Abstract
A hallmark feature of cognitive aging is a decline in the ability to form new memories. Parallel to these cognitive impairments are marked disruptions in sleep physiology. Despite recent evidence in young adults establishing a role for sleep spindles in restoring hippocampal-dependent memory formation, the possibility that disrupted sleep physiology contributes to age-related decline in hippocampal-dependent learning remains unknown. Here, we demonstrate that reduced prefrontal sleep spindles by over 40% in older adults statistically mediates the effects of old age on next day episodic learning, such that the degree of impaired episodic learning is explained by the extent of impoverished prefrontal sleep spindles. In addition, prefrontal spindles significantly predicted the magnitude of impaired next day hippocampal activation, thereby determining the influence of spindles on post-sleep learning capacity. These data support the hypothesis that disrupted sleep physiology contributes to age-related cognitive decline in later life, the consequence of which has significant treatment intervention potential.
Keywords: aging, fMRI, hippocampus, learning, sleep
Introduction
A hallmark feature of cognitive decline in later life is a progressive impairment in the ability to form new episodic memories, further associated with deficits in hippocampal-encoding activity (Sperling et al. 2003; Miller et al. 2008). In parallel with these cognitive changes are prominent disruptions in non-rapid eye movement (NREM) sleep, including reduced expression of sleep spindle oscillations (Dijk et al. 1989; Landolt et al. 1996; Carrier et al. 2001; Crowley et al. 2002; De Gennaro and Ferrara 2003). Moreover, this age-related spindle impairment appears to be topographically distinct across the head, maximal over frontal lobe derivations (De Gennaro and Ferrara 2003; Martin et al. 2013).
Independent of aging, a growing literature in healthy young adults supports a preparatory role for sleep before learning in the restoration of next day encoding capacity (Campbell et al. 2002; Yoo et al. 2007). In support of this hypothesis, sleep loss prior to learning impairs hippocampal functioning and associated measures of neuroplasticity (Campbell et al. 2002; Yoo et al. 2007). Conversely, sleep and specifically fast frequency sleep spindles (13.5–15 Hz) over the left prefrontal cortex restores the capacity for hippocampal-dependent episodic learning in young adults (Mander et al. 2011). These data are consistent with studies demonstrating that faster sleep spindle oscillations are associated with both greater hippocampal activation and hippocampal–cortical functional connectivity (Schabus et al. 2007; Andrade et al. 2011). They are also congruent with studies in rodents linking sleep spindle oscillations with coincident hippocampal sharp-wave ripple events (Siapas and Wilson 1998). Such evidence has lead to the proposal that fast frequency sleep spindles represent part of a coordinated NREM sleep mechanism (Siapas and Wilson 1998; Diekelmann and Born 2010), capable of restoring next day hippocampal-dependent learning (Walker 2009).
Given that older adults exhibit reduced sleep spindles, prominent over the prefrontal cortex (De Gennaro and Ferrara 2003; Martin et al. 2013), in combination with compromised hippocampal-dependent episodic learning (Sperling et al. 2003), it is possible that these independently recognized features of aging are functionally related (Fogel et al. 2012), representing a neuropathological pathway that may contribute to cognitive decline in later life. Building on these findings, and combining functional magnetic resonance imaging (fMRI) with physiological sleep electroencephalography (EEG) recordings, here we test the related hypotheses that (1) prefrontal fast sleep spindles predict next day episodic encoding capacity by way of a mediating influence on hippocampal-encoding activation, and (2) deficits in prefrontal fast sleep spindles in older adults consequently diminish this beneficial influence on hippocampal-dependent episodic learning ability.
Materials and Methods
Thirty healthy adults (young adults: n = 16, 8 females, mean ± SD, 20.5 ± 2.1 years; and older adults: n = 14, 11 females, mean ± SD, 71.9 ± 6.7 years) were recruited for the study. The study was approved by the local human studies committee, with all participants providing written informed consent. Participant exclusion criteria included a history of neurologic, psychiatric, or sleep disorders, current use of antidepressant or hypnotic medications, or being left handed (Supplementary Material). All participants abstained from caffeine, alcohol, and daytime naps for the 48 h before and during the entire course of the study. Participants kept normal, habitual sleep-wake rhythms and averaged 7–9 h of reported time in bed per night prior to study participation, verified by sleep logs (Table 1).
Table 1.
Demographic and neuropsychological measures (mean ± SD)
| Variable | Young (N = 16) | Older (N = 14) |
|---|---|---|
| Age (years) | 20.5 ± 2.1 | 71.9 ± 6.7 |
| Gender | 8 Female | 11 Female |
| MMSE | 29.7 ± 0.4 | 29.4 ± 0.8 |
| Mean bed time | 0:31 ± 0:50 | 22:45 ± 1:04*** |
| Mean wake time | 8:33 ± 0:51 | 7:04 ± 1:00*** |
| Mean prestudy time in bed (hours) | 8.05 ± 0.61 | 8.32 ± 0.85 |
| Mean prestudy sleep time (hours) | 7.74 ± 0.61 | 6.85 ± 1.05* |
| Mean prestudy sleep latency (min) | 14.7 ± 9.3 | 40.2 ± 56.8 |
| Mean prestudy sleep efficiency (%) | 96.3 ± 2.2 | 82.9 ± 13.8** |
| Scan time relative to prestudy wake time | 1:49 ± 1:17 | 2:09 ± 1:00 |
| Neuropsychological measures | ||
| Education (year) | 17.5 ± 1.3 | |
| CVLT (long delay, # free recalled) | 11.6 ± 3.3 | |
| WMS (visual reproduction %) | 83.6 ± 13.6 | |
| Trailmaking B (s) | 71.3 ± 43.3 | |
| Stroop (# correct in 60 s) | 52.7 ± 12.2 | |
CVLT denotes the California verbal learning test; WMS denotes the Wechsler memory scale.
*P < 0.05.
**P < 0.01.
***P < 0.001.
General Experimental Design
Participants entered the lab in the evening on the first experimental night and were given an 8-h sleep opportunity, measured with polysomnography (PSG), starting at their habitual bed time, based on sleep logs 5 days before the study date (Table 1), helping to limit the influence of age-related circadian differences (Chang et al. 2009). PSG recording included a 19-channel EEG array (details below). Approximately 2-h post-awakening, participants performed an event-related functional MRI scanning session on the episodic associative memory encoding task, followed 30 min later by a self-paced recognition test outside the scanner (details below). Subjective measures of sleepiness and alertness and overall reaction times during face-name encoding and recognition testing were used to determine whether subjective and objective alertness predicted learning in young and older adults (Supplementary Methods).
Episodic Face-Name Learning Task
The episodic associative memory-encoding task, utilized in the current study, is a well-validated, face-name task sensitive to age and sleep effects (Sperling et al. 2003; Miller et al. 2008; Mander et al. 2011). Encoding was administered inside the MRI scanner, composed of 120 face-name learning trials using an event-related design, and followed by an immediate associative recognition test outside the scanner (Fig. 1 and Supplementary Methods).
Figure 1.
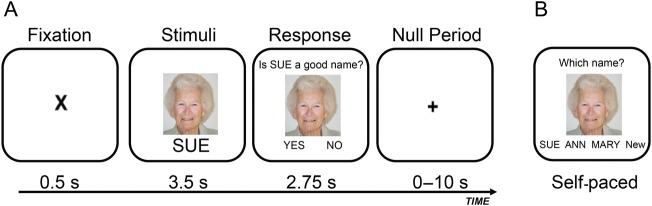
(A) Example face-name encoding fMRI trial (read left to right). During each trial, a fixation was first presented for 0.5 s, followed by a face-name pair for 3.5 s. This was followed by a screen for 2.75 s during which participants had to determine whether the name “fits” the face while still presenting the face-name pair, allowing for a confirmation that participants attended to each face-name pair, and promoted deeper encoding of each face-name pair. Sixty null events, consisting of a fixation display and varying in duration from 1.5 to 10 s, were interspersed to jitter trial onsets. (B) Example face-name recognition trial, presenting a studied face-name pair. During each self-paced recognition trial, a face was first presented on the screen with 4 options presented below that participants chose among allowing for a determination of associative memory recognition: (1) the original name previously paired with that face (correct “Hit” response), (2) a name previously seen before at encoding, but with a different face (incorrect “Lure” response), (3) a new name never shown during encoding (incorrect response), or (4) an option “new” rejecting the trial as a foil trial. Face-name recognition memory was calculated by subtracting the proportion of new faces endorsed as “studied” faces and incorrectly paired with a name (false alarm rate) from the proportion of studied faces correctly named (hit rate).
MRI Scanning
Scanning was performed on a Siemens Trio 3-T scanner equipped with a 32-channel head coil. Functional scans were acquired using a susceptibility-weighted, single-shot, echo-planar imaging method to image the regional distribution of the blood oxygenation level-dependent signal [time repetition (TR)/time echo (TE) 2000/23 ms; flip angle 90°; FatSat, field of view (FOV) 224 mm; matrix 64 × 64; 37 3 mm slices with 0.3 mm slice gap, descending sequential acquisition], and using parallel imaging reconstruction (GRAPPA) with acceleration factor 2. Four functional runs were acquired (138 volumes, 4.6 min). Following functional scanning, 2 high-resolution, T1-weighted anatomical images were acquired using a 3-dimensional magnetization-prepared rapid acquisition with gradient echo protocol (TR 1900 ms; TE 2.52 ms; flip angle 9°; FOV 256 mm; matrix 256 × 256; slice thickness 1.0 mm, 176 slices).
fMRI Analysis
fMRI data were analyzed using SPM8 (Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) beginning with standardized preprocessing (realignment, slice timing correction, and coregistration), and with normalization accomplished using a mixed young–old template generated from 400 individual T1-weighted anatomical images (Bollinger et al. 2011), consistent with recommendations for use in studies comparing young and older adults (Buckner et al. 2004; Bollinger et al. 2011). Each study participant's T1 scan was first normalized to this mixed-age template and then normalized again to the Montreal Neurological Institute (MNI) template using the normalization parameters generated from moving the mixed template into the MNI space (Bollinger et al. 2011).
Following preprocessing, at the single-subject level, face-name encoding trials during scanning were sorted into the category types based on recognition testing performance: Hits (studied trials resulting in correct face-name recognition), Lures (studied trials resulting in an incorrect face-name response, due to selection of the incorrect name previously studied), and Misses (studied trials where either an incorrect name was chosen or the “studied” face was endorsed as “new”; and see Fig. 1). Hit events were contrasted with Lure events (Hit–Lure), reflecting activation in the brain specific for successful versus unsuccessful associative face-name encoding (Henson et al. 1999; Paller and Wagner 2002; Miller et al. 2008). These activation maps generated at the single-subject level were then taken through to a second-level, random-effects analysis to examine group effects. Analyses focused a priori on an anterior hippocampal region of interest (ROI, 8 mm sphere), selected based on its sensitivity to associative face-name episodic encoding in young and older adults (MNI coordinates: x = −21, y = −6, z = −15; Miller et al. 2008). A left prefrontal cortical ROI was also examined, based on its selectivity to memory encoding (10 mm sphere; MNI coordinates: x = −48, y = 22, z = 14; Spaniol et al. 2009). ROI activations were considered significant at the voxel level of P < 0.05 family-wise error (FWE), corrected for multiple comparisons (Lieberman and Cunningham 2009; Poldrack and Mumford 2009). To offer a more complete examination of the data, whole-brain maps, presented at P < 0.001 uncorrected with a 10 voxel threshold, are reported in Supplementary Results.
Structural MRI Analysis
For structural analysis of hippocampal anatomy, optimized voxel-based morphometry (VBM) of gray matter volume was utilized (Ashburner and Friston 2000), employing the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) and the Diffeomorphic Anatomical Registration through Exponentiated Lie algebra (DARTEL) toolbox (Ashburner 2007) in order to improve the registration of older brains to the normalized MNI template (see Supplementary Methods).
Sleep Monitoring
PSG sleep monitoring was recorded including 19-channel EEG, scored using standard criteria (Rechtschaffen and Kales 1968), and sleep spindles were detected utilizing an established algorithm, characterizing fast- and slow-frequency spindle events (Ferrarelli et al. 2007; Mander et al. 2011; see Supplementary Methods).
Statistical Analysis
An independent, 2-sample t-test was used to compare sleep and behavioral measures between young and older adults. Associations between sleep measures and learning were assessed using Pearson's correlations. Spindle-learning correlations were examined and considered significant if they were significant P < 0.05 false discovery rate (FDR) corrected (Benjamini and Hochberg 1995) across all electrode derivations (n = 19). To control for the effects of age, gender, neurocognitive status, and hippocampal structural anatomy, a multiple regression model was employed with age group, gender, mini-mental status exam (MMSE) score, and VBM-measured hippocampal gray matter volume included in the model with fast prefrontal spindle density to predict episodic learning ability. An additional multiple regression model included encoding-related hippocampal activation as an additional predictor of episodic learning ability. While some of these measures may not predict episodic encoding ability, or differ between age groups, they were nevertheless included in the model to control for any potential influence on episodic encoding ability, spindle density, or the association between spindles and encoding ability.
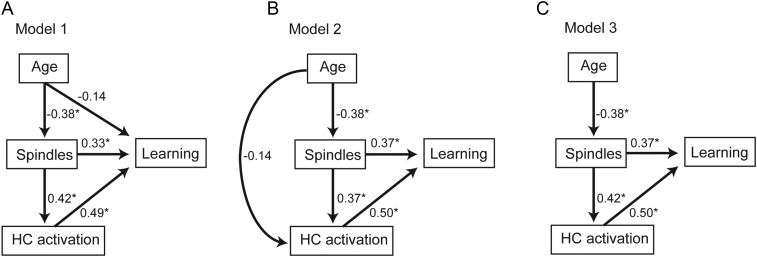
To test the hypotheses that: (1) Age effects on prefrontal fast sleep spindles contributed to that on hippocampal-dependent episodic learning, and (2) effects of prefrontal fast sleep spindles on encoding-related hippocampal activation contributed to that on hippocampal-dependent episodic learning, path analysis was performed using structural equation modeling (Stage et al. 2004; Kline 2011). This technique calculates the path coefficients, that is, coupling between model variables, given a specified model. Path coefficients reflect the direct and proportional influence of one variable on another while controlling for other variables in the model. Three hypothesized models were specified and compared with saturated and independence models as well as to each other. All 3 hypothesized models allowed for age to impact learning through its influence on sleep spindles and hippocampal activation, as this was the primary hypothesis of the current study (for visual representations, see later Fig. 5A–C). The first hypothesized model also allowed age to impact learning independently of sleep spindles via a direct path to learning. Similar to the first model, the second hypothesized model allowed age to impact learning independently of sleep spindles, but this time indirectly through the effects of age on hippocampal activation. Finally, model 3 had neither of these sleep spindle-independent pathways, instead requiring age to impact learning solely through its influence on sleep spindles. If age impacts learning independently of spindles, then either model 1 or 2 should outperform model 3, with sleep spindle-independent paths being significant and sleep spindle-dependent paths not being significant. Should model 3 outperform models 1 and 2, then learning deficits in older adults statistically depend on the degree of sleep spindle impairment.
Figure 5.
Path analysis models examining the relative contributions of age, frontal fast sleep spindles (spindles), and hippocampal (HC) activation to learning ability (learning) in 3 hypothesized models (A–C). Values represent standardized regression weights. Models are estimated and model fit for model 1 (A, AICc = 40.191; BIC = 31.191; RMR = 0.019; GFI = 0.990), model 2 (B, AICc = 40.658; BIC = 31.658; RMR = 0.003; GFI = 0.983), and model 3 (C, AICc = 36.837; BIC = 28.837; RMR = 0.019; GFI = 0.973) are compared against a saturated model (AICc = 44.012; BIC = 34.012; RMR = 0.000; GFI = 1.000) and an independence model (AICc = 52.320; BIC = 48.320; RMR = 0.070; GFI = 0.602). * denotes path significance at P < 0.05.
Four validated metrics were used to compare model fits: Corrected Akaike's information criterion (AICc), Bayesian information criterion (BIC), root mean square residual (RMR), and goodness-of-fit index (GFI; Bozdogan 1987; Raftery 1995; Kline 2011). In short, models with RMR near 0, GFI above 0.9, were considered sufficient model fits (Kline 2011). The model with the lowest AICc and BIC values was considered the superior model, with a difference in BIC of over 10 suggesting marked differences between the models, a difference of 6–10 suggesting a strong difference, and a difference of 2–6 suggesting a marginal difference is present (Raftery 1995). Within the superior model, individual path coefficients were then examined for significance.
To formally test for the presence of indirect effects, mediation analyses were additionally performed using established methods (MacKinnon et al. 1995). In short, these analyses determine whether the influence of independent variable X on dependent variable Y is accounted for by mediator M. Specifically, is the value of the direct path coefficient between X and Y reduced by the inclusion of M? Reduction to 0 is interpreted as mediation, partial reduction is interpreted as partial mediation, and nonsignificant reduction is interpreted as no evidence for mediation. Effects were formally tested using the Sobel test of mediation, applying the Goodman correction (MacKinnon et al. 1995). To account for data dependency and potential nonnormality of data distribution given the smaller sample size, mediation effects were verified using an additional established mediation method utilizing bootstrapping to estimate the associated P-values (Preacher and Hayes 2004, 2008; Preacher et al. 2007). Analyses were completed using the SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA), and path analyses were performed using the Amos version 21 (IBM Corp., Armonk, NY, USA).
Results
Age Effects on Hippocampal-Dependent Memory Encoding
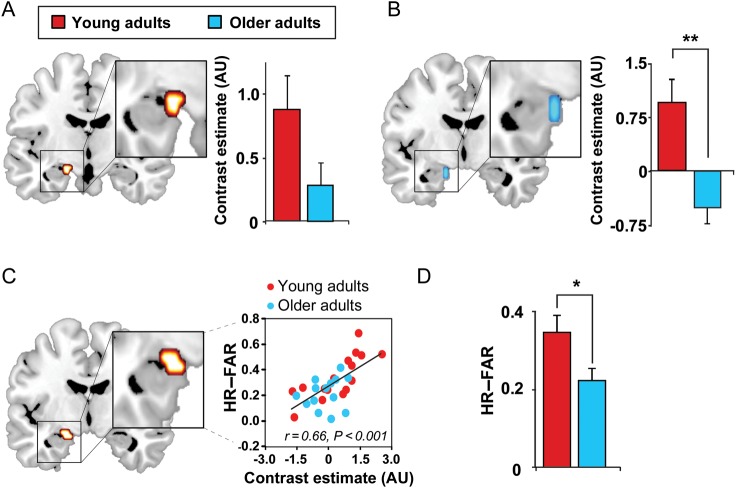
First, and consistent with prior findings (Sperling et al. 2003; Miller et al. 2008), we established that successful encoding was not only associated with hippocampal activation (Fig. 2A), but that older adults exhibited significantly less hippocampal-encoding activation than young adults (Fig. 2B). Effects were maximal in the left hippocampus (and present bilaterally at a lower statistical threshold; Supplementary Fig. 1). Moreover, hippocampal activation during successful encoding relative to unsuccessful encoding significantly predicted episodic learning ability across participants (P < 0.001; Fig. 2C). To determine that this association was not driven solely by age differences in hippocampal activation and episodic learning ability, age group was included in an analysis of covariance model with hippocampal activation. Hippocampal activation still significantly predicted episodic learning ability (P < 0.001), while age, per se, did not (P = 0.120). These analyses suggest that decreasing hippocampal activation with increasing age contributes to impaired episodic encoding ability in older adults. This deficient hippocampal-encoding activation was mirrored by a significant impairment in episodic learning in older, relative to young, adults (>35%, P = 0.031; Fig. 2D and Table 2). These analyses verified that (1) successful episodic encoding of face-name pairs is dependent on the hippocampus to a similar degree in young and older adults, with greater hippocampal activation predicting more successful episodic encoding, and (2) older adults exhibited deficient episodic learning due, in part, to reduced hippocampal recruitment during encoding.
Figure 2.
Effects of age on hippocampal functioning. (A) Hippocampal activation greater during successful associative episodic encoding than unsuccessful associative encoding (Hits–Lures; 8-mm sphere ROI: x = −21, y = −6, z = −15) (Miller et al. 2008) and (B) corresponding age effects (Hits–Lures; older < young adults) in the same ROI. (C) Regression between episodic learning and encoding-related hippocampal activation (Hits–Lures) in the above-mentioned ROI. (D) Episodic learning in young and older adults, both performing significantly greater than chance (HR-FAR: hit rate to originally studied faces–false alarm rate to new, unstudied faces). Activations are displayed and considered significant at the voxel level of P < 0.05 FWE, corrected for multiple comparisons within the a priori hippocampal ROIs (Miller et al. 2008). Hot colors represent the extent of activation in both young and older adults, and cold colors represent the extent of decreased activation in older, relative to younger, adults. While the 8-mm sphere ROI used to correct for multiple comparisons did extend outside the hippocampus, no effects were detected or presented outside the hippocampus in the current report. Bilateral effects were detected in the hippocampus, when using an anatomical hippocampal mask, albeit at a lower statistical threshold (P < 0.005 uncorrected; Supplementary Fig. 2). *P < 0.05, **P < 0.001.
Table 2.
Face-name task statistics (mean ± SEM)
| Variable | Young (N = 16) | Older (N = 14) |
|---|---|---|
| Hit rate | 0.45 ± 0.04 | 0.39 ± 0.03 |
| Miss rate | 0.55 ± 0.04 | 0.61 ± 0.03 |
| Correct rejection rate | 0.90 ± 0.02 | 0.83 ± 0.04 |
| False alarm rate | 0.10 ± 0.02 | 0.17 ± 0.04 |
| Hit rate–false alarm rate | 0.35 ± 0.04 | 0.22 ± 0.03* |
*P < 0.05.
Age Effects on Sleep
We next sought to test the hypothesis that fast sleep spindles over the prefrontal cortex, previously demonstrated to restore both episodic learning in young adults (Mander et al. 2011) and exhibit maximal disruption with age (Martin et al. 2013), were significantly reduced in older adults. Consistent with prior evidence (De Gennaro and Ferrara 2003; Martin et al. 2013), older adults exhibited marked, yet regionally selective, deficits in fast sleep spindle density relative to young adults, with the greatest impairment (>40%) over prefrontal derivations (Fig. 3A–C). These group differences in frontal sleep spindles could be due to decreased spindle generation in older adults, or a decreased ability to detect spindles using EEG methods due to increased brain to scalp distance, particularly in frontal atrophied brain regions, leading to increased conductance distance. However, neither frontal gray matter volume relative to total intracranial volume, nor left frontal gray matter volume relative to total intracranial volume (both r2 < 0.06), significantly predicted spindle density at F3. These data support the interpretation (and prior findings cf. De Gennaro and Ferrara 2003; Martin et al. 2013) that differences in spindle density between young and older adults reflect changes in spindle generation rather than detection using EEG methods. Further details of sleep physiology in young and older adults are provided in Table 3 and Supplementary Table 1.
Figure 3.
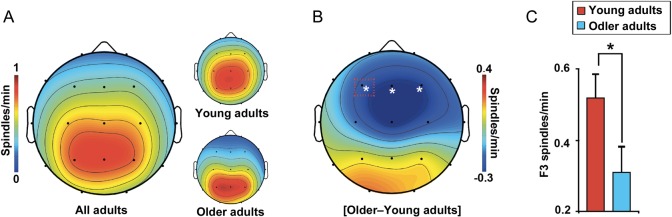
Age effects on fast sleep spindle density. (A) electroencephalography (EEG) topographic plots of fast spindle density in all adults with young (top) and older (bottom) adult plots to the right, and (B) the fast sleep spindle density difference between young and older adults, significant over prefrontal EEG derivations. Red box indicates the stage 2 NREM sleep fast spindle density at the F3 derivation, plotted as a bar graph (C) in young (red) and older (blue) adults. * denotes significance at P < 0.05.
Table 3.
Sleep statistics (mean ± SEM)
| Variable | Young (N = 16) | Older (N = 14) |
|---|---|---|
| Total recording time (min) | 480.4 ± 0.2 | 481.0 ± 0.4 |
| Total sleep time (min) | 433.1 ± 7.2 | 365.5 ± 14.7** |
| Sleep latency (min) | 13.3 ± 2.4 | 25.8 ± 9.6 |
| Wake after sleep onset | 29.2 ± 7.2 | 88.6 ± 11.5** |
| Stage 1 (min) | 14.1 ± 2.0 | 21.4 ± 1.9* |
| Stage 2 (min) | 202.6 ± 8.2 | 201.3 ± 13.8 |
| Slow-wave sleep (min) | 115.5 ± 8.1 | 65.9 ± 9.0** |
| Rapid eye movement sleep (min) | 100.9 ± 6.2 | 76.8 ± 7.9* |
| Global absolute slow-wave activity (μV2) | 315.9 ± 36.6 | 77.1 ± 6.1** |
| Global relative slow-wave activity (%) | 93.7 ± 0.4 | 86.0 ± 1.0** |
*P < 0.05.
**P < 0.001.
Sleep Associations with Hippocampal-Dependent Memory Encoding
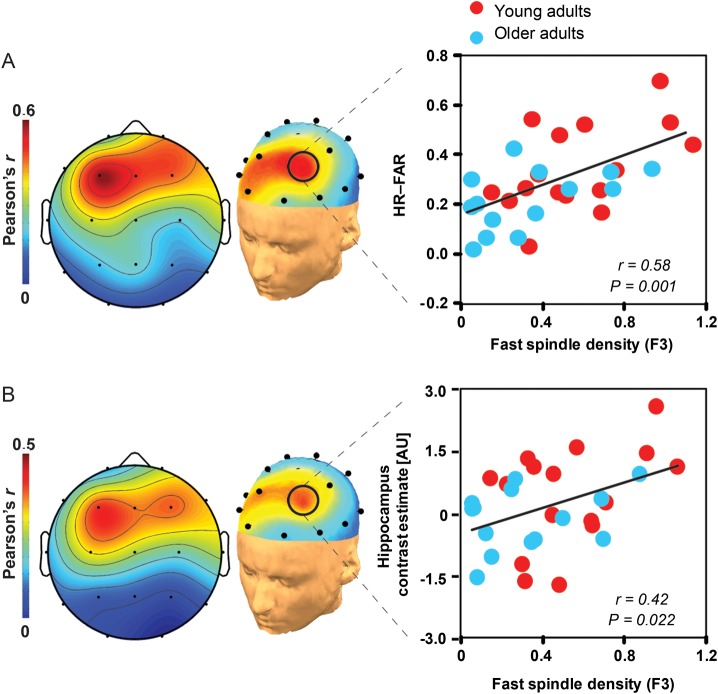
We subsequently investigated whether prefrontal fast sleep spindles predicted episodic learning across participants. Spatially congruent with previous evidence in young adults (Mander et al. 2011), a positive and selective association was identified between left prefrontal fast sleep spindles (F3 derivation) and the extent of episodic encoding success across all participants, which remained significant when corrected across all electrode derivations (Fig. 4A) (Benjamini and Hochberg 1995). This association was specific to fast sleep spindles, as no significant relationships were detected for slow sleep spindles (all P > 0.05 FDR corrected). Given the left prefrontal specificity of these spindle-learning associations, and those previously reported in healthy young adults (Mander et al. 2011), subsequent mediation and multiple regression analyses focused on the role of left prefrontal (F3) fast spindle density in next day hippocampal encoding.
Figure 4.
Associations between sleep spindles, hippocampal activation, and episodic learning. (A) EEG topographic plots of the association between fast sleep spindle density and episodic learning, with the corresponding regression for young (red) and older (blue) adults plotted to the right, focusing on the left prefrontal EEG derivation (F3) where the spindle-learning association is significant false discovery rate corrected (Benjamini and Hochberg 1995). (B) EEG topographic plots of the association between fast sleep spindle density and encoding-related hippocampal activation (described in Fig. 2C), with the corresponding regression for young (red) and older (blue) adults plotted to the right, focusing on the left prefrontal EEG derivation (F3). Associations were specific to fast sleep spindles, as subjective measures of sleepiness and alertness (all P > 0.12), objective alertness response times collected during encoding and recognition testing (all P > 0.20), absolute and relative slow-wave activity (0.8–4.6 Hz calculated as described previously, Mander et al. 2011, all P > 0.10), circadian preference (all P > 0.72), and scanning time relative to wake time (all P > 0.16) did not correlate with: (1) Episodic learning, (2) hippocampal activation, or (3) prefrontal fast sleep spindles, in either young or older adults. Associations were also specific to the hippocampus, as activation within an encoding-relevant region of the left prefrontal cortex (Spaniol et al. 2009) did not differ between age groups (P = 0.244) and was not associated with either frontal fast sleep spindles (r2 < 0.01, P = 0.969) or episodic learning ability across participants (r2 < 0.1, P = 0.156; see Supplementary Results).
To additionally determine the relative contribution of fast sleep spindles on episodic learning when controlling for potential cofactors, a multiple regression model was constructed with fast spindle density at F3, age group, gender, hippocampal structure (VBM gray matter volume; Supplementary Fig. 2), and neurocognitive status (MMSE scores) as independent variables predicting episodic learning. The overall regression model significantly predicted episodic learning (r = 0.66, P = 0.013). Critically, only fast sleep spindles remained a significant predictor of learning when all other factors were taken into account (F3 fast spindle density, P = 0.007; age group, P = 0.960; gender, P = 0.128; hippocampal gray matter volume, P = 0.351; MMSE score, P = 0.880). These findings indicate that reduced prefrontal fast sleep spindles contribute to impaired episodic encoding ability in older adults, independently of other factors including hippocampal structure and neuropsychological mental status.
Modeling Associations Between Age, Sleep Spindles, Hippocampal Activation, and Learning
To expressly examine how age, sleep spindles, and encoding-related hippocampal activation influence episodic encoding ability, path analyses were performed. Specifically, 3 models were constructed (Fig. 5A–C) and compared with saturated and independence models. Of all models tested, the model requiring age to influence learning through sleep spindles was most robust (Fig. 5C, see Supplementary Results). In this model, age was significantly associated with frontal fast sleep spindles (P = 0.029), spindles were significantly associated with both learning (P = 0.006) and encoding-related hippocampal activation (P = 0.013), and hippocampal activation was significantly associated with learning (P < 0.001).
Given these results, and to formally test whether age exerts its influence on learning through an indirect effect of age on frontal sleep spindles, mediation analyses were performed. While age negatively predicted episodic learning ability (r = 0.41, P = 0.025), age no longer significantly predicted episodic learning ability when F3 fast spindles were included in the statistical model (F3 fast spindles P = 0.006, Δβ = −0.08; age P = 0.248, Δβ = +0.21), reflecting a significant mediation effect for F3 fast spindles (Sobel test; MacKinnon et al. 1995; p = 0.047). This mediation effect was further verified using an independent method that does not assume a normal data distribution and is robust against data dependency (indirect effects test, Preacher and Hayes 2008; P = 0.0018). These mediation results, in conjunction with the above path analyses, support the hypothesis that reduced prefrontal fast spindles in older adults contribute to age-related decline in next day episodic learning ability.
Building on this fast sleep spindle-learning association, we sought to formally test the hypothesis that the influence of sleep spindles on episodic learning ability was statistically mediated, in part, by the effects of sleep spindles on next day hippocampal functioning. To address this, we first examined whether activation in the hippocampal voxels that significantly predicted episodic encoding ability across participants also exhibits a relationship with left prefrontal fast sleep spindles (F3 derivation), as evidenced in the path analysis above. A significant association was detected between left prefrontal fast sleep spindles and hippocampal activation (Fig. 5B), with more left prefrontal fast sleep spindles yielding greater next day encoding-related hippocampal activation.
To determine whether the impact of frontal fast sleep spindles on episodic learning was determined by an influence on hippocampal activation, mediation analyses were performed. Hippocampal activation was included in a multiple regression model along with fast sleep spindle density at F3, age group, gender, hippocampal gray matter volume, and neurocognitive status as independent variables predicting episodic learning. The overall regression model significantly predicted episodic learning (r = 0.80, P < 0.001), with only hippocampal activation remaining a significant predictor of learning (hippocampal activation, P = 0.002; F3 fast spindle density, P = 0.061; age group, P = 0.816; gender, P = 0.053; hippocampal gray matter volume, P = 0.306; MMSE score, P = 0.564). Since left prefrontal fast sleep spindles predicted encoding-related hippocampal activation, and since left prefrontal fast sleep spindles no longer predicted learning when hippocampal activation was included in the model, it is possible that hippocampal activation statistically mediates the influence of left prefrontal fast sleep spindles on episodic learning. This possibility was formally examined by a Sobel test of mediation. Supporting the above possibility (1) prefrontal fast spindles predicted episodic learning (Fig. 4A), even when effects were adjusted for age, gender, hippocampal structure, and neurocognitive status (see above), (2) prefrontal fast spindles no longer significantly predicted episodic learning ability when encoding-related hippocampal activation was included in the statistical model (hippocampal activation P = 0.002, Δβ = −0.11 and F3 fast spindles P = 0.061, Δβ = −0.21), and (3) path analyses demonstrating that age influences learning partially through the effects of spindles on hippocampal activation, a significant mediation effect for encoding-related hippocampal activation was observed (Sobel test; MacKinnon et al. 1995; P = 0.039). This mediation effect was further verified using an independent method that does not assume a normal data distribution and is robust against data dependency (indirect effects test; Preacher and Hayes 2008; P < 0.001). Taken together, this collection of results, along with the path analysis support a model in which the effects of age on prefrontal fast sleep spindles compromises the restoration of next day hippocampal functioning, resulting in significantly diminished episodic encoding in older adults.
Given that gender was not balanced in this study, it is possible that gender may have influenced these results. However, gender was not associated with a significant difference in memory when examined directly (P = 0.17), nor a difference in fast spindle density at F3 (P = 0.85), encoding-related hippocampal activation (P = 0.90), or hippocampal gray matter volume (P = 0.242). Furthermore, if all male participants are excluded, left frontal fast sleep spindles still significantly predict episodic memory-encoding ability (r = 0.69, P < 0.001). This relationship within females remains significant when adjusting for age (P = 0.008, for age P = 0.886). Taken together, these results suggest that gender does not appear to parsimoniously explain the age-related difference in hippocampal-related memory encoding and the associations with sleep spindles, nor was hippocampal structure significantly different between genders in the current sample.
Alertness and Circadian Measures
To assess possible age-related differences in alertness and circadian preference between groups, and their association with learning, a collection of validated subjective and objective metrics were evaluated including subjective ratings of sleepiness and alertness (Monk 1989), reaction times during face-name encoding and recognition testing, a validated metric of circadian preference (Horne and Ostberg 1976), and the time between habitual wake and learning and recognition testing times.
For measures of sleepiness and alertness, using a repeated-measures, 2-way analysis of variance, with Time as a within-subjects factor and Group (young/old) as a between-subjects factor, no main effect of Group or Group × Time interaction (all P > 0.15) was detected for subjective sleepiness or alertness. A main effect of Time in both groups was detected for both sleepiness and alertness (all P < 0.001), with sleepiness being highest in the evening before sleep and alertness being highest in the morning after sleep in both young and older adults. Importantly, none of the subjective sleepiness or alertness measures described above predicted episodic learning (all r2 < 0.15, P > 0.2), hippocampal activation (all r2 < 0.05, P > 0.45), or frontal fast spindles (all r2 < 0.05, P > 0.45) in young or older adults. In addition, neither overall reaction times during face-name encoding nor recognition testing predicted either learning (all r2 < 0.1, P > 0.2), hippocampal activation (all r2 < 0.1, P > 0.2), or frontal fast spindles in young or older adults (all r2 < 0.15, P > 0.2).
As expected (Czeisler et al. 1992), circadian preference assessed using subjective measures of habitual circadian preference differed between young and older adults (young adults mean ± SEM, 46.4 ± 2.1 and older adults mean ± SEM, 63.1 ± 3.1, P < 0.001), with older adults reporting a greater morning preference than young adults. This was consistent with significantly earlier habitual bed times and wake times for the 5 days leading up to the study (Table 1). However, time at encoding in the scanner relative to both prestudy habitual wake time, and wake time on the day of scanning, did not differ between groups (all P > 0.56, Table 1). These data indicate that episodic learning took place at similar circadian times in both young and older adults. Further, circadian preference and time of encoding in the scanner relative to habitual and experimental wake times did not predict learning, hippocampal activation, or frontal fast spindles in either group (all P > 0.35). While these data do not discount the possibility that alertness and circadian rhythms contribute to age-related memory decline, the impact of age on sleep-dependent memory reported in the present study is not parsimoniously explained by age differences in alertness or circadian rhythms.
Discussion
Our findings characterize an association between prefrontal fast sleep spindles and next day hippocampal functioning, the interaction of which significantly determines next day learning ability. Such data are consistent with recent theoretical proposals that fast sleep spindles represent part of a coordinated NREM sleep-dependent memory mechanism (Siapas and Wilson 1998; Diekelmann and Born 2010; Mander et al. 2011), one that is capable of restoring hippocampal-dependent neuroplasticity and associated encoding ability (Walker 2009; Mander et al. 2011). For example, rodent studies suggest that sleep spindles not only coincide with the onset of phasic sharp-wave ripple events within the hippocampus (Siapas and Wilson 1998), but also can trigger sharp-wave ripples through excitation of cortical output layers (Sirota et al. 2003). Additionally, hippocampal sharp-wave ripples provide feedback excitation that instantiates neuroplasticity in spindle-activated cortical neurons (Sirota and Buzsaki 2005), corroborating the notion of a coordinated hippocampal–neocortical mechanism of memory processing (Walker 2009; Diekelmann and Born 2010; Fogel and Smith 2011). Moreover, human neuroimaging studies demonstrate that, relative to slow sleep spindles, fast sleep spindle events are associated with greater hippocampal activation and greater hippocampal–cortical functional connectivity (Schabus et al. 2007; Andrade et al. 2011). Combined with the current findings, this collection of data advance a framework in which fast sleep spindles can promote bidirectional communication between the hippocampus and the neocortex during sleep, one benefit of which may be the transfer of prior hippocampal-dependent representations to greater cortical dominance (Siapas and Wilson 1998; Diekelmann and Born 2010) and, symbiotically, promote the post-sleep restoration of sparse hippocampal-encoding capacity (Walker 2009).
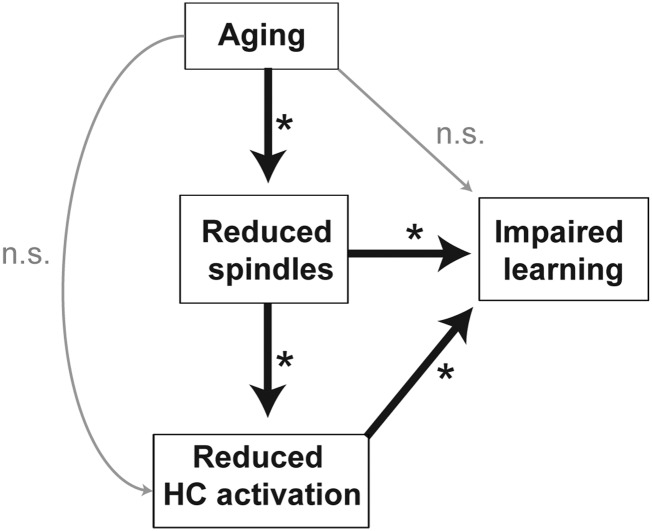
Secondly, and in the context of aging, our findings establish neural evidence consistent with the proposal that sleep disruption is a factor contributing to cognitive decline in later life (Van Der Werf et al. 2009; Fogel et al. 2012; Mander et al. 2013). In particular, our data suggest that the topographically specific reduction in prefrontal fast spindles in older adults (by over 40%) leads to an impoverished sleep-dependent restoration of next day learning ability. Both path and mediation analyses corroborate that the reduction in fast sleep spindles in older adults statistically mediates age effects on episodic learning, with this interaction being governed by a diminished sleep spindle influence on next day hippocampal functioning. That is, older adults, expressing fewer prefrontal fast sleep spindles than younger adults, exhibited a proportional impairment in next day hippocampal functioning, and with that impairment, a deficit in the ability to form new episodic memories. Such findings suggest the potential decline of a sleep spindle-dependent hippocampal memory mechanism in aging, one that may contribute to canonical learning impairments observed in the elderly (Craik 1977; Grady et al. 1995; Sperling et al. 2003; Miller et al. 2008; Mormino et al. 2009). Indeed, we were not able to identify a significant sleep spindle-independent path linking aging to learning, suggesting that the erosion of sleep-dependent memory processing is a substantive contributor to memory impairment in the elderly (Fig. 6).
Figure 6.
Model schematic of path and mediation analyses findings. Aging is associated with reduced frontal fast sleep spindles, which mediates the degree of reduced next day, encoding-related hippocampal (HC) activation and impaired episodic learning ability in older adults. Furthermore, age did not significantly impact learning or hippocampal activation independently of its impact on frontal fast sleep spindles in the present study. *Denotes paths that are significant at P < 0.05.
While remaining speculative, one potential candidate underlying diminished prefrontal fast sleep spindles in older adults may be age-related atrophy in select corticothalamic networks that generate and/or regulate the expression of spindle oscillations. For example, aging has already been demonstrated to disrupt white matter tracts linking the prefrontal cortex with the hippocampus (through the thalamus and parietal cortex) (Nordahl et al. 2006; Greicius et al. 2009), and represents a pathway through which prefrontal spindles have (independent of aging) been proposed to transact interactions with the hippocampus (Sirota and Buzsaki 2005).
Clinically, these data suggest that the disruption of sleep physiology is an under-appreciated factor contributing to cognitive aging, which, together with already recognized factors (Buckner 2004; Hedden and Gabrieli 2004), may further lead to age-related memory deficits. Such a proposal is supported by evidence that sleep spindle impairments predate the recognized decline of memory in the elderly (Dijk et al. 1989; Carrier et al. 2001; De Gennaro and Ferrara 2003). Furthermore, our data endorse the possibility that improvement of sleep quality in the elderly may be a future treatment target capable of minimizing the onset and/or progression of cognitive decline in later life.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by awards R01-AG031164 (M.P.W.), R01-AG034570 (W.J.), R01-AG08415 (S.A.), and F32-AG039170 (B.A.M.) from the National Institutes of Health.
Supplementary Material
Notes
We thank Meghna Bhatter, Michelle Binod, Sam Bowditch, Catherine Dang, Amynta Hayenga, April Horn, Emily Hur, Jack Lindquist, Candace Markeley, Elizabeth Mormino, Molly Nicholas, Lily Zhang, and Alyssa Zhu for their assistance in recruitment and data collection; and Michael Rubens and Adam Gazzaley for use of their aging template brain. Conflict of Interest: None declared.
References
- Andrade KC, Spoormaker VI, Dresler M, Wehrle R, Holsboer F, Samann PG, Czisch M. Sleep spindles and hippocampal functional connectivity in human NREM sleep. J Neurosci. 2011;31:10331–10339. doi: 10.1523/JNEUROSCI.5660-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Ser B Method. 1995;57:289–300. [Google Scholar]
- Bollinger J, Rubens MT, Masangkay E, Kalkstein J, Gazzaley A. An expectation-based memory deficit in aging. Neuropsychologia. 2011;49:1466–1475. doi: 10.1016/j.neuropsychologia.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdogan H. Model selection and Akaike Information Criterion (AIC)—the General-Theory and its analytical extensions. Psychometrika. 1987;52:345–370. [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old) Psychophysiology. 2001;38:232–242. [PubMed] [Google Scholar]
- Chang AM, Reid KJ, Gourineni R, Zee PC. Sleep timing and circadian phase in delayed sleep phase syndrome. J Biol Rhythms. 2009;24:313–321. doi: 10.1177/0748730409339611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FI. Age differences in human memory. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. New York: Van Nostrand Reinhold; 1977. pp. 384–420. [Google Scholar]
- Crowley K, Trinder J, Kim Y, Carrington M, Colrain IM. The effects of normal aging on sleep spindle and K-complex production. Clin Neurophysiol. 2002;113:1615–1622. doi: 10.1016/s1388-2457(02)00237-7. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Dumont M, Duffy JF, Steinberg JD, Richardson GS, Brown EN, Sanchez R, Rios CD, Ronda JM. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG, van den Hoofdakker RH. All night spectral analysis of EEG sleep in young adult and middle-aged male subjects. Neurobiol Aging. 1989;10:677–682. doi: 10.1016/0197-4580(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Martin N, Lafortune M, Barakat M, Debas K, Laventure S, Latreille V, Gagnon J, Doyon J, Carrier J. NREM sleep oscillations and brain plasticity in aging. Front Neurol. 2012;3:176. doi: 10.3389/fneur.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35:1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Smith CT, Beninger RJ. Evidence for 2-stage models of sleep and memory: learning-dependent changes in spindles and theta in rats. Brain Res Bull. 2009;79:445–451. doi: 10.1016/j.brainresbull.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. New York (NY): The Guilford Press; 2011. [Google Scholar]
- Landolt HP, Dijk DJ, Achermann P, Borbely AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738:205–212. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Warsi G, Dwyer JH. A simulation study of mediated effect measures. Multivariate Behav Res. 1995;30:41–62. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Santhanam S, Saletin JM, Walker MP. Wake deterioration and sleep restoration of human learning. Curr Biol. 2011;21:R183–R184. doi: 10.1016/j.cub.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Lafortune M, Godbout J, Barakat M, Robillard R, Poirier G, Bastien C, Carrier J. Topography of age-related changes in sleep spindles. Neurobiol Aging. 2013;34:468–476. doi: 10.1016/j.neurobiolaging.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34:1411–1421. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH. A visual analogue scale technique to measure global vigor and affect. Psychiatry Res. 1989;27:89–99. doi: 10.1016/0165-1781(89)90013-9. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA. Independence in ROI analysis: where is the voodoo? Soc Cogn Affect Neurosci. 2009;4:208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS And SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behav Res. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Raftery AE. Bayesian model selection in social research. Soc Methodol. 1995;25:111–163. [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques an scoring system of sleep stages in human subjects. Los Angeles: UCLA Brain Information Services; 1968. [Google Scholar]
- Saletin JM, Goldstein AN, Walker MP. The role of sleep in directed forgetting and remembering of human memories. Cereb Cortex. 2011;21:2534–2541. doi: 10.1093/cercor/bhr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, Darsaud A, Degueldre C, Desseilles M, Gais S, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci USA. 2007;104:13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Sirota A, Buzsaki G. Interaction between neocortical and hippocampal networks via slow oscillations. Thalamus Relat Syst. 2005;3:245–259. doi: 10.1017/S1472928807000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci USA. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stage FK, Carter HC, Nora A. Path analysis: an introduction and analysis of a decade of research. J Educ Res. 2004;98:5–12. [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Tamaki M, Matsuoka T, Nittono H, Hori T. Fast sleep spindle (13–15 Hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep. 2008;31:204–211. doi: 10.1093/sleep/31.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helm E, Gujar N, Nishida M, Walker MP. Sleep-dependent facilitation of episodic memory details. PLoS One. 2011;6:e27421. doi: 10.1371/journal.pone.0027421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf YD, Altena E, Schoonheim MM, Sanz-Arigita EJ, Vis JC, De Rijke W, Van Someren EJ. Sleep benefits subsequent hippocampal functioning. Nat Neurosci. 2009;12:122–123. doi: 10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.