t(176) = 3.79, P < 0.001], suggesting that patients did move more. To verify that this did not affect our analyses, we used the proportion of removed frames as a covariate for the identified dysconnectivity patterns. Results remained unchanged when the proportion of flagged frames was used as a variable, and it did not explain a significant portion of the variance for any between-group effects. All subjects across both clinical and control samples (schizophrenia discovery, N = 90/90; schizophrenia replication, N = 23/23, bipolar illness, N = 67/47, total N = 340) passed these criteria.
Given emerging concerns that GMS removal can induce negative relationships (Murphy et al. 2009), which could complicate between-group interpretation (Saad et al. 2012), we repeated the main effect analyses (presented in Fig. 1) without GMS removal to ensure the stability of findings. Results remained largely unchanged when repeated within the regions defined via original analyses (Supplementary Fig. 13), as well as when repeated at the whole-brain level. We also repeated the across-subject analyses presented in Figure 3 and Supplementary Figures 6 and 7 with and without GMS removal, given the observed stable anticorrelated relationships in control subjects (and the possibility that this anticorrelation could be artifactual). Key effects remained unchanged without removing GMS. Based on these stable patterns of results, prior reports that GMS removal is critical to optimize the specificity of functional connectivity findings (Fox et al. 2009), and its wide-spread use by other leading groups conducting similar analyses (Biswal et al. 2010), we computed all follow-up analyses with GMS removed. Moreover, as noted in our prior work (Anticevic, Brumbaugh, et al. 2012), across all analyses groups underwent identical preprocessing steps, making it less likely that observed differences are driven by GMS removal exclusively. Nevertheless, we acknowledge that prospective formal simulation and clinical studies are needed to fully resolve complex considerations pertaining to GMS removal in functional connectivity work (Saad et al. 2012) and possible nuanced but important differences when using this analysis step (Supplementary Fig. 13).
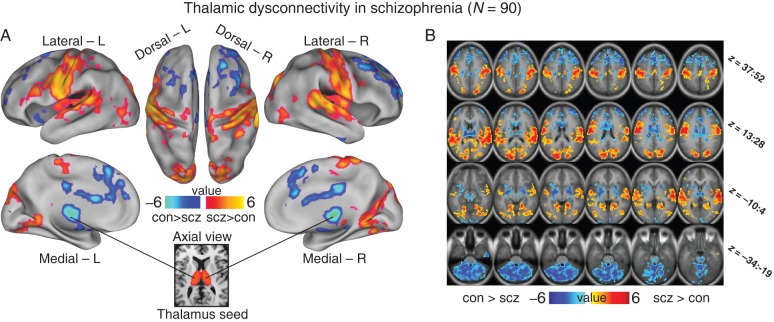
Figure 1.
Thalamic dysconnectivity in schizophrenia. (A) Significant whole-brain between-group differences in thalamic connectivity between healthy controls (CON) and individuals with schizophrenia (SCZ). Red-orange foci mark areas where patients exhibited stronger thalamic coupling; blue foci mark areas where patients exhibited reduced thalamic coupling relative to healthy controls (Supplementary Tables 1 and 2 list all foci showing significant between-group differences). The bottom inset illustrates a thalamic seed. (B) Volume-based axial view with Z-coordinate ranges (each slice in each row increments by 3 mm). For group-specific unthresholded connectivity patterns see Supplementary Figure 1; and for comprehensive between-group contrasts across samples see Supplementary Figures 2 and 4. For a formal conjunction analysis with a priori-defined sensory–motor networks see Supplementary Figure 12.
Figure 3.
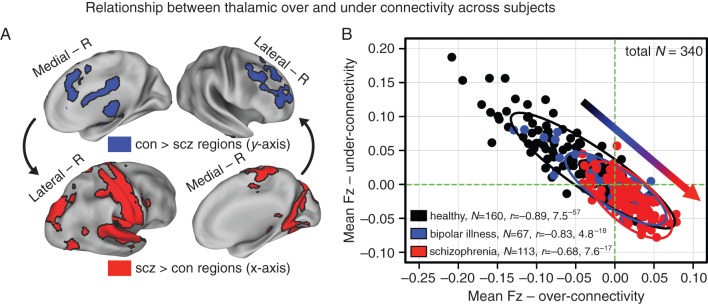
Relationship between thalamic over- and under-connectivity across subjects. (A) Regions showing reduced (blue, top panel) and increased (red, bottom panel) thalamic connectivity for the original discovery sample (N = 90). (B) A significant negative relationship evident across all healthy controls (gray-black data points, N = 160; r = −0.89, P < 7.5−57) collapsing across all 3 samples (discovery, replication, and healthy controls matched to bipolar patients). The same pattern was evident for bipolar patients (blue data points, N = 67; r = −0.83, P < 4.8−18), whereas an attenuated and shifted correlation was found for schizophrenia patients (red data points, N = 113; r = −0.68, P < 7.6−17, collapsing across both discovery and replication samples). Vertical/horizontal green lines mark the zero points. Schizophrenia patients showed a “shift” across the zero lines, indicative of weaker prefrontal–cerebellar–thalamic coupling, but stronger sensory–motor–thalamic coupling. Bipolar patients showed an intermediate degree of disruption, suggesting a “gradient” (inset arrow for qualitative illustration). Ellipses for each group mark the 95% confidence interval. Supplementary Figures 6 and 7 show sample-specific analyses.
Thalamus Seed-Based Connectivity (fcMRI) Analyses
The seed-based fcMRI approach closely followed prior studies using subcortical anatomically defined nuclei (Anticevic, Repovs, Barch, 2010). We started with the entire thalamic seed analyses to test whether there is a robust, wide-spread dysconnectivity that can be observed even when taking the thalamus as a whole. We then progress to more complex analyses to characterize this core effect (see Supplementary Information).
In-house Matlab tools (Repovs et al. 2011; Anticevic, Repovs, et al. 2012) were used to examine the thalamus coupling with all voxels in the brain. For complete details on clustering, dysconnectivity, anatomy-based and classification thalamic analyses please see Supplementary Materials and Methods. We computed a seed-based thalamus correlation map by extracting average time-series across all voxels in each subject's bilateral thalamus (which was anatomically defined through Freesurfer-based segmentation; Fischl et al. 2002, 2004). This thalamic signal was then correlated with each gray matter voxel, and the computed Pearson correlation values were transformed to Fisher Z-values (Fz) using a Fisher r-to-Z transform. This yielded a map for each subject, where each voxel's value represents connectivity with the thalamus. To examine between-group differences, Fz maps were entered into an independent samples t-test. Whole-brain type I error correction was implemented via threshold-free cluster enhancement nonparametric techniques implemented in the FSL's “Randomise” tool (Smith and Nichols 2009). Results were visualized using both the Caret 5.5 software (http://brainvis.wustl.edu/wiki/index.php/Caret) and NeuroLens software (http://www.neurolens.org).
Results
Thalamic Dysconnectivity in Schizophrenia
We computed an independent sample t-test on whole-brain connectivity maps using subject-specific anatomical thalamus seeds (see Materials and Methods). Results revealed robust between-group differences best described as increased coupling between thalamus and sensory cortices, but decreased coupling between the thalamus and prefrontal cortex (PFC), and striatum and cerebellum in schizophrenia (Fig. 1, see Supplementary Tables 1 and 2 for foci coordinates and Supplementary Fig. 1 for threshold-free maps across groups). We quantitatively verified that the observed patterns largely followed a dichotomy between sensory versus prefrontal–striatal–cerebellar systems (Supplementary Fig. 12). We computed a conjunction between voxels showing over-/under-connectivity and the sensory–motor map identified independently via resting-state by Power et al. [obtained with permission from Power et al. (2011)]. Results revealed a 61% spatial overlap between a priori-defined sensory–motor networks and regions showing over-connectivity (red foci) in patients. Conversely, less than 1% of all voxels identified as under-connected with the thalamus fell within the sensory–motor network boundaries. Both proportions significantly exceeded chance (binomial tests for proportions, P < 0.000001). While there are a few exceptions (see Supplementary Tables 1 and 2), these results quantitatively confirm that thalamic over-/under-connectivity found in schizophrenia follows a general anatomical dissociation between sensory–motor networks and prefrontal–striatal–cerebellar networks. We fully replicated this pattern in an independent sample, with comparable effect sizes (Fig. 2, see below for detail), and similar but attenuated patterns were found for bipolar patients (Supplementary Figs 1–3, later used for classification; see Supplementary Fig. 4 for a direct schizophrenia–bipolar contrast).
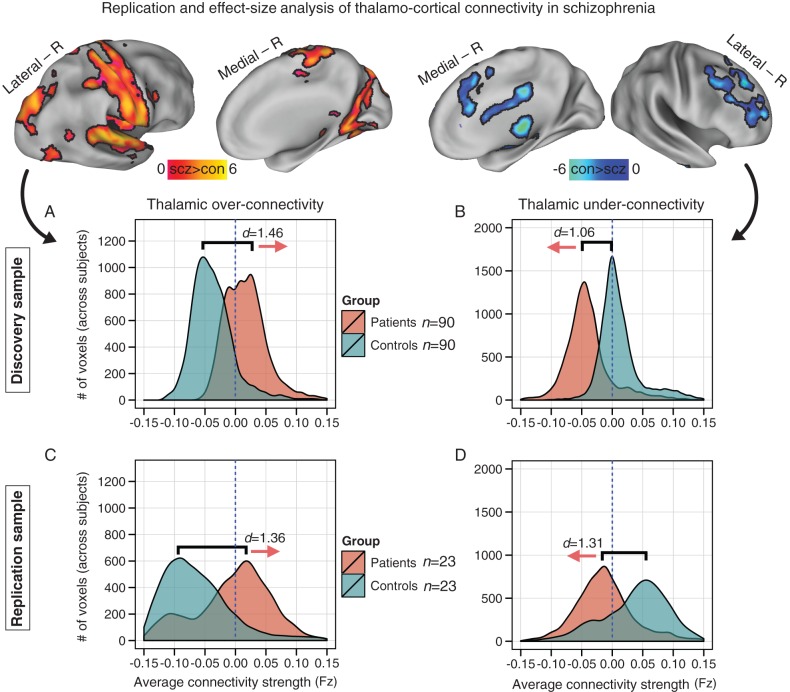
Figure 2.
Replication and effect-size analysis of thalamo-cortical connectivity in schizophrenia. Top panels show increased (left) versus reduced (right) thalamic coupling in schizophrenia. Distributions of average connection strengths for each voxel showing (A) increased and (B) reduced thalamic coupling in schizophrenia. (C and D) Independently diagnosed replication sample. Effect sizes (Cohen's d) indicate robust effects across samples. Blue vertical dashed lines mark the zero point, highlighting increased thalamic coupling with sensory–motor networks and decreased coupling with prefrontal–striatal and cerebellar regions for patients. Supplementary Figure 3 shows distributions for the bipolar sample; Supplementary Figure 4 shows schizophrenia versus bipolar contrast maps.
Relationship Between Symptoms and Thalamic Over-Connectivity in Schizophrenia
To test the functional significance of increased/reduced thalamic connectivity, we examined its association with symptom severity in schizophrenia (see Materials and Methods). We focused on overall symptom severity (PANSS total score) given: (1) No a priori predictions for any symptom class as to the expected pattern of thalamo-cortical coupling; (2) overall symptom severity provides a test for the functional significance of observed patterns, while avoiding stringent type I error correction needed for exploratory analyses. We correlated symptomatology with connectivity measures separately for areas showing reduced versus increased thalamic coupling in schizophrenia. A significant positive correlation between the PANSS total score and regions showing over-connectivity in patients [r = 0.22, P < 0.036, 2-tailed] indicates that patients with more severe symptoms exhibit stronger thalamic coupling with sensory cortices (Supplementary Fig. 5). Signal in regions showing reduced thalamic connectivity was not significantly related to symptoms (r = −0.09, P < 0.4). We conducted 2 exploratory follow-up analyses for positive and negative symptoms: There was an attenuated relationship for both positive (r = 0.11, P = 0.3, 2-tailed) and negative (r = 0.16, P = 0.13, 2-tailed) symptoms. However, the general psychopathology PANSS subscale showed a significant relationship with over-connectivity (r = 0.24, P < 0.023, 2-tailed). These results highlight a relationship between symptoms and whole-brain thalamic over-connectivity in schizophrenia, but also suggest that nonspecific illness severity may be more related to observed thalamic disturbance. We did not repeat analyses for the bipolar sample, because patients were remitted at the time of assessment (see Limitations and Supplementary Table 3).
Replication and Effect-Size Analysis of Thalamo-Cortical Dysconnectivity
We replicated findings in an independent sample of 23 schizophrenia patients and 23 matched healthy controls, collected, and diagnosed independently (Anticevic et al. 2011; Washington University School of Medicine; (Supplementary Table 4). The key reason for this independent replication is to highlight the robustness of present effects (via formal effect-size analyses) and provides a guide for future smaller and focused clinical or treatment outcome studies extending the present findings. Because identified regions showing increased/reduced thalamic coupling in the discovery sample were independent from the replication sample, we repeated the replication analysis within the mask identified with the discovery sample. All replication subjects met identical methodological stringency criteria as the discovery sample. We observed shifts in thalamic coupling across schizophrenia samples (Supplementary Figs 1 and 2), with comparable between-group effects sizes (Fig. 2). A similar, but reduced pattern was identified for bipolar patients (Supplementary Fig. 3, see Supplementary Fig. 4 for a direct schizophrenia–bipolar contrast), consistent with possibly attenuated disturbances in bipolar patients. These independent replications highlight the robust nature of the identified thalamo-cortical disturbances in schizophrenia and extend prior ROI-restricted reports.
Relationship Between Over- and Under-Connectivity
One possibility is that observed findings constitute dissociable disturbances in sensory–motor networks versus prefrontal–striatal–cerebellar nodes. To determine whether these findings represent functionally related or separable system-level disruptions, we correlated the thalamic coupling magnitude of these networks across subjects for regions showing reduced versus increased thalamic connectivity (Fig. 3A). As a baseline, we used healthy controls across all samples (N = 160), to test if coupling with thalamus is correlated across subjects. Controls with the lowest thalamo-prefrontal–cerebellar coupling showed the highest thalamo-sensory–motor coupling (r = −0.89, P < 7.5−57, Fig. 3B), suggesting that perturbations in thalamo-cortical information flow across these networks may represent related system-level phenomena. Schizophrenia patients (N = 113) exhibited a negative relationship (r = −0.68, P < 7.6−17, Fig. 3B), which was significantly reduced relative to healthy controls (Z = 4.86, P < 1.2−6) and relative to bipolar patients (Z = 2.22, P < 0.027). The coefficients between bipolar patients and healthy controls did not differ (P = 0.09, NS). Due to concerns about global signal removal potentially inducing some negative relationships (Murphy et al. 2009), we repeated analyses without this step, which did not alter the key patterns or group differences (see Materials and Methods). Note that all significant correlations survived Bonferroni correction and all findings held when examining schizophrenia and bipolar samples separately (Supplementary Figs 6 and 7).
Connectivity Differences Across Thalamic Nuclei in Schizophrenia
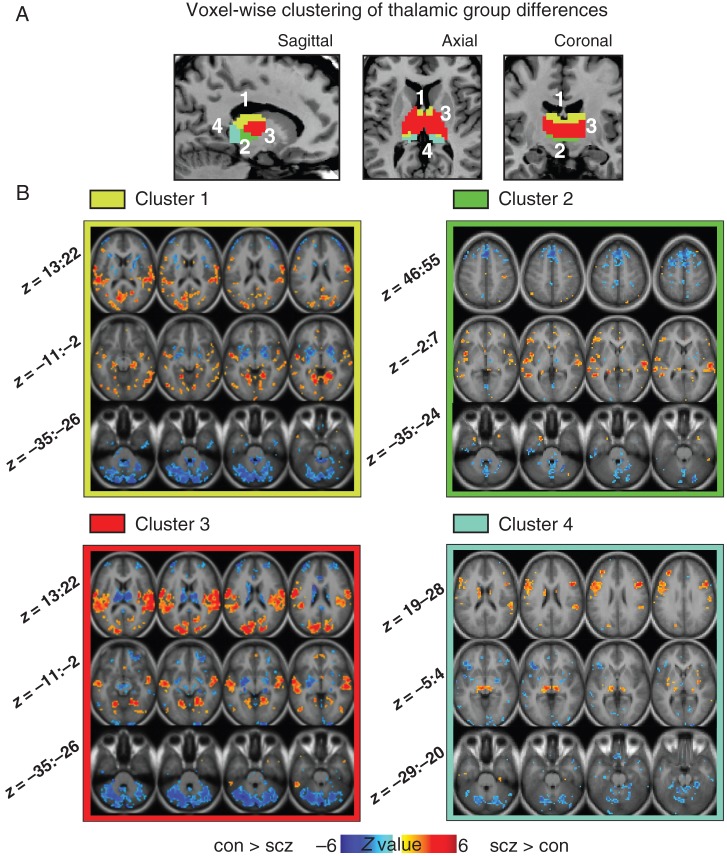
The thalamus is comprised of nuclei with dissociable communication patterns within thalamo-cortico-striatal loops (Haber and McFarland 2001). To pinpoint unique disturbances across thalamic subdivisions, we computed a cluster-based parcellation of between-group thalamic coupling patterns (Cauda et al. 2011; Yeo et al. 2011; see Materials and Methods, and Supplementary Fig. 8). We highlight a 4-cluster solution of between-group differences based on k-means clustering (Fig. 4A; see Supplementary Fig. 9 for a 6-cluster solution). Irrespective of clustering solution, results revealed a large cluster centered on the medio-dorsal thalamus (red cluster 3 in Fig. 4). Cluster 3, anatomically closest to higher-order associative thalamic nodes, showed a pattern of connectivity disturbance most similar to the main effect shown in Figure 1. Interestingly, certain clusters deviated somewhat from the generally identified pattern (Fig. 4B). Notably, the posterior cluster, corresponding to the pulvinar and lateral geniculate nucleus (Fig. 4, cyan), showed stronger connectivity with voxels around itself in patients, and stronger coupling with frontal eye fields (a similar pattern was reported previously; Woodward et al. 2012).
Figure 4.
Voxel-wise clustering of group differences in thalamic connectivity. (A) Results of 4-cluster solution identifying thalamic voxels with similar patterns of whole-brain connectivity differences between groups (see Supplementary Fig. 8 for workflow and Supplementary Fig. 9 for a 6-cluster solution). (B) Between-group difference maps when a given cluster is used as a seed. The pattern of between-group differences for cluster 3 (red) was qualitatively most similar to main effects (see Fig. 1), which roughly corresponds to higher-order associative thalamic nodes (Behrens et al. 2003). Z-coordinates as in Figure 1.
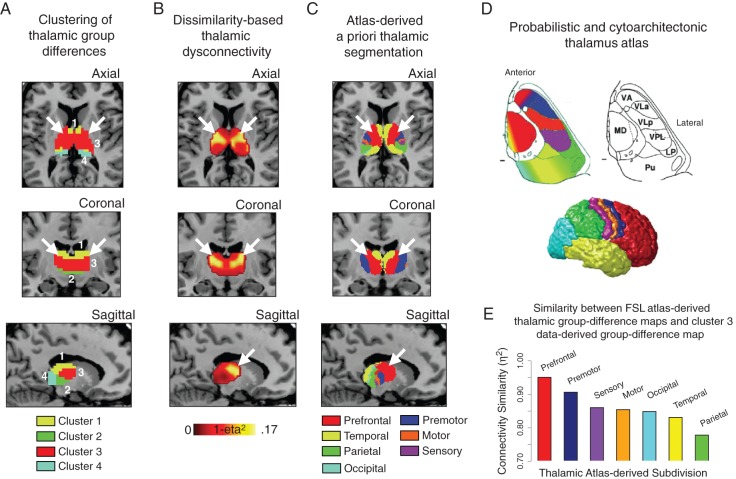
We also computed a voxel-wise dissimilarity index (1 − η2) (Cohen et al. 2008), showing the extent of between-group differences in thalamic connectivity patterns (see Supplementary Materials and Methods). Figure 5B shows the identified between-group thalamic dissimilarity gradient. Consistent with the clustering analysis, thalamic divisions associated with greatest group dissimilarity were centered on the medio-dorsal nucleus. To facilitate interpretation, we juxtaposed findings against previously validated atlas-based human thalamic subdivisions defined using diffusion tractography-based segmentation (Johansen-Berg et al. 2005) (Fig. 5C). This qualitatively confirmed that higher-order associative thalamic nuclei, previously established to project densely to the PFC, matched the most prominent data-driven dysconnectivity patterns. We verified this quantitatively by calculating a seed map from each anatomical subdivision, revealing that voxels with previously established strong anatomical PFC connections (Behrens et al. 2003) (Fig. 5C,D, red voxels) showed the highest similarity with overall between-group differences and clustering results (see Supplementary Fig. 10). A formal similarity index (η2) between dysconnectivity patterns of each anatomical subdivision and cluster 3 confirmed that nodes densely projecting to the PFC were most similar with data-driven clustering results centered on medio-dorsal thalamic aspects (Fig. 5E, see Supplementary Fig. 11 for a comprehensive similarity matrix of clustering vs. anatomy). That is, we selected each atlas-derived seed across thalamic nuclei (independent of this sample altogether; Behrens et al. 2003) to compute a group difference. We then quantified how similar each atlas-derived map was to the map identified from the data-derived medio-dorsal cluster. These effects collectively highlight—in both a data-driven and anatomically based fashion—that observed thalamic dysconnectivity in schizophrenia may be most severe for PFC-projecting thalamic nuclei.
Figure 5.
Intrinsic thalamic dysconnectivity in schizophrenia. (A) Results of 4-cluster solution identifying thalamic voxels with similar patterns of whole-brain connectivity differences between groups (as in Fig. 4). (B) Intrinsic thalamic dysconnectivity pattern based on group dissimilarity (1 − η2) (Jenkinson et al. 2012). Brightest voxels are associated with highest between-group differences. (C) Thalamus subdivisions based on the FSL thalamic atlas, to facilitate the comparison of data-driven dysconnectivity relative to the anatomy. White arrows show the correspondence across results for thalamic nodes with strong PFC connectivity. (D) Adapted with permission (Smith and Nichols 2009), to allow inspection of thalamic segmentation in comparison to between-group findings: (top-left), thalamic nuclei color-coded based on the major cortical connection site, (top-right) cytoarchitectonic atlas subdivisions (Saad et al. 2012), and (bottom) cortical sectors showing different patterns of thalamic anatomical connectivity. (E) Quantitative comparison of similarity (η2) between each anatomically based between-group differences map and cluster 3 from panel A. We used an FSL-based atlas-derived seeds across thalamic nuclei (independent of this sample altogether) to compute a group difference map. We then quantified how similar each atlas-derived seed result was to those identified from the medio-dorsal cluster in our data (to be distinguished from any anatomy-based analyses that are defined based on subject-specific data in the current sample). For all atlas-derived maps of between-group differences see Supplementary Figure 10; for similarity matrix comparing 4- and 6-cluster solutions relative to independent atlas-derived subdivisions see Supplementary Figure 11.
Diagnostic Classification Based on Thalamo-Cortical Dysconnectivity via MVPA
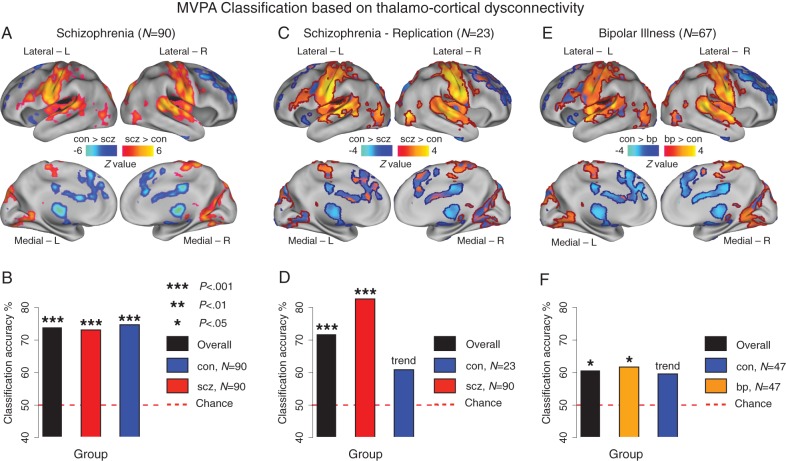
To establish whether thalamic dysconnectivity in schizophrenia has utility as a biomarker and possibly a diagnostic tool, we conducted an MVPA based on previously validated approaches (Norman et al. 2006; Cole, Etzel, et al. 2011). Classifying subjects as either a patient or control using whole-brain thalamic connectivity maps, with linear support vector machines and leave-one-subject-out cross-validation, we found that discovery sample subjects (N = 90) could be classified with 73.9% accuracy (P < 0.001) (73.1% for schizophrenia, P < 0.001 and 74.7% for controls, P < 0.001; Fig. 6B). We repeated analyses on the schizophrenia replication sample (N = 23) with similar results (71.7%, P < 0.001; 82.6% for patients, P < 0.001; 60.9% for controls, P = 0.06, trend; Fig. 6D). “Sensitivity” and “specificity” were 75.5 and 72.2 for the discovery sample and 67.9 and 77.8 for the replication sample, respectively.
Figure 6.
MVPA classification based on thalamo-cortical dysconnectivity. (A) As in Figure 1, schizophrenia (SCZ) results used to train the classifier. (B) Discovery sample results showing above-chance classification accuracy. (C) Between-group map for the SCZ replication sample (N = 23), shown unthresholded, masked with regions from panel A (to allow inspection relative to original findings). Red and blue borders mark whole-brain corrected increased and decreased thalamic connectivity, respectively, identified in the original SCZ sample. (D) SCZ replication classification. (E) Between-group difference map for bipolar patients (N = 67), again shown unthresholded, masked with panel A regions and surrounded by borders. (F) Bipolar disorder (BP) and control (CON) classification. For comprehensive visualization of volume-based type I error corrected group differences and unthresholded surface group contrast maps across samples, see Supplementary Figs 1, 2, and 4. Of note, sensitivity and specificity were 75.5 and 72.2, respectively, for the discovery sample and 67.9 and 77.8 for the replication sample.
Extending schizophrenia findings, we examined whether thalamic connectivity patterns in remitted bipolar patients may be similar to those observed in schizophrenia and sensitive to diagnostic classification. We specifically tested the hypothesis, motivated by NIMH RDoC initiative (Cuthbert and Insel 2010), whether observed dysconnectivity may be predictive across diagnostic categories that share clinical features (Glahn et al. 2007) and genetic risk (Potash 2006), given the co-occurrence of psychosis in bipolar illness (Glahn et al. 2007; Anticevic, Brumbaugh, et al. 2012). First, we computed seed-based thalamic connectivity for bipolar patients relative to matched controls within voxels identified in the discovery sample, which we juxtaposed with schizophrenia results for comparison. Results revealed similar patterns, marked by both increased and reduced thalamic connectivity (Fig. 6E and Supplementary Figs 1–3, see Supplementary Fig. 4 for a schizophrenia–bipolar contrast). Secondly, classification results revealed slightly reduced, but above-chance accuracy for bipolar patients (61.7%, P < 0.038) and matched controls (59.6%, P = 0.055, trend) (Fig. 6F), suggesting that thalamic dysconnectivity is sensitive to classification across diagnoses that may share clinical features but perhaps indicting a less-severe patter of disturbance in bipolar patients. Taken together, findings suggest that thalamic connectivity-based classification was successful when applied to schizophrenia, and that thalamic connectivity patterns were somewhat predictive of classification for bipolar disorder, supporting the hypothesis that there may be shared neural disturbances across these diagnoses (Keshavan et al. 2011).
Discussion
In one of the largest schizophrenia neuroimaging samples to date, followed by a smaller-independent replication sample, we found a complex pattern of increased thalamic connectivity with all sensory–motor cortices, but reduced thalamic connectivity with the PFC, dorsal striatum, and cerebellum, extending recent focused ROI-based investigations (Welsh et al. 2010; Woodward et al. 2012). Across samples, we showed that the 2 disturbances are likely functionally related. We localized thalamic disturbances in schizophrenia to nuclei densely connected to the PFC and higher-order associative cortical regions (medio-dorsal regions). Present findings relate to the long-standing focus on PFC-mediated executive deficits (Goldman-Rakic 1991; Weinberger et al. 1991; Carter et al. 1998; Barch and Ceaser 2012), stressing the important role of higher-order multimodal cortex in schizophrenia (Pearlson et al. 1996; Ross and Pearlson 1996; Cannon et al. 2002). These results also need to be considered in the context of proposals detailing thalamic filtering functions that have emerged from animal (Carlsson and Carlsson 1990b) and computational models (Lisman et al. 2010; Lisman 2012), as well as hypotheses relating altered sensory processing to core symptoms of the disorder (Hoffman et al. 1995; Kapur et al. 2005; Corlett, Murray, et al. 2007; Ford et al. 2007). Finally, classification findings suggest that thalamo-cortical disruptions may provide a sensitive marker across psychotic conditions with shared clinical features and potential genetic risk. Collectively, this manuscript is the first examination of whole-brain thalamo-cortical disturbances across psychiatric conditions that may present with psychotic symptoms. In addition, these results are the first to show the most prominent locus of thalamic dysconnectivity in schizophrenia is centered on the medio-dorsal nucleus.
Disrupted Thalamic Information Flow in Schizophrenia
Disruptions in the interplay of thalamic nuclei with their afferent and efferent cortical connections in schizophrenia may profoundly affect large-scale cortical function (Lisman et al. 2010). Indeed, prior studies focusing on the medio-dorsal thalamus, and prefrontal regions showed reduced connectivity in schizophrenia (Woodward et al. 2012). However, Woodward et al. note that (p. 1097) “…use of large cortical areas as seeds, while useful for functionally segregating the thalamus, does not allow for a more fine-grained analysis at the cortical level.” In contrast, Welsh et al. (2010) used a thalamic seed-based approach, but given restricted sample size did not have sufficient power to detect subtle disturbances. We addressed both concerns with a large discovery and an independent replication sample. Across samples, schizophrenia was associated with significantly reduced prefrontal–cerebellar–thalamic coupling, but also increased coupling with all bilateral sensory–motor cortices. This complex shift in thalamic information flow, without the constraint of large cortical ROIs, provides the first whole-brain evidence that, in schizophrenia, thalamic connectivity may not differentiate between the prefrontal and sensory cortex, possibly reflective of a “blurring” between sensory–prefrontal signals flowing through the thalamus.
We further show that these over-/under-connectivity disruptions are likely related: Control subjects with lower prefrontal–striatal–cerebellar–thalamic coupling exhibited increased sensory–motor–thalamic coupling, a robust effect replicated across independent well-powered samples. In schizophrenia, there was a drop in this relationship, but not for bipolar patients (Supplementary Figs 6 and 7), suggesting that functional interactions between large-scale thalamo-cortical systems may be particularly perturbed in schizophrenia. The strength of identified correlation in controls suggests that these are functionally connected processes linked by some, yet unknown, mechanisms. This is an important consideration related to possible neurodevelopmental anomalies in specific patterns of thalamo-cortical communication in schizophrenia. Such functionally related alterations in thalamo-cortical connectivity may also have implications for treatments designed to restore neural function. For instance, drugs that may diffusely reduce cortical connectivity, perhaps like benzodiazepines or group II metabotropic glutamate receptor agonists might exacerbate some executive cognitive deficits. In contrast, interventions with regional selectivity, like low-frequency transcranial magnetic stimulation, might treat some symptoms without this potential risk (Hoffman et al. 1999; Fox et al. 2012; Demirtas-Tatlidede et al. 2013). These are speculative possibilities, but prospective investigations incorporating detailed computational models of thalamo-cortical loops may deepen our intuition for such complex dynamics, particularly in response to possible treatment regiments (Lisman et al. 2010).
Prior studies could not distinguish if there is anatomical overlap between portions of the thalamus exhibiting decreased connectivity with the PFC and that exhibiting increased connectivity with the sensory–motor cortex. While ROI-based analyses were an important starting point, here, we show that the same cluster exhibiting decreased prefrontal–cerebellar connectivity also exhibits increased connectivity with motor, temporal, and sensory cortices. Dorso-lateral and anterior cingulate cortex also exhibited severe disruptions in schizophrenia rather than in the entire PFC (see Supplementary Figs 1 and 2 for a juxtaposition of group maps). Both regions have been linked to cognitive control and are compromised in schizophrenia (Barch and Ceaser 2012). In addition, results suggest no “clean” division in lower connectivity of one thalamic node and stronger connectivity of another node. Rather, information flow in the same thalamic division is altered with both PFC and sensory cortex—again suggesting that these are likely not independent network-level disturbances in schizophrenia.
We also found that increased thalamo-sensory coupling predicted schizophrenia symptoms. We did not parse symptom categories to avoid stringent type I error correction, given no a priori predictions with regard to symptom dimensions. The overarching aim was to elucidate a central disruption in information flow across thalamo-cortical networks, thus avoiding the “single symptom–single localization” strategy (Andreasen 1997). Thalamo-cortical communication disturbance could provide such a mechanism (Carlsson and Carlsson 1990b). The observed individual differences in symptoms highlight the functional relevance of increased thalamic coupling—the effect was subtle but significant given the sample size. Such a subtle relationship raises the possibility that, while thalamo-cortical disturbances scale with symptoms, they may be present at a given level in most of the patients. Importantly, the thalamo-sensory coupling predicted overall psychopathology in schizophrenia subjects, but not positive or negative symptom clusters, per se. Perhaps, this functional connectivity measure better assesses the nonspecific psychopathology that cooccurs in schizophrenia subjects rather than reflecting any one specific feature of the illness. This possibility needs to be verified in prospective studies. Collectively, these findings extend prior reports and indicate robust sensory–motor–thalamic and prefrontal–cerebellar–thalamic disturbances, consistent with theoretical models of thalamic alterations in schizophrenia (Andreasen 1997).
Clustering and Dissimilarity Findings Point to Medio-Dorsal Nucleus in Schizophrenia
Initially, we examined the thalamus as a whole, defined using subject-specific anatomical segmentation. This provided large and precise individual-specific seeds to test group differences irrespective of a given nucleus. However, theoretical and empirical schizophrenia studies have implicated disturbances in specific thalamic nodes communicating with prefrontal networks (Lynall et al. 2010; van den Heuvel et al. 2010; Salomon et al. 2011). Thus, we localized thalamic nodes associated with large between-group differences via 3 complementary approaches: (1) Clustering of between-group differences in thalamic coupling; (2) voxel-wise thalamic dysconnectivity assessment via a quantitative dissimilarity index; (3) direct comparison to all thalamic nuclei defined via a well-validated human diffusion tractography-based segmentation atlas (Johansen-Berg et al. 2005). All approaches converged on a disturbance in thalamic nodes projecting densely to the PFC (Johansen-Berg et al. 2005). This does not imply that other thalamic subdivisions were disturbance-free in schizophrenia (Woodward et al. 2012) nor does it minimize the functional importance of sensory–motor system disturbance. Indeed, interesting patterns emerged across clustering solutions and from thalamic anatomy analyses. For instance, posterior thalamic nodes centered on the pulvinar exhibited a pattern, whereby patients showed stronger coupling with voxels around the pulvinar itself and stronger coupling with frontal eye fields. This dissociation of local over-connectivity perhaps reflects the role of posterior thalamic nuclei in sensory processing versus thalamic nodes with dense prefrontal projections. Yet, the pattern most consistent with the overall effects, and thus likely the biggest source of disturbance in schizophrenia, was found for thalamic nodes that are known to be connected with the PFC. This is consistent with a theoretical model postulating a functional disturbance in thalamo-cortical loops in schizophrenia that encompass prefrontal networks (Lisman et al. 2010). An open question, difficult to address here, is the directionality of thalamo-cortical disturbance. Below we discuss hypothesized mechanisms and theoretical possibilities in light of present findings, which may ultimately be helpful for understanding the direction/locus of disruption and guide treatment strategies.
Thalamic Connectivity and Diagnostic Classification
Discovery and replication analyses support the inference for thalamo-cortical coupling disturbances in schizophrenia. A fundamental challenge in clinical neuroscience is identifying biomarkers (Meyer-Lindenberg 2010) reflective of common alterations in neural systems across neuropsychiatric conditions exhibiting similar symptoms. We hypothesized that thalamo-cortical coupling may be sensitive to such shared disturbances, and that some alterations found in schizophrenia may occur in bipolar disorder. We tested this hypothesis in 2 ways: (i) Seed-based replication, providing qualitative illustration of patterns across samples, and (2) quantitative group membership prediction via support vector machine classification. Bipolar individuals exhibited a pattern of thalamic disturbances similar to schizophrenia (Fig. 6E), although there was a quantitatively less-profound disruption (Supplementary Figs 3, 6, and 7). Perhaps, a disturbance in thalamo-cortical systems across these illnesses reflects a severity index. Indeed, a “graded” pattern of disturbances across diagnostic categories would be in accord with proposals that psychosis may arise due to processes that overlap in their functional anatomy across phenotypes. This raises the possibility that these apparently separate clinical illnesses represent different endpoint phenomenological expressions of similar underlying neural circuit problems, consistent with the NIMH RDoC initiative (Cuthbert and Insel 2010). Nevertheless, it is certainly noteworthy that there were differences between bipolar individuals and those diagnosed with schizophrenia. Therefore, we cannot definitively conclude that bipolar illness is indeed “schizophrenia-like” in every respect. Perhaps, those bipolar individuals with a psychosis history are closer to alterations found in schizophrenia. Of note, in the present bipolar sample, 33 patients also had a history of cooccurring psychosis. While we did not pursue additional analyses distinguishing these subgroups of bipolar patients, it will be critical to establish whether cooccurrence of psychosis in bipolar illness is associated with a more “schizophrenia-like” pattern. Another key challenge here is taking into account the accuracy of behaviorally based diagnostic classification (First et al. 2002) relative to the sensitivity of the imaging methods. Future studies should investigate whether altering the diagnostic stringency criteria changes classification accuracy, to pinpoint the sensitivity/specificity of imaging markers. Alternatively, prospective investigations could hone in on clinically significant symptoms within a given symptom domain and could test whether the classifier analysis picks them out accurately. Such finer-grained classification along the symptom spectrum will be vital to refine the precision of our imaging markers. Similarly, future studies investigating siblings of patients could help refine our understanding of the severity gradient of thalamo-cortical disturbances.
Toward Understanding the Mechanisms of Thalamo-Cortical Disruptions in Schizophrenia
Identified thalamo-cortical disruptions in schizophrenia seem to follow a functional anatomical dissociation between frontal–control and sensory cortices, in line with recent anatomical evidence (Marenco et al. 2012). While compelling, these patterns cannot speak to neuronal mechanisms that may underlie the observed thalamo-cortical abnormalities. It is tempting to speculate how hypothesized neuropathological disturbances in schizophrenia may relate to identified patterns. There is increasing evidence from preclinical, pharmacological, and postmortem clinical studies implicating disrupted excitation and inhibition balance (E/I balance) within cortical microcircuitry in schizophrenia (Marin 2012). This could reflect a number of altered pathways, involving a confluence of glutamate (Krystal et al. 2003; Macdonald and Chafee 2006), γ-aminobutyric acid, (Lewis et al. 2005) and dopamine disturbance (Laruelle et al. 2003). How can we reconcile hypothesized cellular-level disruption with observed system-level cortico-thalamic abnormalities? One emerging hypothesis in schizophrenia implicates disruption of inhibitory interneurons, which results in disinhibition of cortical circuits. We recently detailed a computational model, where we identified cortical disinhibition as a key mechanism for disrupted long-range interactions between cortical areas that may operate in schizophrenia (Anticevic, Gancsos, et al. 2012). As an extension of this hypothesis, a cortex-wide disruption in appropriate E/I balance might de-stabilize thalamo-cortical information flow in ways observed presently. Better understanding of such hypothetical mechanisms awaits future studies designed to close the gaps between our emerging cellular hypotheses and system-level observations.
In light of complex thalamo-cortical disruptions in schizophrenia, a possible locus and direction of disturbed information flow remains to be determined. Can the observed dysconnectivity be linked to one node or does it involve a dynamical disturbance across interconnected systems that makes directionality less central (Loh et al. 2007)? We previously identified increased connectivity between dorso-lateral PFC and sensory regions in schizophrenia (Cole, Anticevic, et al. 2011). Emerging information regarding disruptions of both cortico-cortical and thalamo-cortical connectivity suggests 3 patterns in schizophrenia: (1) Reduced prefrontal–thalamic coupling; (2) increased prefrontal–sensory coupling; and (3) increased thalamus–sensory coupling. PFC is involved in gating information flow and can exert inhibitory top-down control over thalamic nuclei through projections via the basal ganglia (Haber and McFarland 2001), whereas sensory regions are considered as a source of drive onto the thalamus. Within this framework, we could hypothesize a primary top-down disruption, with secondary, thalamo-sensory hyper-connectivity; or a thalamic locus. This is not meant to imply that the sensory–motor effects are unimportant, as they, in fact, scaled with symptoms (whereas the prefrontal one did not). Nevertheless, a symptom-related finding does not necessarily imply that the sensory–motor disturbance is the “source” or “cause,” as this effect is correlational. One possibility is that every patient has a thalamic-prefrontal disturbance, but the magnitude of sensory gating abnormalities predicts psychopathology. Given the architecture of prefrontal and medio-dorsal thalamic connections, and the focally more severe pattern of disturbances in this nucleus, it is perhaps likely that a PFC and/or thalamic locus may be implicated, as supported by preclinical evidence (Parnaudeau et al. 2013). While future studies may shed light on hypothesized directionality/locus of present observations, we need to consider explanatory schemes in light of identified system-level disruptions, whereby a parsimonious mechanism could account for both increased and reduced thalamo-cortical coupling in schizophrenia. Irrespective of a particular pathogenesis mechanism (Jaaro-Peled et al. 2009), observed and replicated thalamo-cortical disruptions may reflect, at least at the neural system level, a final common pathway in schizophrenia. Detailed computational models of low-frequency cortical dynamics captured with BOLD, which also incorporate noted cortical perturbations hypothesized to occur in schizophrenia (Anticevic, Gancsos, et al. 2012), will be crucial to understand complex dynamical disturbances in thalamo-cortical information flow.
Limitations
Some limitations need to be considered. The schizophrenia sample, although well characterized and one of the largest to date, was associated with comorbid history of drug/alcohol abuse/dependence to provide more generalizability to patients typically encountered in the population. However, history of substance abuse is a complex issue in clinical populations, which may partially confound imaging studies. Patients were also medicated. Although medication dose did not alter effects statistically, long-term medication use might impact the pattern of thalamo-cortical connectivity independently of the illness [given known dopamine and glutamate influence on thalamo-cortical function (Carlsson et al. 2001)]. This could not be addressed in our replication sample as those patients were also receiving stable medication (Anticevic et al. 2011). Effects in bipolar illness suggest that reported findings are unlikely to occur purely due to schizophrenia medications (because bipolar patients are primarily treated by a different medication class). Alternatively, perhaps the type of mediations the bipolar subjects were tasking had an attenuated effect compared with the effect of the type of mediation schizophrenia subjects were on. Also of note, the mean chlorpromazine equivalents (229 mg/day) were relatively lower for the larger sample of SZ subjects, compared with the replication sample (585 mg/day). If anything, this would argue for the robustness of present effects, as the patterns replicate despite a difference in medication levels across sites. However, chlorpromazine equivalents for atypical neuroleptics remain somewhat controversial, as it is not straightforward how one would calculate comparable dosing and relative potency, given presumed differences in mechanisms of action. Thus, carefully follow-up studies will need to consider differences in typical and atypical medication. For these reasons, it will be critical to replicate findings across unmedicated, prodromal, at-risk, or first-degree relatives of patients. In addition, it is important to establish whether medication of any kind has the potential to reverse observed effects. We could not address this question in our study. While present control analyses argue against medication confounds, medication remains a vital consideration for future studies.
Schizophrenia patients across discovery and replication samples were generally quite symptomatic, which may have restricted the range of thalamo-cortical disturbances that scale with symptoms. Future studies should examine whether identified connectivity patterns remain unaltered or worsen during acute psychosis. Such investigations would help disambiguate whether observed disturbances are stable within a subject or scale as a function of symptom exacerbation. This is vital, because bipolar results suggest that observed patterns might be somewhat stable (bipolar patients were in remission at the time of the scan). Relatedly, the absence of mania measures needs to be considered: Future work should dissociate the features of bipolar illness that are schizophrenia-like, and in turn, examine features of schizophrenia that may be bipolar-like. Also, we did not examine thalamic localization in bipolar illness nor can we conclude from classification analyses what precisely discriminates schizophrenia from bipolar disorder. Carefully matched follow-up studies should ascertain if similar or distinct thalamic circuits are involved across diagnoses and determine whether schizophrenia and bipolar illness are dissociable based on thalamic connectivity (although Supplementary Fig. 4 provides clues). It is important to consider that our IQ measures, while valid at estimating premorbid intellectual functioning, likely do not reflect the full complexity of the IQ construct if we were to employ a more complete cognitive battery. While group IQ differences did not explain present effects, it remains possible that observed thalamic dysconnectivity may relate to more complex cognitive deficits in schizophrenia (Parnaudeau et al. 2013), which future studies should investigate.
Because of correlational measures, it is unclear whether changes reflect the cause or the consequence of the illness, which may be associated with dynamical circuit alterations over time. To disambiguate these causal possibilities, it will be important to determine if observed disturbances relate to illness duration, number of psychotic episodes, and occur in at-risk populations. Although beyond the scope of this investigation, future connectivity studies also need to carefully consider the use of correlation coefficients as indexes of neuronal communication (see Friston 2011). Finally, a methodological concern relates to tracing of the thalamus as an ROI, which can be challenging as there is a good deal of partial volume effects (with voxels containing different tissue classes, e.g., both gray matter and white matter) around thalamus edges. This makes delineation of this subcortical structure particularly challenging, either manually or automatically. We addressed this issue partially via our clustering and thalamic dysconnectivity analyses, as all thalamic ROIs were nonlinearly registered to the same template (removing possible volume size/shape concerns). Even with this step results remained unchanged. Moreover, prior studies argue against gross registration/volume issues for subcortical nuclei in schizophrenia (Anticevic, Repovs, Van Snellenberg, et al. 2010). Nevertheless, subtle inaccuracies in the tracing of the thalamus might influence functional connectivity results.
Conclusion
This is the first study to comprehensively characterize, in a data-driven and anatomically based fashion, thalamic connectivity alterations in schizophrenia and bipolar illness. This is also one of the largest functional connectivity schizophrenia investigations in the literature, also providing a fully independent replication and evidence for cross-diagnostic relevance of reported findings. We found robust reductions in thalamo-prefrontal–striatal–cerebellar coupling, but also a symptom-related increase in thalamo-sensory–motor coupling in schizophrenia. Both schizophrenia samples indicated strong and likely functionally related thalamic connectivity disturbances across these large-scale networks. Clustering and data-driven efforts revealed a complex pattern based on intrinsic thalamic differences, but a key motif emerged—thalamic nodes associated with strongest disturbances were previously found to densely project to the PFC. Classification and cross-diagnostic results suggest that thalamic dysconnectivity may provide a sensitive large-scale neural system measure for classification across schizophrenia and bipolar illness. Collectively, these effects suggest that disruptions in thalamo-cortical networks may have promise as a marker for treatment effects in future clinical studies and might constitute a final common pathway of neural system disturbances in schizophrenia.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Financial support for this study was provided by NIH grant MH080912 (PI: D.C.G.), NIH grant DP5OD012109-01 (PI: A.A.), NIAAA grant 2P50AA012870-11 (PI: J.H.K.), NIH grant MH096801 (PI: M.W.C.), and NIH grants MH43775, MH077945, and MH074797 (PI: G.D.P.), Fulbright Foundation (A.S.) and the Brain and Behavior Research Foundation Young Investigator Award (PI: A.A.).
Supplementary Material
Notes
We thank Drs Deanna M. Barch and David Van Essen for helpful comments and feedback during preparation of the manuscript as well as for assistance with replication sample data collection. We thank Jonathan Power for providing us with the previously published data. Lastly, we thank two anonymous reviewers for their constructive feedback and helpful suggestions on how to improve the manuscript. Conflict of Interest: J.H.K. consults for several pharmaceutical and biotechnology companies with compensation less than $10 000 per year. All other authors declare that they have no conflict of interest.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The role of the thalamus in schizophrenia. Can J Psychiatry. 1997;42:27–33. doi: 10.1177/070674379704200104. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Swayze V, Cizadlo T, Flaum M, O'Leary D, Ehrhardt JC, Yuh WTC. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho B-C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, Kober H, Gruber J, Repovs G, Cole MW, et al. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 2012;73:565–573. doi: 10.1016/j.biopsych.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, Niciu MJ, Morgan PT, Surti TS, Bloch MH, et al. NMDA receptor function in large-scale anti-correlated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci USA. 2012;109:16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Barch DM. Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophr Bull. 2012;38:967–980. doi: 10.1093/schbul/sbq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Barch DM. Resisting Emotional Interference: Brain Regions Facilitating Working Memory Performance During Negative Distraction. Cogn Affect Behav Neurosci. 2010;10:159–173. doi: 10.3758/CABN.10.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Corlett PR, Barch DM. Negative and Non-emotional Interference with Visual Working Memory in Schizophrenia. Biol Psychiatry. 2011;70:1159–1168. doi: 10.1016/j.biopsych.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Van Snellenberg JX, Csernansky JG, Barch DM. Subcortical alignment precision in patients with schizophrenia. Schizophr Res. 2010;120:76–83. doi: 10.1016/j.schres.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Braver TS. Cognitive control in schizophrenia: psychological and neural mechanisms. In: Engle RW, Sedek G, von Hecker U, McIntosh AM, editors. Cognitive limitations in aging and psychopathology. Cambridge: Cambridge University Press; 2007. pp. 122–159. [Google Scholar]
- Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, Lonnqvist J, Standerskjold-Nordenstam CG, Narr KL, Khaledy M, et al. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci USA. 2002;99:3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia—implications for schizophrenia and Parkinson's disease. Trends Neurosci. 1990a;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Schizophrenia: a subcortical neurotransmitter imbalance syndrome? Schizophr Bull. 1990b;16:425–432. doi: 10.1093/schbul/16.3.425. [DOI] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Cohen AL, Fair DA, Dosenbach NU, Miezin FM, Dierker D, Van Essen DC, Schlaggar BL, Petersen SE. Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage. 2008;41:45–57. doi: 10.1016/j.neuroimage.2008.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Anticevic A, Repovs G, Barch DM. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70:43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Etzel JA, Zacks JM, Schneider W, Braver TS. Rapid transfer of abstract rules to novel contexts in human lateral prefrontal cortex. Front Hum Neurosci. 2011;5:142. doi: 10.3389/fnhum.2011.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Honey GD, Fletcher PC. From prediction error to psychosis: ketamine as a pharmacological model of delusions. J Psychopharmacol. 2007;21:238–252. doi: 10.1177/0269881107077716. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Murray GK, Honey GD, Aitken MR, Shanks DR, Robbins TW, Bullmore ET, Dickinson A, Fletcher PC. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain. 2007;130:2387–2400. doi: 10.1093/brain/awm173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronenwett WJ, Csernansky J. Thalamic pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:509–528. doi: 10.1007/7854_2010_55. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Schindler MK, Splinter NR, Wang L, Gado M, Selemon LD, Rastogi-Cruz D, Posener JA, Thompson PA, Miller MI. Abnormalities of thalamic volume and shape in schizophrenia. Am J Psychiatry. 2004;161:896–902. doi: 10.1176/appi.ajp.161.5.896. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36:1061–1062. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566–578. doi: 10.1016/j.neuropharm.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M. Whole brain segmentation automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Ségonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Ford JM, Krystal JH, Mathalon DH. Neural synchrony in schizophrenia: from networks to new treatments. Schizophr Bull. 2007;33:848–852. doi: 10.1093/schbul/sbm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Zhang D, Snyder A, Raichle M. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain Connectivity. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62:910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Prefrontal cortical dysfunction in schizophrenia: the relevance of working memory. In: Carroll BJ, Barrett JE, editors. Psychopathology and the brain. New York: Raven Press, Ltd; 1991. pp. 1–23. [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guller Y, Ferrarelli F, Shackman AJ, Sarasso S, Peterson MJ, Langheim FJ, Meyerand ME, Tononi G, Postle BR. Probing thalamic integrity in schizophrenia using concurrent transcranial magnetic stimulation and functional magnetic resonance imaging. Arch Gen Psychiatry. 2012;69:662–671. doi: 10.1001/archgenpsychiatry.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist. 2001;7:315–324. doi: 10.1177/107385840100700408. [DOI] [PubMed] [Google Scholar]
- Harms MP, Wang L, Mamah D, Barch DM, Thompson PA, Csernansky JG. Thalamic shape abnormalities in individuals with schizophrenia and their nonpsychotic siblings. J Neurosci. 2007;27:13835–13842. doi: 10.1523/JNEUROSCI.2571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RE, Boutros NN, Berman RM, Roessler E, Belger A, Krystal JH, Charney DS. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated "voices". Biol Psychiatry. 1999;46:130–132. doi: 10.1016/s0006-3223(98)00358-8. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Rapaport J, Ameli R, McGlashan TH, Harcherik D, Servan-Schreiber D. A neural network simulation of hallucinated “voices” and associated speech perception impairments in schizophrenic patients. J Cogn Neurosci. 1995;7:479–496. doi: 10.1162/jocn.1995.7.4.479. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Lakshmanan B, Freudenreich O, Goff DC, Rauch SL, Kuperberg GR. Dysfunction of a cortical midline network during emotional appraisals in schizophrenia. Schizophr Bull. 2011;37:164–176. doi: 10.1093/schbul/sbp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62 doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, Matthews PM. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15:31–39. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- Kapur S. Li M, editor. R. M. From dopamine to salience to psychosis–linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Morris DW, Sweeney JA, Pearlson GD, Thaker G, Seidman LJ, Eack SM, Tamminga C. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res. 2011;133:250–254. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 2003;1003:138–158. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Is there a neuropathology of schizophrenia? Recent findings converge on altered thalamic-prefrontal cortical connectivity. Neuroscientist. 2000;6:208–218. [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol. 2012;22:537–544. doi: 10.1016/j.conb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry. 2010;68:17–24. doi: 10.1016/j.biopsych.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh M, Rolls ET, Deco G. A dynamical systems hypothesis of schizophrenia. PLoS Comput Biol. 2007;3:e228. doi: 10.1371/journal.pcbi.0030228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall M-E, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald AW, Chafee MV. Translational and developmental perspective on N-methyl-d-aspartate synaptic deficits in schizophrenia. Development Psychopathol. 2006;18:853–876. [PubMed] [Google Scholar]
- Marenco S, Stein JL, Savostyanova AA, Sambataro F, Tan HY, Goldman AL, Verchinski BA, Barnett AS, Dickinson D, Apud JA, et al. Investigation of anatomical thalamo-cortical connectivity and fMRI activation in schizophrenia. Neuropsychopharmacology. 2012;37:499–507. doi: 10.1038/npp.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- Meda SA, Gill A, Stevens MC, Lorenzoni RP, Glahn DC, Calhoun VD, Sweeney JA, Tamminga CA, Keshavan MS, Thaker GK, et al. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry. 2012;71:881–889. doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Nature. 2010;468:194–202. doi: 10.1038/nature09569. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD Harvard School of Public Health., World Health Organization., World Bank. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Cambridge (MA): Harvard School of Public Health on behalf of the World Health Organization and the World Bank; Distributed by Harvard University Press; 1996. [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Oltmanns TF, Neale JM. Schizophrenic performance when distractors are present: attentional deficit or differential task difficulty? J Abnorm Psychol. 1975;84:205–209. doi: 10.1037/h0076721. [DOI] [PubMed] [Google Scholar]
- Parnaudeau S, O'Neill PK, Bolkan SS, Ward RD, Abbas AI, Roth BL, Balsam PD, Gordon JA, Kellendonk C. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77:1151–1162. doi: 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson GD, Petty RG, Ross CA, Tien AY. Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology. 1996;14:1–17. doi: 10.1016/S0893-133X(96)80054-6. [DOI] [PubMed] [Google Scholar]
- Potash JB. Carving chaos: genetics and the classification of mood and psychotic syndromes. Harv Rev Psychiatry. 2006;14:47–63. doi: 10.1080/10673220600655780. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012a;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage. 2012b;76:439–441. doi: 10.1016/j.neuroimage.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- Repovs G, Barch DM. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Front Hum Neurosci. 2012;6:137. doi: 10.3389/fnhum.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 2011;15:967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Pearlson GD. Schizophrenia, the heteromodal association neocortex and development: potential for a neurogenetic approach. Trends Neurosci. 1996;19:171–176. doi: 10.1016/s0166-2236(96)10022-9. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connectivity. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R, Bleich-Cohen M, Hahamy-Dubossarsky A, Dinstien I, Weizman R, Poyurovsky M, Kupchik M, Kotler M, Hendler T, Malach R. Global functional connectivity deficits in schizophrenia depend on behavioral state. J Neurosci. 2011;31:12972–12981. doi: 10.1523/JNEUROSCI.2987-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Begovic A, Rakic P. Selective reduction of neuron number and volume of the mediodorsal nucleus of the thalamus in macaques following irradiation at early gestational ages. J Comp Neurol. 2009;515:454–464. doi: 10.1002/cne.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RCW, Stam CJ, Kahn RS, Pol HEH. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2010;30:15915–15926. doi: 10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snellenberg JX, de Candia T. Meta-analytic evidence for familial coaggregation of schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2009;66:748–755. doi: 10.1001/archgenpsychiatry.2009.64. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Effects of a mediodorsal thalamus lesion on prefrontal inhibitory circuitry: Implications for schizophrenia. Biol Psychiatry. 2003;53:385–389. doi: 10.1016/s0006-3223(02)01547-0. [DOI] [PubMed] [Google Scholar]
- Walker E, Kestler L, Bollini A, Hochman KM. Schizophrenia: etiology and course. Annu Rev Psychol. 2004;55:401–430. doi: 10.1146/annurev.psych.55.090902.141950. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Daniel DG. Prefrontal cortex dysfunction in schizophrenia. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal lobe function and dysfunction. New York: Oxford University Press; 1991. pp. 276–285. [Google Scholar]
- Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36:713–722. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Tamir D, Minzenberg MJ, Ragland DJ, Ursu S, Carter CS. Multivariate pattern analysis of functional magnetic resonance imaging data reveals deficits in distributed representations in schizophrenia. Biol Psychiatry. 2008;64:1035–1041. doi: 10.1016/j.biopsych.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20:1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.