Abstract
Limb immobilization and nonuse are well-known causes of corticomotor depression. While physical training can drive the recovery from nonuse-dependent corticomotor effects, it remains unclear if it is possible to gain access to motor cortex in alternative ways, such as through motor imagery (MI) or action observation (AO). Transcranial magnetic stimulation was used to study the excitability of the hand left motor cortex in normal subjects immediately before and after 10 h of right arm immobilization. During immobilization, subjects were requested either to imagine to act with their constrained limb or to observe hand actions performed by other individuals. A third group of control subjects watched a nature documentary presented on a computer screen. Hand corticomotor maps and recruitment curves reliably showed that AO, but not MI, prevented the corticomotor depression induced by immobilization. Our results demonstrate the existence of a visuomotor mechanism in humans that links AO and execution which is able to effect cortical plasticity in a beneficial way. This facilitation was not related to the action simulation, because it was not induced by explicit MI.
Keywords: action observation, direct-matching hypothesis, immobilization, internal simulation, motor imagery
Introduction
The plasticity of the sensorimotor system has been studied using 2 main approaches (see Pascual-Leone et al. 2005; Sanes and Donoghue 2000 for reviews). The first approach investigates motor practice or peripheral stimulation as means of enhancement of the sensorimotor input/output (e.g. Elbert et al. 1995; Pascual-Leone et al. 1995; Sterr et al. 1998; Naito et al. 1999; Naito, Kochiyama, et al. 2002; Kalisch et al. 2010). The second concerns the effects of transient deprivation of these signals, for example, in the case of amputation (Cohen et al. 1991; Elbert et al. 1994; Kew et al. 1994), anesthesia (e.g. Rossini et al. 1994; Rossini, Rossi, et al. 1996; Rossini, Tecchio, et al. 1996; Kristeva-Feige et al. 1996; Rossi et al. 1998), or immobilization (e.g. Liepert et al. 1995; Huber et al. 2006; Lissek et al. 2009; Weibull et al. 2011). It is well known that both limb nonuse and immobilization induce corticomotor depression, which is reflected by a decrease of excitability of motor areas (Huber et al. 2006; Avanzino et al. 2011; Langer et al. 2012). After limb nonuse, cortical efficiency can be restored through physical training by targeting the increased use of the inactive body part. Alternative ways to avoid the suppression of corticomotor functioning remain an open question. One possibility is based on neurophysiologic evidence that the motor cortex can be activated not only during actual movements, but also when actions are merely observed or mentally simulated in the absence of motor output. In fact, previous works reveal that motor imagery (MI; e.g. Abbruzzese et al. 1999; Fadiga et al. 1999; Hashimoto and Rothwell 1999; Ridding and Rothwell 1999; Rossini et al. 1999) and action observation (AO; see Fadiga et al. 1995, for reviews Rizzolatti and Craighero 2004; Fadiga et al. 2005) induce cortical facilitation in the motor cortex. If combined with physical practice, MI has been shown to produce beneficial effects on athletes' (e.g. Roure et al. 1996; Homes and Calmels 2008) and musicians' (e.g. Lotze and Halsband 2006) performances and, similarly, AO also seems to improve motor recovery in brain-damaged patients (see for instance, Page et al. 2001; Buccino et al. 2006; Lotze and Cohen 2006; Sharma et al. 2006; Ertelt et al. 2007).
Given this evidence, one could postulate that MI and AO could prevent the corticomotor depression that typically follows the nonuse of a limb and may prove to be a viable means of activating the motor system even during limb inactivity.
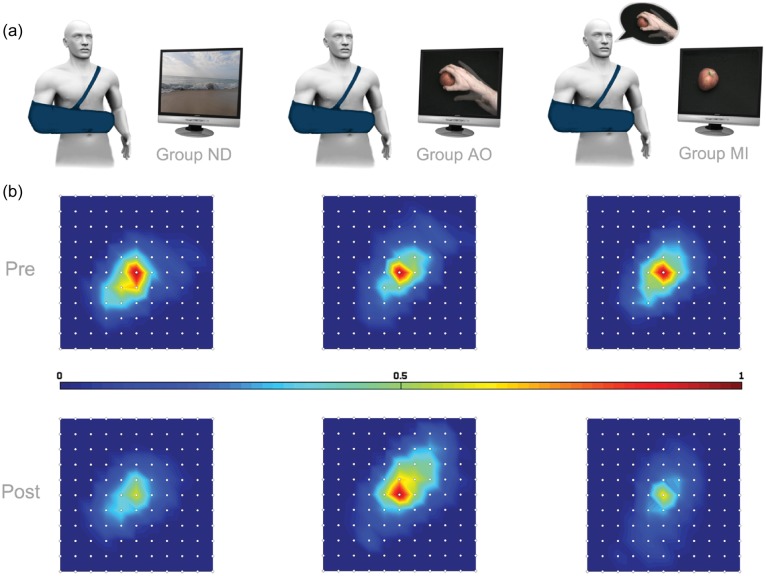
To investigate this, a reversible cortical depression was induced in normal participants through immobilization (Bassolino et al. 2012) in order to assess the effects of AO and MI on motor plasticity and cortical reorganization. Participants were randomly assigned to 3 groups according to the type of task that had to be performed during immobilization. The first group watched nature documentaries (ND) without human agents; the second group observed videos of reaching-to-grasp actions performed with the right hand of an adult in a first-person perspective (AO), and the third group was asked to mentally simulate similar grasping actions upon visual presentation of a target object on a computer screen (MI; Fig. 1a) without the presence of a reaching hand. Transcranial magnetic stimulation (TMS) was used before and after 10 h of right upper limb immobilization to map participants' corticomotor representation of intrinsic muscles of the right hand (specifically, first dorsal interosseous, FDI). Recruitment curves (RCs) were determined on the basis of motor evoked potentials (MEPs) acquired at different stimulus intensities.
Figure 1.
Mean FDI corticomotor maps recorded before (Pre) and after (Post) immobilization in the 3 groups. (a) In ND (on the left), AO (in the middle), and MI (on the right) groups, participants performed the requested task for 10 hourly sessions during the immobilization period (from around 8 AM to 6 PM). (b) Each map was centered on the maximal response obtained for each participant in every condition, regardless of antero-posterior (here corresponding to above–below directions) and medio-lateral (here right-left) coordinates. Colors indicate the amplitude of MEP, normalized with respect to the maximal response obtained in the Pre-condition in every subject, from “blue” (the lowest values) to “red” (the highest responses).
Materials and Methods
Subjects
The 3 groups of subjects were matched for age and gender (ND: 8 subjects, 3 females, mean age, 23.9 ± 2 years; AO: 8 subjects, 3 females, mean age 24.5 ± 2.07 years; and MI: 8 subjects, 3 females, mean age 24.8 ± 2.30 years). All participants showed strong right-hand dominance (all subjects scores of more than +60) as determined by the Handedness Inventory (Oldfield 1971). They had normal or corrected-to-normal vision. All subjects had no contraindication to TMS and participated in this study after having signed a written informed consent. They reported no previous history of orthopedic problems for the right hand and arm. The study was performed with approval of the local ethics committee and in accordance with the Declaration of Helsinki. All participants were naive to the purposes of the experiment and received an attendance fee.
The subjects' capability to perform MI was evaluated by an Italian version (Fourkas et al. 2008) of the Revised Movement Imagery Questionnaire (MIQ-R; Hall and Martin 1997) the day before immobilization. The Italian MIQ-R measures the difficulty (on a scale of 1, “very easy,” to 7, “very difficult”) of forming visual and kinesthetic images of movements on 8 test items (all “very easy” answers: 8 and all “very difficult” answers: 56). The subjects in this study as a group considered MI to be an “easy” or “fairly easy” task, with an average score of 19.38 ± 2.74.
Immobilization Procedure
The immobilization procedure used here was the same as previously reported (Avanzino et al. 2011; Bassolino et al. 2012). The subjects' hand and forearm were wrapped with a soft bandage covered by a tissue support that further limited the arm movement holding the elbow in a comfortable position at 90° of flexion (Fig. 1a). Subjects were instructed not to move their right hand over the immobilization period, that is 10 h, from 8 AM to 6 PM.
Experimental Protocol
During the immobilization period, participants pertaining to each of the 3 experimental groups performed the requested task for hourly sessions during the whole period of immobilization (i.e. 10 sessions, one session every hour). The 3 tasks were: Watching ND, mentally simulating hand actions (MI), and observing actions performed by others (AO; Fig. 1a). In these 3 groups, the duration of each session was established on the basis of the number of the observed videos (in AO and ND) or of the simulated actions (MI). Precisely, in every group, each session always included 80 items (videos/actions).
In the ND group, subjects watched videos representing scenes from documentary without human agents.
In the AO group, subjects observed videos showing a right hand (first-person perspective) reaching and grasping various objects with either power grip (all fingers) or precision grip (thumb and index finger). A male or female hand was displayed in agreement with the subject's gender. In ND and AO, each video lasted 3 s and 80 videos were displayed in every session.
In the MI group, in order to standardize the task among participants, the objects to grasp and the precise instructions related to the kind of grip requested (i.e. power grasp or precision grip) were displayed by videos. Eighty videos were displayed for each session. Subjects were instructed to carefully observe the static objects and then to mentally simulate the action by keeping their eyes closed trying to “feel the same as during actual execution” (kinesthetic imagery). Precisely, they had to close their eyes before starting the imagery and open them at the end. To estimate the duration of simulated movements (see below), eyes closure occurrence and duration were monitored by a webcam and recorded by means of a keyboard button pushed by the same experimenter for all participants.
To keep high the level of attention during the tasks, the subjects sometimes had to answer if the last seen video (in ND and AO) or imagined action (in MI) was the same as the previous trial. This question was displayed on the computer screen at the end of randomly selected videos (10 questions during each hourly session). Subjects were instructed to respond as soon as possible “yes” or “no” by pushing 1 of 2 pedals. The correspondence between the pedals and the yes/no responses was displayed on the monitor and was counterbalanced over the experiment.
Transcranial Magnetic Stimulation
Cortical excitability (RCs and cortical mapping, see below) was assessed by TMS both the day before and immediately after the immobilization period, always starting at around 6 PM. TMS was applied by means of a single Magstim 200® stimulator (Magstim Company, Whitland, Dyfed, UK) with a 70-mm figure-of-eight coil. The coil was placed tangentially to the scalp with the handle pointing backward and laterally to form a 45° with the sagittal plane. This coil orientation induced a posterior–anterior current in the brain. We determined the hotspot of the left cortical representation of the right FDI muscle by moving the coil in 0.5 cm steps and by assessing MEP amplitude at each location. Resting motor threshold (RMT, on the hotspot) of FDI muscle was defined as the minimum stimulus intensity able to produce a MEP of at least 0.05 mV in 5 of 10 consecutive trials, and was expressed as a percentage of maximum stimulator output (MSO).
Electromyography (EMG) was recorded with silver disc surface electrodes positioned on the FDI in a tendon-belly configuration. EMG signals were amplified and filtered (20 Hz to 1 kHz) thanks to a D360 amplifier (Digitimer). The signals were sampled at 5000 Hz, digitized using a laboratory interface (Power1401; Cambridge Electronics Design), and stored on a personal computer for display and later off-line data analysis. Each recording epoch lasted 400 ms, of which 100 ms preceded the TMS. Trials with a EMG background activity or latency variability >1.5 ms (e.g. Starr et al. 1988; Rossi et al. 2008) were excluded from analysis.
The mapping procedure was similar to that previously described (see Uy et al. 2002). First, we determined the position of the vertex (using nasion–inion and preauricular creases as references), and we marked it on the subjects' head. Then, participants wore a tight swimming cap with a little hole (0.5 cm of diameter) positioned on their vertex by the experimenter, which allowed to keep visible the vertex landmark over the procedure. To guide the coil positioning on the scalp, a 1 × 1-cm grid centered on the vertex (point 0,0) was drawn on the cap. The points on the grid represented the predefined stimulation sites. The cortical maps were assessed at 120% of RMT by moving the TMS coil at 1 cm step (following the grid) around the muscle area until no discernible responses (≥0.05 mV) were evoked. Each scalp site was stimulated 5 times and in a pseudorandom sequence. Data were normalized with respect to the maximal response obtained in the Pre-condition in each subject. A custom Visual Basic software generated a trigger for TMS pulse, sent it through an acquisition board (National Instruments 6009), and stored the EMG signals on a personal computer for display and later off-line data analysis.
The RCs were determined by measuring the peak-to-peak amplitude (expressed in mV) of MEPs elicited at stimulus intensities of 5%, 10%, 15%, 20%, and 25% of MSO above RMT, in 5% of MSO steps. Individual RMT was always assessed before and after immobilization and RCs were drawn based on the corresponding RMT. Ten trials were recorded for each stimulus intensity and then averaged for further analysis (see Avanzino et al. 2011). For each subject, MEP amplitude was normalized with respect to the mean MEP value obtained before immobilization (Pre-condition).
Statistical Analysis
To evaluate the presence of task-related effects in the 3 groups, for each parameter (RMT, RC, and volume) we run repeated-measures (RM) analyses of variance (ANOVAs) with the corresponding specific within-subjects factors (RMT: “TIME” Pre, Post; RC: “TIME” Pre, Post and “INTENSITY” 5%, 10%, 15%, 20%, and 25%; map-volume: “TIME” Pre, Post) and with “GROUP” (ND, AO, and MI) like between-subjects factors.
In addition, in order to deepen the effects observed in each group, data on the RC and hand cortical representation were separately analyzed in every group. Precisely, RMT was compared before (Pre) and after (Post) immobilization by means of paired t-tests in ND, MI, and AO, separately. Then, TMS RC data (RC, normalized) were analyzed separately for each group by means of RM-ANOVA with “TIME” (Pre and Post) and “INTENSITY” (5%, 10%, 15%, 20%, and 25%) as within-subjects factors. Therefore, we compared the map-volume (taken as the sum of the averaged MEPs recorded at all scalp sites at which a response was evoked, see Uy et al. 2002; Schabrun and Ridding 2007) recorded before and after immobilization using paired t-tests in ND, MI, and AO, separately.
Significance threshold was set at P < 0.05. If RM-ANOVA showed a significant interaction effect, we performed post hoc comparisons using the least significant difference (Fisher's) test. Data are presented as mean ± SE.
Finally, a General Linear Model (GLM), originally conceived for brain imaging studies (Friston et al. 1994), was applied to TMS maps data. Data analysis was carried out using SPM2 (http://www.fil.ion.ucl.ac.uk/spm/). Typically, to describe the whole extension of a cortical map, a unique parameter is extrapolated, such as here the map-volume (see for a methodological description, Uy et al. 2002). Then, the volume of maps recorded in different conditions is compared through statistical tests. Conversely, applying the GLM approach, it was possible to directly and statistically contrast the maps point-by-point, taking into account the complete time profiles of MEPs from all scalp sites. Scalp points were first spatially aligned, subject-by-subject, on individual maximal response location. Then, we averaged the repetitions of MEPs acquired in a certain scalp point for one subject in one condition. The resulting 2 mean time courses (corresponding to Pre- and Post-conditions) of each stimulated site were concatenated in time (Fig. 2b). Finally, these data were used to fill a 2-dimensional (2D) image time series including all the stimulated scalp points for each subject in the 2 conditions. Hence, the obtained 2D image time series was fitted with an event-related model. Here, the event considered was the single TMS pulse. To overcome the difference in the temporal evolution of the EMG response evoked by TMS (∼ ms) and of the hemodynamic response (HR) (∼ sec), we set an arbitrary repetition time (1 s). This led to adopt the default SPM canonical HR as a basis function in the GLM analysis, improving the statistical power of the analysis and bringing to more easily interpretable results.
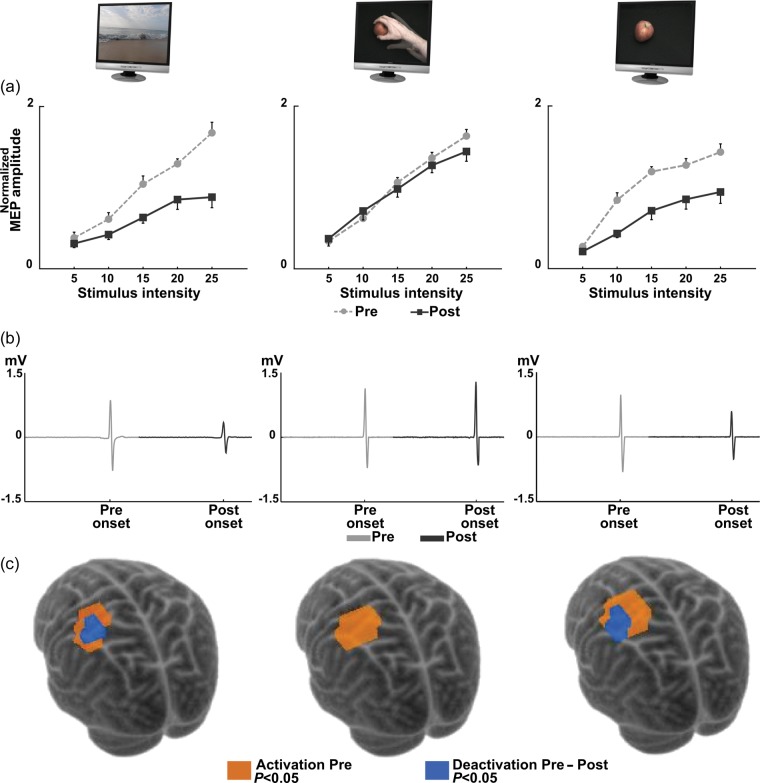
Figure 2.
Effect of AO in preventing corticomotor modifications induced by immobilization. (a) MEP RCs of right FDI in ND, AO, and MI before (dashed light line, Pre) and after (solid dark line, Post) immobilization. On the abscissa, TMS stimulus intensity above RMT (% of MSO); on the ordinate, mean values of the normalized MEP ± standard error. A significant Pre–Post difference is present in ND and MI, but not in AO. (b) A typical MEP from one scalp site of one exemplificative subject from each group averaged among the repeated recordings before (light gray) and after (dark gray) immobilization. Two-dimensional image time series were filled with these data from all scalp sites. (c) Scalp sites significantly responding to TMS before immobilization (orange, P < 0.05) and significantly deactivated after nonuse (blue, P < 0.05, Pre - Post) are shown (overlaid onto a 3D rendering of the Montreal Neurological Institute, MNI, template brain by coregistering FDI cortical coordinates onto the MNI space, Niyazov et al. 2005). In ND and MI was evident a significant deactivated area (blue) that was completely absent in AO.
We aimed at identifying within each map a region of significant activated scalp sites that are points in which a statistically significant response was evoked by the TMS (“activation maps”). Then, in order to evaluate the effect of immobilization, the activation map estimated for each subject before nonuse was statistically compared with the corresponding activation map obtained after immobilization (GLM analysis, first level). Finally, in order to evaluate the effect of AO and MI during immobilization, 3 groups (ND, MI, and AO) statistical maps were assessed by following a group random-effects analysis (second level).
Motor Imagery: Duration of Simulated Actions
Given that imagined and executed movements follow the same rules of motor control (e.g. Sirigu et al. 1996; Papaxanthis et al. 2002), to check the accuracy of the MI during immobilization, we evaluated if in line with the speed/accuracy trade-off (Fitts' law, Fitts, 1992), the time required to mentally simulate actions during immobilization was shorter for easy rather than for complex movements. Indeed, it is well known that grasping big objects with the whole hand (power grasp) is more rapid in time than grasping small objects with 2 fingers (precision grip, e.g. Castiello 2005). Thus, we considered the time spent to mentally grasp objects that are so small or so big to unambiguously required a precision or power grip, respectively. These objects were selected among all displayed during MI task. Additionally, to compare the duration between imagined actions during immobilization and really executed movements in the normal condition, a new group of 6 naïve participants [real execution (RE) group, 2 females, mean age 24.2 ± 2.15 years] were recruited to actually perform the same actions mentally simulated in the MI group. Subjects in the RE group never underwent the immobilization procedure. They had to grasp the same subset of objects selected to compare precision or power grip during MI in a single afternoon session. For mimicking the eyes' closure/opening of the MI group, the subjects in the RE group stamped the right foot before starting the reaching-to-grasp and lifted it at the end of the action. Foot movement was recorded as described above for eyes' closure/opening in the MI. Since it has been shown that the isochrony between the executed and imagined movements was evident between 2 PM and 8 PM (Gueugneau et al. 2009), among all the sessions recorded across the whole immobilization period in MI, we considered those performed in the afternoon. We run a mixed-model analyses of variance on the duration of movements in order to check any effect due to the complexity of the task (“small objects with precision grip” and “big objects with power grasp,” within-subjects factor) in the 2 groups of participants (MI and RE, between-subjects factor). Significant interactions between factors were examined with the least significance difference (Fisher's) test.
Results
To check participants' compliance in the tasks performed during nonuse, subjects of ND, MI, and AO groups were instructed to answer some questions randomly displayed among videos. For every subject in the 3 groups, the accuracy was always >85% of the total number of questions displayed in all sessions.
Comparison Between Imagined and Real Actions
Findings showed that, in both the MI and RE groups, subjects took more time when they grasped small objects with precision grip than big objects with the whole hand (COMPLEXITY × GROUP: F1, 11 = 23.8, P = 0.0005; precision grip vs. power grasp in RE: P < 0.00001 and in MI: P = 0.039). This indicates that Fitt's law (on speed/accuracy trade-off, Fitts 1992) was obeyed during MI and RE. However, independently of the type of grip considered, a general increase in the duration of the imagined actions with respect to that of the actual movements was observed (COMPLEXITY × GROUP: F1, 11 = 23.8, P = 0.0005): Indeed, mentally executed actions in MI were globally longer than really performed movements in RE (MI vs. RE in precision, P = 0.015 and in power grasp, P < 0.0001; precision grip: MI = 1958 ± 108 ms, RE = 1727 ± 53 ms; power grasp: MI = 1738 ± 78 ms, RE = 1284 ± 36 ms). See Supplementary Material for a further detailed analysis on MI times.
Comparison Between Groups: Action Observation, but not Motor Imagery, Compensates for the Immobilization-Related Changes
Comparing RC data in the ND, MI, and AO groups (Fig. 2a), RM-ANOVA revealed that, independently of the stimulus intensity (GROUP × TIME × INTENSITY, F8, 84 = 1.4, P = 0.23), the GROUP × TIME interaction was significant (F2,21 = 7.0, P = 0.005). Post hoc (Fisher's least significant difference, P < 0.05) showed that the excitability of the motor cortex was significantly different in the 3 groups after immobilization. In fact, MEP amplitude in ND and MI after nonuse was lower than the one recorded in the same condition in AO (ND-Post vs. AO-Post: P < 0.0001 and MI-Post vs. AO-Post: P < 0.0001). Importantly, MEP amplitude in AO after immobilization was not statistically different from the one recorded before nonuse in the 3 groups (ND-Pre vs. AO-Post: P = 0.52 and MI-Pre vs. AO-Post: P = 0.52; AO-Pre vs. AO-Post: P = 0.53).
Differently, a small but significant increase of RMT was found in the 3 groups (TIME: F1, 21=27.68, P = 0.00003), with no condition-dependent differences (GROUP × TIME, F2,21 = 0.89, P = 0.42).
Figure 1b qualitatively shows the extension of the corticomotor representation of FDI muscle as determined by TMS stimulation of the 1-cm mesh grid on the scalp. Visual inspection of the corticomotor maps reveals an immobilization-dependent shrinkage of the hand motor area in the ND and MI groups that was compensated only in the AO group. This was confirmed by RM-ANOVA on map-volume. The GROUP × TIME interaction (F2,21 = 3.51, P = 0.048) was significant. Post hoc comparisons showed that map-volume after immobilization was different in the 3 groups. Indeed, the map-volume measured after nonuse in ND and MI was significantly smaller than the one obtained in the same condition (Post) in the AO (ND-Post vs. AO-Post: P = 0.012; MI-Post vs. AO-Post: P = 0.007). Crucially, the map-volume after nonuse in AO was similar to that recorded before immobilization in the 3 groups (ND-Pre vs. AO-Post: P = 0.38; MI-Pre vs. AO-Post: P = 0.12; AO-Pre vs. AO-Post: P = 0.13).
Effects of Immobilization in Each Group
In the ND group, after immobilization, RMT significantly increased (P = 0.026, Pre: 36.5 ± 1.04; Post: 37.6 ± 1.34). Importantly, cortical excitability of the motor cortex contralateral to the immobilized arm decreased. Indeed, RM-ANOVA revealed that MEP amplitude recorded after immobilization was significantly reduced (TIME × INTENSITY: F4, 28 = 5.27, P = 0.003) at +15% (P = 0.002), +20% (P = 0.001), and +25% (P < 0.0001) of MSO. Moreover, the volume of the FDI cortical map decreased after immobilization (P = 0.001).
In the MI group, similar to the ND group, cortical excitability of the motor cortex contralateral to the immobilized arm and the cortical representation of hand area significantly decreased after nonuse. Precisely, RMT increased (P = 0.018, Pre: 34 ± 0.89; Post: 35 ± 0.87) and the MEP amplitude was reduced after immobilization (TIME × INTENSITY: F4, 28 = 3.66, P = 0.016) at the stimulation intensity of +10% (P = 0.0001), +15% (P = 0.00002), +20% (P = 0.0001), and +25% (P = 0.00001) of MSO. Corticomotor hand representation shrank as indicated by the significant reduction in the map-volume (P = 0.005).
In AO, no significant effects of immobilization were observed either considering RC data (TIME × INTENSITY: F4,28 = 1.15, P = 0.36; TIME: F1, 7 = 0.46, P = 0.52) either the volume of the FDI map (P = 0.37). Besides this, a significant increase of RMT was found (P = 0.013, Pre: 36.25 ± 1.16; Post: 38 ± 1.28).
Effect of Immobilization in the ND, MI, and AO Groups: the GLM Approach
Additionally, in order to further statistically assess the modulation of cortical excitability on corticomotor representation, we analyzed our TMS data by using the GLM (see Statistical Analysis). Among all the stimulated points around hand area, this analysis permitted to successfully identify in each group a cortical activation map, differentiating scalp sites where TMS produced a response from those at which no discernible MEPs were recorded (Fig. 2c). Then, contrasting these activation maps estimated before and after nonuse, we obtained statistical parametric maps of the decrease of motor responses (i.e. deactivation) after immobilization. Results confirmed that the modifications of hand cortical representation described using the map-volume (see above) were reflected also by the spatial extension of the FDI cortical deactivation maps (Fig. 2c, SPM{t}, P < 0.05 uncorrected). Indeed, the significant deactivated area found in the ND and MI groups after immobilization was prevented in the AO group.
Discussion
In the present work, we showed that AO prevents the cortical effects induced by immobilization. In particular, the decrease of cortical excitability (measured through RCs) and the shrinkage of hand cortical representation (evaluated through map-volume) found in the control group (ND) after immobilization were no more present when subjects observed hand action during nonuse. Conversely, MI did not compensate for the immobilization-related changes. These results were also confirmed by analyzing the TMS map data through an innovative approach based on the GLM.
In spite of the different effects observed in the RCs and in hand cortical representations after AO and MI, a small but significant increase of the RMT was equally found in the 3 groups. This apparent contradiction could be explained referring to the fact that MEP amplitude and RMT are related to different neurophysiological mechanisms. Indeed, it has been previously demonstrated that MEP amplitude could be considered as an index of both synaptic and postsynaptic activity, while RMT is related to the excitability of a central core region of neurons (Ziemann et al. 1996; Hallett et al. 1999; see also Facchini et al. 2002). Thus, this weak modification of RMT similarly present in the 3 groups after nonuse, even confirming the efficacy of the immobilization procedure in all subjects, seems not related to the compensation of the decrease of MEP amplitude found only in the AO group.
The effect of AO in reverting the immobilization-related changes could be explained referring to the evidences that the observation of other actions induces specific motor facilitation of the observer's corticospinal system (Fadiga et al. 1995; see also Fadiga et al. 2005). This motor facilitation, revealed by the modulation of MEP amplitude as evoked by TMS applied to the precentral motor cortex, preserves the temporal structure (Gangitano et al. 2001) and the muscular organization (Borroni and Baldissera 2008) of the observed action. Motor facilitation induced by AO may exert plastic changes as well, both in terms of kinematic (Stefan et al. 2005) and of dynamic (Porro et al. 2007) modifications. Similar results have been classically obtained with MI. The kinesthetic simulation of one's own action is known to precisely reflect the timing of RE, as assessed by mental chronometry (e.g. Sirigu et al. 1996; Papaxanthis et al. 2002), and to simulate the phasic (e.g. Abbruzzese et al. 1999; Fadiga et al. 1999) and plastic (e.g. Decety 1996; Naito, Roland, et al. 2002) modifications of corticomotor excitability occurring during actual execution.
Despite this apparent similarity, suggesting a common substrate underlying AO and MI (Jeannerod 2001), the present results show important differences between MI and AO. While MI has no compensatory effects on immobilization (i.e. reduction of corticomotor maps and excitability as in the control condition, ND), AO completely prevents the nonuse-induced corticomotor depression.
One possible interpretation of this difference is that, during immobilization, subjects did not perform the requested MI task. This explanation can, in our view, be fully discarded for the following reasons. First, only participants with a high score at the questionnaire related to imagery abilities (Fourkas et al. 2008) were included in the study. Secondly, all MI participants showed high accuracy when asked to compare 2 following imagined actions. Thirdly, MI participants showed a significant difference in the time required to mentally perform various types of grasping, being slower in the simulated execution of more complex movements (see Results, “Comparison between imagined and real actions”). This is in line with the previous studies, demonstrating that MI obeys the same physical constraints (e.g. Fitt's law on speed/accuracy trade-off, Fitts, 1992) applied to real movements (e.g. Sirigu et al. 1996; Papaxanthis et al. 2002; Papaxanthis et al. 2012), and suggests that they carefully performed the task during the immobilization period. Such result confirms that a motor representation of the grasping movement subserving MI is still accessible even during arm inactivity. In this view, the inefficacy of MI to prevent corticomotor depression seems not dependent on a general impossibility to perform the task, but it could also be due to a less efficiency of MI in activating the motor cortex when the involved body part is prevented to move, as here because of immobilization (Crews and Kamen 2006). Indeed, it has been demonstrated that: (1) MI is less accurate after long-term lower limb immobilization (Malouin et al. 2009); (2) subjects requested to evaluate whether a visually presented hand was a right or a left one were faster in mentally rotate the stimulus when their own hand was kept in a canonical posture (Parsons 1994; de Lange et al. 2006); (3) in neuroimaging studies, compatible bodily states produce an activation in motor and motor-related structures stronger than that evoked while keeping incompatible postures (de Lange et al. 2006; Lorey et al. 2009); (4) in TMS experiments, abnormally low corticospinal facilitation was induced by MI when participant's hand posture was incongruent with the imagined movement (Vargas et al. 2004; Fourkas et al. 2006). In this vein, the present posture imposed by the bandage, the reduction of sensorimotor information during immobilization (Huber et al. 2006) and the impossibility to act with the restricted body part could have affected the motor planning routines normally activated during MI. In further support to this hypothesis, we found here that mentally simulated actions during immobilization (in MI) lasted longer than corresponding actual movements (in RE) (see Results, “Comparison between imagined and real actions”). With regard to this result, it worth nothing that the posology of the trainings in the 3 groups was planned on the number of the observed videos (in AO and ND) or of the simulated actions (MI), but not on the duration of the required tasks. This was designed to rule out the possibility that any difference in the duration/difficulty of the tasks could affect the present findings.
However, all these aspects that may justify the low effectiveness of MI during immobilization did not influence the efficacy of AO.
The result that immobilization-dependent corticomotor depression was prevented in subjects observing hand actions, but not through MI, is new to our knowledge and has important theoretical and practical implications. Theoretically, it suggests that different neural mechanisms underlie MI and AO. MI is an explicit covert mental state during which participants internally simulate a movement without actually performing it (Jeannerod 2001). As during RE, mental simulation of movement involves expectations about sensory and motor effects of that action. Precisely, the framework of internal models suggests that, during both actual and imagined actions, the future sensorimotor state is predicted given the efferent copy of the motor command and the current state of the body (for a review, Wolpert and Flanagan 2001). Thus, the actual body state plays a crucial role during MI (de Lange et al. 2006; Lorey et al. 2009). It has been proposed that such kinesthetic feeling related to the limb is typically processed through parietal region that modulates, in turn, motor cortex facilitation during MI (Sirigu et al. 1996; Blakemore and Sirigu 2003; Tian and Poeppel 2010). When information about the initial state of the limb is lacking (as in amputees) or incompatible with the action to imagine (because of the posture or here because of immobilization), a decrease of activation in the parietal cortex would consequently reduce the MI -related facilitation (Nico et al. 2004; Vargas et al. 2004).
In contrast, during AO, cortical facilitation is not modulated by the initial state of the limb (i.e. the postural similarity between the seen model's moving hand and the observer's own hand, Urgesi et al. 2006), suggesting that bodily information is not essential as in MI process. The visual inputs of the movement performed by the others would have a nonmediated access to observer's motor system. In view of that the so-called “direct-matching hypothesis” (Rizzolatti et al. 2001) proposes that such access is built at the “hardware level,” not relaying on explicit simulation of action as during MI (Jeannerod 2001). A large number of neurons responding to both AO and execution in premotor and parietal cortices support this mechanism (Fadiga et al. 2000). Here, the compensation of immobilization-related effect through AO could be driven by a motor facilitation induced via a direct mapping of the visual representation of the observed action into the corresponding motor representation of the observer, regardless of proprioceptive or postural inputs from the constrained arm.
Furthermore, such reasoning fits also with another previously proposed difference between AO and MI: While the former is based on an automatic mechanism, the latter could involve a more voluntary/active process (Macuga and Frey 2012). This could also explain the increase of cortical facilitation induced by repeated sessions of MI, but not of AO recently showed in healthy subjects by Bianco et al. (2012). Interestingly, however, session-by-session enhancement occurs only in the nondominant, less practiced, hemisphere. Thus, while in some conditions the active nature of MI could produce greater effects, in other cases like the present study, when the motion of a limb is prevented, the automatic matching promoted through AO seems more successful. This appears relevant as far as clinical applications are concerned. Indeed, in agreement with a recent work on stroke patients (Liepert et al. 2012), our data suggest that the intactness of somatosensory input could influence the efficacy of the interventions involving MI. Differently, the simplicity of AO and its effectiveness in cortical remapping encourage the use of this method to powerfully modulate brain plasticity during inactivity, even when sensorimotor information from the constrained limb is reduced.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work has been supported by the grants EU Poeticon++ and “Regione Emilia Romagna–Università” to L.F. Funding to pay the Open Access publication charges for this article was provided by Istituto Italiano di Tecnologia, Robotics, Brain and Cognitive Sciences Department.
Supplementary Material
Notes
We thank L. Avanzino for help with TMS protocol, M. Jacono and R. Canto for the technical support, and L. Taverna for the 3D images, V. Pippo, S. Ghiotto, and M. Jazayeri for their contribution to data collection, and Shannon Hennig for manuscript editing. Conflict of Interest: None declared.
References
- Abbruzzese G, Assini A, Buccolieri A, Marchese R, Trompetto C. Changes in intracortical inhibition during motor imagery in human subjects. Neurosci Lett. 1999;263:113–116. doi: 10.1016/s0304-3940(99)00120-2. [DOI] [PubMed] [Google Scholar]
- Avanzino L, Bassolino M, Pozzo T, Bove M. Use-dependent hemispheric balance. J Neurosci. 2011;31(9):3423–3428. doi: 10.1523/JNEUROSCI.4893-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassolino M, Jacono M, Bove M, Fadiga L, Pozzo T. Functional effect of short term immobilization: kinematic changes and recovery on reaching-to-grasp. Neuroscience. 2012;215:127–134. doi: 10.1016/j.neuroscience.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Bianco G, Feurra M, Fadiga L, Rossi A, Rossi S. Bi-hemispheric effects on corticospinal excitability induced by repeated sessions of imagery versus observation of actions. Restor Neurol Neurosci. 2012;30(6):481–489. doi: 10.3233/RNN-2012-120241. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Sirigu A. Action prediction in the cerebellum and in the parietal lobe. Exp Brain Res. 2003;153:239–245. doi: 10.1007/s00221-003-1597-z. [DOI] [PubMed] [Google Scholar]
- Borroni P, Baldissera F. Activation of motor pathways during observation and execution of hand movements. Soc Neurosci. 2008;3:276–288. doi: 10.1080/17470910701515269. [DOI] [PubMed] [Google Scholar]
- Buccino G, Solodkin A, Small S. Functions of the mirror neuron system: implications for neurorehabilitation. Cogn Behav Neurol. 2006;19:55–63. doi: 10.1097/00146965-200603000-00007. [DOI] [PubMed] [Google Scholar]
- Castiello U. The neuroscience of grasping. Nat Rev Neurosci. 2005;6:726–736. doi: 10.1038/nrn1744. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Bandinelli S, Findley TW, Hallett M. Motor reorganization after upper limb amputation in man (a study with focal magnetic stimulation) Brain. 1991;114:615–627. doi: 10.1093/brain/114.1.615. [DOI] [PubMed] [Google Scholar]
- Crews RT, Kamen G. Motor-evoked potentials following imagery and limb disuse. Int J Neurosci. 2006;116:639–651. doi: 10.1080/00207450600592198. [DOI] [PubMed] [Google Scholar]
- Decety J. Do imagined and executed actions share the same neural substrate? Cogn Brain Res. 1996;3:87–93. doi: 10.1016/0926-6410(95)00033-x. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Helmich RC, Toni I. Posture influences motor imagery: an fMRI study. Neuroimage. 2006;33:609–617. doi: 10.1016/j.neuroimage.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Elbert T, Flor H, Birbaumer N, Knecht S, Hampson S, Larbig W, Taub E. Extensive reorganization of the somatosensory cortex in adult humans after nervous system injury. Neuroreport. 1994;5:2593–2597. doi: 10.1097/00001756-199412000-00047. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Ertelt D, Small S, Solodkin A, Dettmers C, McNamara A, Binkofski F, Buccino G. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage. 2007;36(2):T164–T173. doi: 10.1016/j.neuroimage.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Facchini S, Romani M, Tinazzi M, Aglioti SM. Time-related changes of excitability of the human motor system contingent upon immobilization of the ring and little fingers. Clin Neurophysiol. 2002;113:367–375. doi: 10.1016/s1388-2457(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Buccino G, Craighero L, Fogassi L, Gallese V, Pavesi G. Corticospinal excitability is specifically modulated by motor imagery: a magnetic stimulation study. Neuropsychologia. 1999;37:147–158. doi: 10.1016/s0028-3932(98)00089-x. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Craighero L, Olivier E. Human motor cortex excitability during the perception of other actions. Curr Opin Neurobiol. 2005;15:213–218. doi: 10.1016/j.conb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Visuomotor neurons: ambiguity of the discharge or “motor” perception? Int J Psychophysiol. 2000;35(2–3):165–177. doi: 10.1016/s0167-8760(99)00051-3. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol Gen. 1992;121:262–269. doi: 10.1037//0096-3445.121.3.262. [DOI] [PubMed] [Google Scholar]
- Fourkas AD, Bonavolontà V, Avenanti A, Aglioti SM. Kinesthetic imagery and tool-specific modulation of corticospinal representations in expert tennis players. Cereb Cortex. 2008;18:2382–2390. doi: 10.1093/cercor/bhn005. [DOI] [PubMed] [Google Scholar]
- Fourkas AD, Ionta S, Aglioti SM. Influence of imagined posture and imagery modality on corticospinal excitability. Behav Brain Res. 2006;168:190–196. doi: 10.1016/j.bbr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:189–210. [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual-Leone A. Phase-specific modulation of cortical motor output during movement observation. Neuroreport. 2001;12:1489–1492. doi: 10.1097/00001756-200105250-00038. [DOI] [PubMed] [Google Scholar]
- Gueugneau N, Mauvieux B, Papaxanthis C. Circadian modulation of mentally simulated motor actions: implications for the potential use of motor imagery in rehabilitation. Neurorehabil Neural Repair. 2009;23:237–245. doi: 10.1177/1545968308321775. [DOI] [PubMed] [Google Scholar]
- Hall CR, Martin KA. Measuring movement imagery abilities: a revision of the movement imagery questionnaire. J Ment Imagery. 1997;21:143–154. [Google Scholar]
- Hallett M, Chen R, Ziemann U, Cohen LG. Reorganization in motor cortex in amputees and in normal volunteers after ischemic limb de-afferentiation. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:183–187. [PubMed] [Google Scholar]
- Hashimoto R, Rothwell JC. Dynamic changes in corticospinal excitability during motor imagery. Exp Brain Res. 1999;125:75–81. doi: 10.1007/s002210050660. [DOI] [PubMed] [Google Scholar]
- Holmes P, Calmels C. A neuroscientific review of imagery and observation use in sport. J Mot Behav. 2008;40:433–445. doi: 10.3200/JMBR.40.5.433-445. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage. 2001;14:S103–S109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- Kalisch T, Tegenthoff M, Dinse HR. Repetitive electric stimulation elicits enduring improvement of sensorimotor performance in seniors. Neural Plast. 2010;2010:690531. doi: 10.1155/2010/690531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JJ, Ridding MC, Rothwell JC, Passingham RE, Leigh PN, Sooriakumaran S, Frackowiak RS, Brooks DJ. Reorganization of cortical blood flow and transcranial magnetic stimulation maps in human subjects after upper limb amputation. J Neurophysiol. 1994;72:2517–2524. doi: 10.1152/jn.1994.72.5.2517. [DOI] [PubMed] [Google Scholar]
- Kristeva-Feige R, Rossi S, Pizzella V, Sabato A, Tecchio F, Feige B, Romani GL, Edrich J, Rossini PM. Changes in movement-related brain activity during transient deafferentation: a neuromagnetic study. Brain Res. 1996;714(1–2):201–208. doi: 10.1016/0006-8993(95)01537-x. [DOI] [PubMed] [Google Scholar]
- Langer N, Hänggi J, Müller NA, Simmen HP, Jäncke L. Effects of limb immobilization on brain plasticity. Neurology. 2012;78:182–188. doi: 10.1212/WNL.0b013e31823fcd9c. [DOI] [PubMed] [Google Scholar]
- Liepert J, Greiner J, Nedelko V, Dettmers C. Reduced upper limb sensation impairs mental chronometry for motor imagery after stroke: clinical and electrophysiological findings. Neurorehabil Neural Repair. 2012;26:470–478. doi: 10.1177/1545968311425924. [DOI] [PubMed] [Google Scholar]
- Liepert J, Tegenthoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroenceph Clin Neurophysiol. 1995;97:382–386. doi: 10.1016/0924-980x(95)00194-p. [DOI] [PubMed] [Google Scholar]
- Lissek S, Wilimzig C, Stude P, Pleger B, Kalisch T, Maier C, Peters SA, Nicolas V, Tegenthoff M, Dinse HR. Immobilization impairs tactile perception and shrinks somatosensory cortical maps. Curr Biol. 2009;19:837–842. doi: 10.1016/j.cub.2009.03.065. [DOI] [PubMed] [Google Scholar]
- Lorey B, Bischoff M, Pilgramm S, Stark R, Munzert J, Zentgraf K. The embodied nature of motor imagery: the influence of posture and perspective. Exp Brain Res. 2009;194:233–243. doi: 10.1007/s00221-008-1693-1. [DOI] [PubMed] [Google Scholar]
- Lotze M, Cohen LG. Volition and imagery in neurorehabilitation. Cogn Behav Neurol. 2006;19(3):135–140. doi: 10.1097/01.wnn.0000209875.56060.06. [DOI] [PubMed] [Google Scholar]
- Lotze M, Halsband U. Motor imagery. J Physiol. 2006;99:386–395. doi: 10.1016/j.jphysparis.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Macuga KL, Frey SH. Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. Neuroimage. 2012;59(3):2798–2807. doi: 10.1016/j.neuroimage.2011.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Durand A, Descent M, Poiré D, Frémont P, Pelet S, Gresset J, Doyon J. Effects of practice, visual loss, limb amputation, and disuse on motor imagery vividness. Neurorehabil Neural Repair. 2009;23:449–463. doi: 10.1177/1545968308328733. [DOI] [PubMed] [Google Scholar]
- Naito E, Ehrsson HH, Geyer S, Zilles K, Roland PE. Illusory arm movements activate cortical motor areas: a positron emission tomography study. J Neurosci. 1999;19:6134–6144. doi: 10.1523/JNEUROSCI.19-14-06134.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E, Kochiyama T, Kitada R, Nakamura S, Matsumura M, Yonekura Y, Sadato N. Internally simulated movement sensations during motor imagery activate cortical motor areas and the cerebellum. J Neurosci. 2002;22:3683–3691. doi: 10.1523/JNEUROSCI.22-09-03683.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E, Roland PE, Ehrsson HH. I feel my hand moving: a new role of the primary motor cortex in somatic perception of limb movement. Neuron. 2002;36:979–988. doi: 10.1016/s0896-6273(02)00980-7. [DOI] [PubMed] [Google Scholar]
- Nico D, Daprati E, Rigal F, Parsons L, Sirigu A. Left and right hand recognition in upper limb amputees. Brain. 2004;127:120–132. doi: 10.1093/brain/awh006. [DOI] [PubMed] [Google Scholar]
- Niyazov DM, Butler AJ, Kadah YM, Epstein CM, Hu XP. Functional magnetic resonance imaging and transcranial magnetic stimulation: effects of motor imagery, movement and coil orientation. Clin Neurophysiol. 2005;116:1601–1610. doi: 10.1016/j.clinph.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Page SJ, Levine P, Sisto S, Johnson MV. A randomized efficacy and feasibility study of imagery in acute stroke. Clin Rehabil. 2001;15:233–240. doi: 10.1191/026921501672063235. [DOI] [PubMed] [Google Scholar]
- Papaxanthis C, Paizis C, Pozzo T, Stucchi N. The relation between geometry and time in mental actions. PLoS One. 2012;7(11):e51191. doi: 10.1371/journal.pone.0051191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaxanthis C, Schieppati M, Gentili R, Pozzo T. Imagined and actual arm movements have similar durations when performed under different conditions of direction and mass. Exp Brain Res. 2002;143:447–452. doi: 10.1007/s00221-002-1012-1. [DOI] [PubMed] [Google Scholar]
- Parsons LM. Temporal and kinematic properties of motor behavior reflected in mentally simulated action. J Exp Psychol Hum Percept Perform. 1994;20:709–730. doi: 10.1037//0096-1523.20.4.709. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74(3):1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Porro CA, Facchin P, Fusi S, Dri G, Fadiga L. Enhancement of force after action observation: behavioural and neurophysiological studies. Neuropsychologia. 2007;45:3114–3121. doi: 10.1016/j.neuropsychologia.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Afferent input and cortical organization: a study with magnetic stimulation. Exp Brain Res. 1999;126:536–544. doi: 10.1007/s002210050762. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rossi S, De Capua A, Pasqualetti P, Ulivelli M, Fadiga L, Falzarano V, Bartalini S, Passero S, Nuti D, Rossini PM. Distinct olfactory cross-modal effects on the human motor system. PLoS One. 2008;3(2):e1702. doi: 10.1371/journal.pone.0001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Tecchio F, Sabato A, Rossini PM. Modulation of corticospinal output to human hand muscles following deprivation of sensory feedback. Neuroimage. 1998;8(2):163–175. doi: 10.1006/nimg.1998.0352. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Martino G, Narici L, Pasquarelli A, Peresson M, Pizzella V, Tecchio F, Torrioli G, Romani GL. Short-term brain ‘plasticity’ in humans: transient finger representation changes in sensory cortex somatotopy following ischemic anesthesia. Brain Res. 1994;642:169–177. doi: 10.1016/0006-8993(94)90919-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Pasqualetti P, Tecchio F. Corticospinal excitability modulation to hand muscles during movement imagery. Cereb Cortex. 1999;9:1047–3211. doi: 10.1093/cercor/9.2.161. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Tecchio F, Pasqualetti P, Finazzi-Agrò A, Sabato A. Focal brain stimulation in healthy humans: motor maps changes following partial hand sensory deprivation. Neurosci Lett. 1996;214(2–3):191–195. doi: 10.1016/0304-3940(96)12940-2. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Tecchio F, Sabato A, Finazzi-Agrò A, Pasqualetti P, Rossi S. The role of cutaneous inputs during magnetic transcranial stimulation. Muscle Nerve. 1996;19(10):1302–1309. doi: 10.1002/(SICI)1097-4598(199610)19:10<1302::AID-MUS7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Roure R, Collet C, Deschaumes-Molinaro C, Delhomme G, Dittmar A, Vernet-Maury E. Imagery quality estimated by autonomic response is correlated to sporting performance enhancement. Physiol Behav. 1996;66(1):63–72. doi: 10.1016/s0031-9384(99)00026-8. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Schabrun SM, Ridding MC. The influence of correlated afferent input on motor cortical representations in humans. Exp Brain Res. 2007;183:41–49. doi: 10.1007/s00221-007-1019-8. [DOI] [PubMed] [Google Scholar]
- Sharma N, Pomeroy VM, Baron JC. Motor imagery: a backdoor to the motor system after stroke. Stroke. 2006;37:1941–1952. doi: 10.1161/01.STR.0000226902.43357.fc. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996;273:1564–1568. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- Starr A, Caramia M, Zarola F, Rossini PM. Enhancement of motor cortical excitability in humans by non-invasive electrical stimulation appears prior to voluntary movement. Electroencephalogr Clin Neurophysiol. 1988;70:26–32. doi: 10.1016/0013-4694(88)90191-5. [DOI] [PubMed] [Google Scholar]
- Stefan K, Cohen LG, Duque J, Mazzocchio R, Celnik P, Sawaki L, Ungerleider L, Classen J. Formation of a motor memory by action observation. J Neurosci. 2005;25:9339–9346. doi: 10.1523/JNEUROSCI.2282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterr A, Muller MM, Elbert T, Rockstroh B, Pantev C, Taub E. Changed perceptions in Braille readers. Nature. 1998;391(6663):134–135. doi: 10.1038/34322. [DOI] [PubMed] [Google Scholar]
- Tian X, Poeppel D. Mental imagery of speech and movement implicates the dynamics of internal forward models. Front Psychol. 2010;1:166. doi: 10.3389/fpsyg.2010.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urgesi C, Candidi M, Fabbro F, Romani M, Aglioti SM. Motor facilitation during action observation: topographic mapping of the target muscle and influence of the onlooker's posture. Eur J Neurosci. 2006;23:2522–2530. doi: 10.1111/j.1460-9568.2006.04772.x. [DOI] [PubMed] [Google Scholar]
- Uy J, Ridding MC, Miles TS. Stability of maps of human motor cortex made with transcranial magnetic stimulation. Brain Topogr. 2002;14:293–297. doi: 10.1023/a:1015752711146. [DOI] [PubMed] [Google Scholar]
- Vargas CD, Olivier E, Craighero L, Fadiga L, Duhamel JR, Sirigu A. The influence of hand posture on corticospinal excitability during motor imagery: a transcranial magnetic stimulation study. Cereb Cortex. 2004;14:1200–1206. doi: 10.1093/cercor/bhh080. [DOI] [PubMed] [Google Scholar]
- Weibull A, Flondell M, Rosén B, Björkman A. Cerebral and clinical effects of short-term hand immobilisation. Eur J Neurosci. 2011;33(4):699–704. doi: 10.1111/j.1460-9568.2010.07551.x. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR. Motor prediction. Curr Biol. 2001;11:729–732. doi: 10.1016/s0960-9822(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.