Abstract
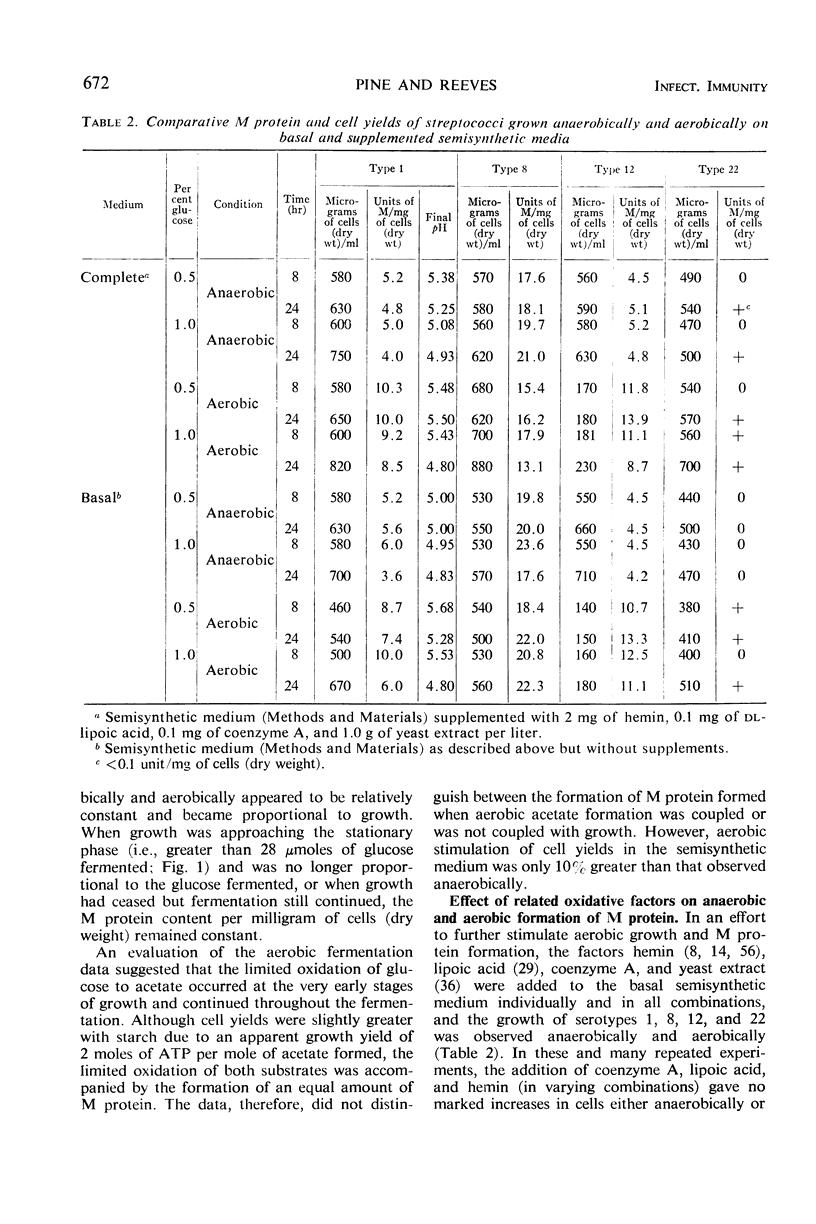
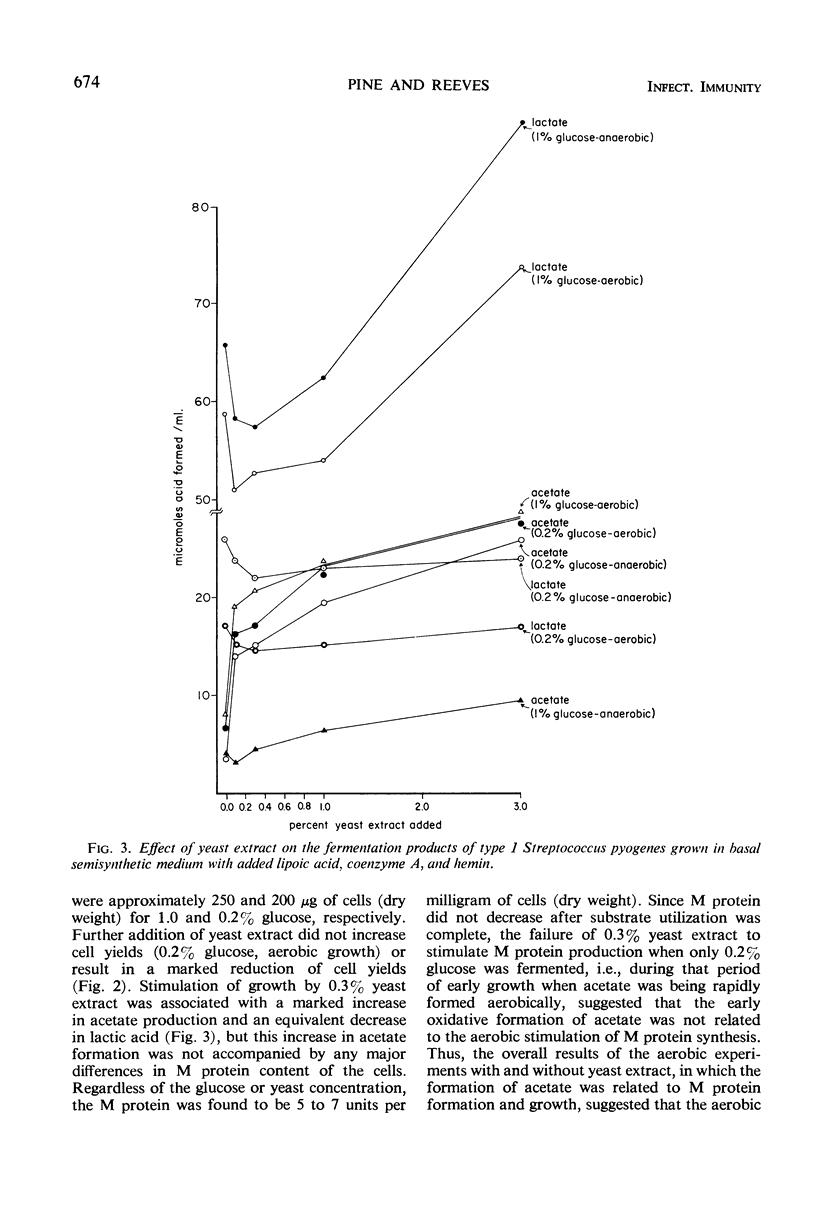
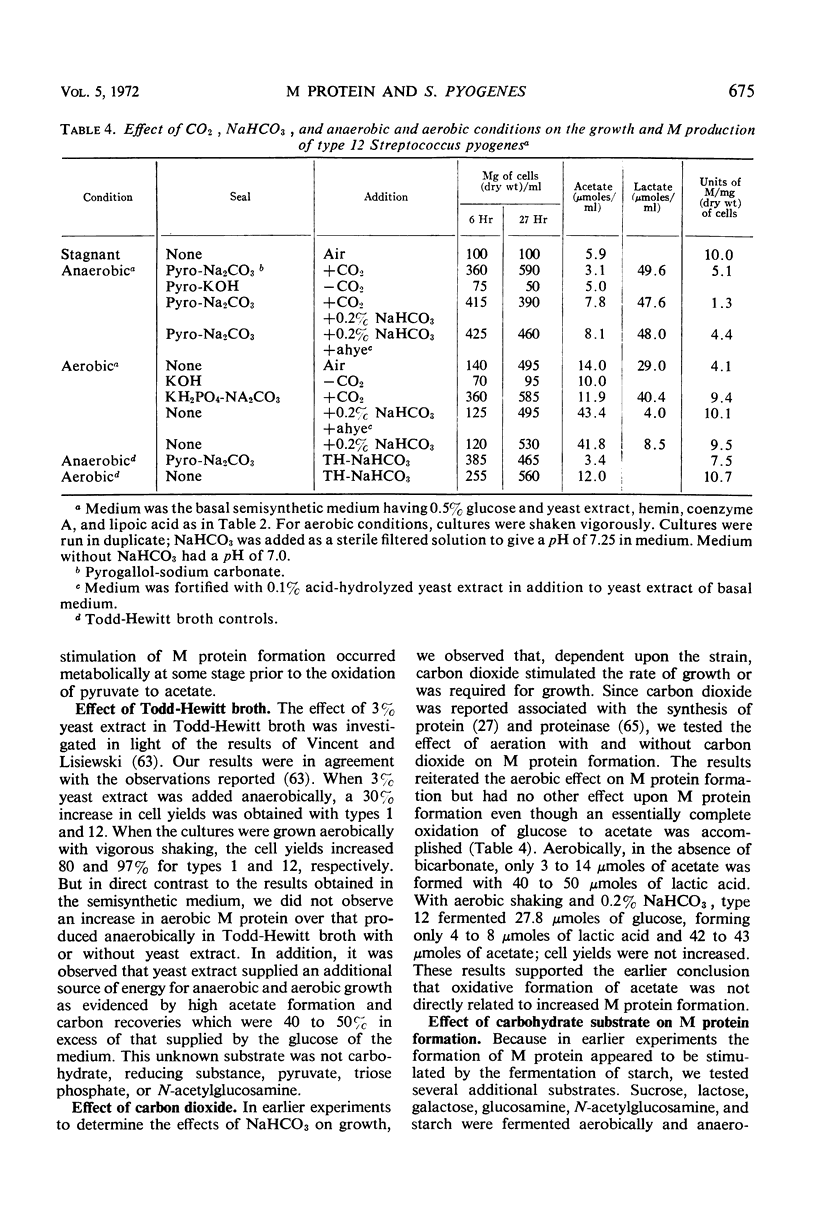
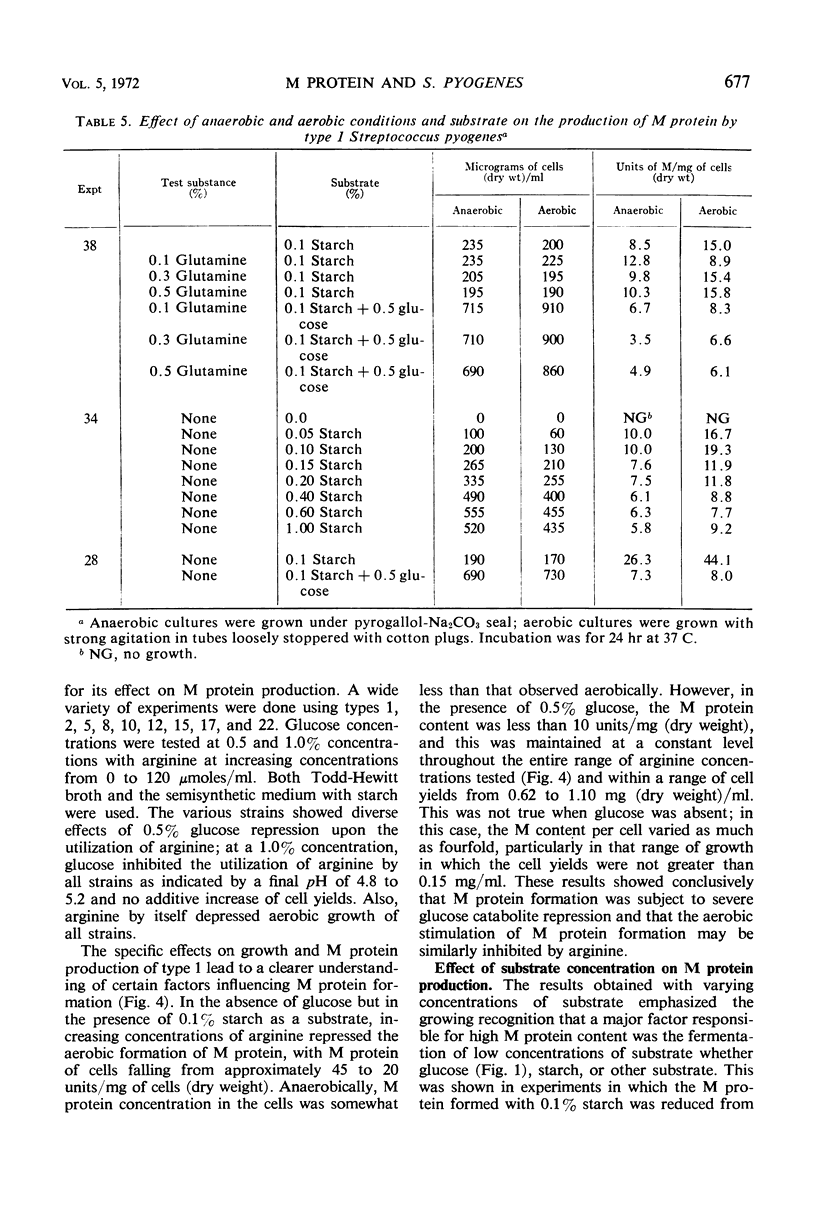
In the absence of proteinase formation, factors reported to influence the growth or fermentation by streptococci have been evaluated to determine their quantitative effect upon the production of M protein during the growth of Streptococcus pyogenes. Buffers, amino acids, peptides, gross organic additions, and carbohydrate substrates were tested under a variety of cultural conditions. The M protein content was remarkably constant throughout the late logarithmic period of growth, i.e., when the cell population doubled, the M protein doubled. However, several factors affected the M protein content per milligram of cells (dry weight). When types 1, 12, and 22 were grown aerobically in a semidefined medium, the M protein content of the cell population essentially doubled; in Todd-Hewitt broth, this aerobic effect on M protein synthesis was not observed. When cells grown on Todd-Hewitt broth were transferred to medium containing 0.1% starch and no added glucose, the M protein content per milligram of cells (dry weight) increased as much as fourfold. When growth was initiated in glucose, the rate of M protein formation was at a maximum in the early logarithmic phase of growth and was comparatively greater than the rate of cellular multiplication. When the amount of substrate fermented was greater than 0.2%, increased M protein was not observed. An evaluation of the effects of medium or conditions of growth showed the units of M per milligram of cells (dry weight) were not influenced by a shift in the stoichiometry of either the anaerobic or aerobic fermentation, substrate used, or adenosine triphosphate utilized for growth. These results show that M protein synthesis is subject to limited glucose repression or substrate catabolite repression.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- BROCK T. D. EFFECT OF ANTIBIOTICS AND INHIBITORS ON M PROTEIN SYNTHESIS. J Bacteriol. 1963 Mar;85:527–531. doi: 10.1128/jb.85.3.527-531.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck R. W., Shugart L. R. Molar growth yields in Streptococcus faecalis var. liquefaciens. J Bacteriol. 1966 Sep;92(3):802–803. doi: 10.1128/jb.92.3.802-803.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Gillman W., Hottle G. A., Pappenheimer A. M. An Improved Medium for the Cultivation of Hemolytic Streptococcus. J Bacteriol. 1942 Apr;43(4):495–498. doi: 10.1128/jb.43.4.495-498.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Pappenheimer A. M. Factors Necessary for Massive Growth of Group A Hemolytic Streptococcus. J Bacteriol. 1942 Apr;43(4):481–494. doi: 10.1128/jb.43.4.481-494.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan-Jones D. G., Whittenbury R. Haematin-dependent oxidative phosphorylation in Streptococcus faecalis. J Gen Microbiol. 1969 Oct;58(2):247–260. doi: 10.1099/00221287-58-2-247. [DOI] [PubMed] [Google Scholar]
- COHN M. Contributions of studies on the beta-galactosidase of Escherichia coli to our understanding of enzyme synthesis. Bacteriol Rev. 1957 Sep;21(3):140–168. doi: 10.1128/br.21.3.140-168.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Physiology of the inhibition by glucose of the induced synthesis of the beta-galactosideenzyme system of Escherichia coli. J Bacteriol. 1959 Nov;78:624–635. doi: 10.1128/jb.78.5.624-635.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. O. Effect of culture medium composition and pH on the production of M protein and proteinase by group A Streptococci. J Bacteriol. 1969 Sep;99(3):737–744. doi: 10.1128/jb.99.3.737-744.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. O., Pine L. Quantitative aspects of the M protein capillary precipitin test. Appl Microbiol. 1968 Jan;16(1):122–127. doi: 10.1128/am.16.1.122-127.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins E. B., Bruhn J. C. Roles of acetate and pyruvate in the metabolism of Streptococcus diacetilactis. J Bacteriol. 1970 Sep;103(3):541–546. doi: 10.1128/jb.103.3.541-546.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES H. C., KARUSH F., RUDD J. H. EFFECT OF AMINO ACIDS ON STEADY-STATE GROWTH OF A GROUP A HEMOLYTIC STREPTOCOCCUS. J Bacteriol. 1965 Feb;89:421–427. doi: 10.1002/path.1700890156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. C., Karush F., Rudd J. H. Synthesis of M protein by group A hemolytic streptococci in completely synthetic media during steady-state growth. J Bacteriol. 1968 Jan;95(1):162–168. doi: 10.1128/jb.95.1.162-168.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT S. D. The crystallization and serological differentiation of a streptococcal proteinase and its precursor. J Exp Med. 1950 Sep;92(3):201–218. doi: 10.1084/jem.92.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX E. N., KRAMPITZ L. O. Studies on the biosynthesis of the M-protein of group A hemolytic streptococci. J Bacteriol. 1956 Apr;71(4):454–461. doi: 10.1128/jb.71.4.454-461.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX E. N. Measurement of streptococcal antigen synthesis with fluorescent antibody. Proc Soc Exp Biol Med. 1962 Mar;109:577–579. doi: 10.3181/00379727-109-27274. [DOI] [PubMed] [Google Scholar]
- FOX E. N. Peptide requirements for the synthesis of streptococcal proteins. J Biol Chem. 1961 Jan;236:166–171. [PubMed] [Google Scholar]
- FREIMER E. H., KRAUSE R. M., McCARTY M. Studies of L forms and protoplasts of group A streptococci. I. Isolation, growth, and bacteriologic characteristics. J Exp Med. 1959 Dec 1;110:853–874. doi: 10.1084/jem.110.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest W. W. Energies of activation and uncoupled growth in Streptococcus faecalis and Zymomonas mobilis. J Bacteriol. 1967 Nov;94(5):1459–1463. doi: 10.1128/jb.94.5.1459-1463.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff R. C., Hartman R. E. Carbon dioxide fixation by cells of Streptococcus faecalis var. liquefaciens. J Bacteriol. 1970 Oct;104(1):27–33. doi: 10.1128/jb.104.1.27-33.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHN J. J., COLE R. M. STREPTOCOCCAL M ANTIGEN LOCATION AND SYNTHESIS, STUDIED BY IMMUNOFLUORESCENCE. J Exp Med. 1963 Nov 1;118:659–666. doi: 10.1084/jem.118.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHN J. J., COLE R. M. Studiey on the mechanism of the long chain phenomenon of group A streptococci. J Exp Med. 1963 Apr 1;117:583–594. doi: 10.1084/jem.117.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Pooley H. M., Shockman G. D. Site of initiation of cellular autolysis in Streptococcus faecalis as seen by electron microscopy. J Bacteriol. 1970 Aug;103(2):504–512. doi: 10.1128/jb.103.2.504-512.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A. Change in the absorbancy of bacterial suspensions before initiation of growth. J Bacteriol. 1969 Mar;97(3):1062–1068. doi: 10.1128/jb.97.3.1062-1068.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLINE L., BARKER H. A. A new growth factor required by Butyribacterium rettgeri. J Bacteriol. 1950 Sep;60(3):349–363. doi: 10.1128/jb.60.3.349-363.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOEPSELL H. J., SHARPE E. S. Micro-determination of pyruvic and alpha-keto-glutaric acids. Arch Biochem Biophys. 1952 Jul;38:443–449. doi: 10.1016/0003-9861(52)90050-7. [DOI] [PubMed] [Google Scholar]
- Katz L., Englesberg E. Hyperinducibility as a result of mutation in structural genes and self-catabolite repression in the ara operon. J Bacteriol. 1971 Jul;107(1):34–52. doi: 10.1128/jb.107.1.34-52.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- LIU T. Y., ELLIOTT S. D. ACTIVATION OF STREPTOCOCCAL PROTEINASE AND ITS ZYMOGEN BY BACTERIAL CELL WALLS. Nature. 1965 Apr 3;206:33–34. doi: 10.1038/206033a0. [DOI] [PubMed] [Google Scholar]
- LIU T. Y., NEUMANN N. P., ELLIOTT S. D., MOORE S., STEIN W. H. Chemical properties of streptococcal proteinase and its zymogen. J Biol Chem. 1963 Jan;238:251–256. [PubMed] [Google Scholar]
- MICKELSON M. N. CHEMICALLY DEFINED MEDIUM FOR GROWTH STREPTOCOCCUS PYOGENES. J Bacteriol. 1964 Jul;88:158–164. doi: 10.1128/jb.88.1.158-164.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall E., Bloom F. R. Catabolite repression in the D-serine deaminase system of Escherichia coli K-12. J Bacteriol. 1971 Jan;105(1):241–248. doi: 10.1128/jb.105.1.241-248.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain H., Fildes P., Gladstone G. P., Knight B. C. Glutamine and the growth of Streptococcus haemolyticus. Biochem J. 1939 Feb;33(2):223–229. doi: 10.1042/bj0330223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain H. The specificity of glutamine for growth of Streptococcus haemolyticus). Biochem J. 1939 Dec;33(12):1942–1946. doi: 10.1042/bj0331942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson M. N., Slade H. D. Absence of type specific M antigen from group A streptococci grown in a chemically defined medium. J Bacteriol. 1964 May;87(5):1251–1251. doi: 10.1128/jb.87.5.1251-.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa H. H., Collins E. B. Molar growth yields of certain lactic acid bacteria as influenced by autolysis. J Bacteriol. 1968 Jul;96(1):117–125. doi: 10.1128/jb.96.1.117-125.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGBURN C. A., HARRIS T. N., HARRIS S. Extracellular antigens in steady-state cultures of the hemolytic Streptococcus: production of proteinase at low pH. J Bacteriol. 1958 Aug;76(2):142–151. doi: 10.1128/jb.76.2.142-151.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- PIERCE W. A., Jr, WHITE A. G. Arginine and glucose metabolism in a strain of Streptococcus pyogenes. J Bacteriol. 1955 Feb;69(2):230–231. doi: 10.1128/jb.69.2.230-231.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINE L., WATSON S. J. Evaluation of an isolation and maintenance medium for Actinomyces species and related organisms. J Lab Clin Med. 1959 Jul;54(1):107–114. [PubMed] [Google Scholar]
- Payne W. J. Energy yields and growth of heterotrophs. Annu Rev Microbiol. 1970;24:17–52. doi: 10.1146/annurev.mi.24.100170.000313. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- ROSENBERGER R. F., ELSDEN S. R. The yields of Streptococcus faecalis grown in continuous culture. J Gen Microbiol. 1960 Jun;22:726–739. doi: 10.1099/00221287-22-3-726. [DOI] [PubMed] [Google Scholar]
- SLADE H. D., KNOX G. A., SLAMP W. C. The amino acid nutrition of group A hemolytic Streptococci, with reference to the effect of glutathione on the cystine requirement. J Bacteriol. 1951 Nov;62(5):669–675. doi: 10.1128/jb.62.5.669-675.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLADE H. D., SLAMP W. C. The formation of arginine dihydrolase by streptococci and some properties of the enzyme system. J Bacteriol. 1952 Oct;64(4):455–466. doi: 10.1128/jb.64.4.455-466.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLADE H. D., SLAMP W. C. The requirement of ovalbumin for the growth of group A hemolytic Streptococcus in a synthetic medium. J Exp Med. 1955 Sep 1;102(3):291–305. doi: 10.1084/jem.102.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijpesteijn A. K. Induction of cytochrome formation and stimulation of oxidative dissimilation by hemin in Streptococcus lactis and Leuconostoc mesenteroides. Antonie Van Leeuwenhoek. 1970;36(3):335–348. doi: 10.1007/BF02069035. [DOI] [PubMed] [Google Scholar]
- Smalley A. J., Jahrling P., Van Demark P. J. Molar growth yields as evidence for oxidative phosphorylation in Streptococcus faecalis strain 10Cl. J Bacteriol. 1968 Nov;96(5):1595–1600. doi: 10.1128/jb.96.5.1595-1600.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., McCarty M. Electron microscopic studies on opaque colony variants of group A streptococci. J Bacteriol. 1969 Oct;100(1):505–511. doi: 10.1128/jb.100.1.505-511.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent W. F., Lisiewski K. J. Improved growth medium for group A streptococci. Appl Microbiol. 1969 Nov;18(5):954–955. doi: 10.1128/am.18.5.954-955.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch P. C., Hartman R. E. Effect of carbon dioxide on the aspartic acid requirement for proteinase biosynthesis by Streptococcus faecalis var. liquefaciens. J Bacteriol. 1969 Nov;100(2):1138–1139. doi: 10.1128/jb.100.2.1138-1139.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


