Abstract
It has consistently been reported that “negative” subsequent memory effects—lower study activity for later remembered than later forgotten items—are attenuated in older individuals. The present functional magnetic resonance imaging study investigated whether these findings extend to subsequent memory effects associated with successful encoding of item–context information. Older (n = 25) and young (n = 17) subjects were scanned while making 1 of 2 encoding judgments on a series of pictures. Memory was assessed for the study item and, for items judged old, the item's encoding task. Both memory judgments were made using confidence ratings, permitting item and source memory strength to be unconfounded and source confidence to be equated across age groups. Replicating prior findings, negative item effects in regions of the default mode network in young subjects were reversed in older subjects. Negative source effects, however, were invariant with respect to age and, in both age groups, the magnitude of the effects correlated with source memory performance. It is concluded that negative item effects do not reflect processes necessary for the successful encoding of item–context associations in older subjects. Negative source effects, in contrast, appear to reflect the engagement of processes that are equally important for successful episodic encoding in older and younger individuals.
Keywords: aging, default mode network, encoding, fMRI, source memory
Introduction
Episodic memory—memory for unique events—declines markedly and, compared with other kinds of memory, disproportionately with increasing age (Nilsson 2003). A considerable body of work, much of it involving functional neuroimaging, has focused on elucidating the cognitive and neural bases of episodic memory decline. Many of these studies have focused on whether there are age-related differences in the neural correlates of episodic memory encoding, employing the functional magnetic resonance imaging (fMRI) “subsequent memory procedure” (Paller and Wagner 2002) in an effort to identify such differences. In this procedure, encoding-related activity associated with study trials that are later remembered is contrasted with the activity elicited on trials that are later forgotten (identifying ‘subsequent memory effects’). Two classes of subsequent memory effect can be identified (see Kim 2011 for review). One class—the most heavily investigated in both studies confined to young subjects and in aging studies—takes the form of enhanced study activity for later remembered relative to later forgotten trials. The other class, known as negative subsequent memory effects, takes the reverse form—namely, a relative diminution of study activity for trials that go on to be remembered. It is this second class of effects that is the focus of the present paper.
As documented in the meta-analysis of Kim (2011; see also Otten and Rugg 2001; Wagner and Davachi 2001; Clark and Wagner 2003; Daselaar et al. 2004; Reynolds et al. 2004; Park and Rugg 2008 for examples), negative subsequent memory effects in young individuals have consistently been reported in a characteristic set of brain regions, including medial parietal and posterior cingulate cortex, medial prefrontal cortex (PFC), and lateral parietal cortex. As noted by several authors (e.g. Daselaar et al. 2004; Turk-Browne et al. 2006; Park and Rugg 2008; Shrager et al. 2008; Kim 2011), these regions form part of the “default mode network,” a set of brain regions that collectively exhibit task-related deactivation (greater activity during “rest” than during task engagement) and whose resting-state activity is intercorrelated (e.g. Raichle et al. 2001; Buckner et al. 2008). This network is of relevance to cognitive aging, because task-induced deactivations in default mode regions are significantly reduced in healthy older subjects compared with young subjects (e.g. Lustig et al. 2003; Grady et al. 2006; Persson et al. 2007; Sambataro et al. 2010) and are further reduced in patients with mild cognitive impairment (Rombouts et al. 2005) and Alzheimer's disease (Lustig et al. 2003; Greicius et al. 2004). Default mode network regions are believed to support “internally-directed” processes that must be disengaged in order to permit optimal allocation of processing resources to an external event (such as a study item). Although the functional significance of negative subsequent memory effects remains to be fully elucidated, negative effects in putative default regions are thought to reflect the benefit to encoding that results from redirecting processing resources from internally directed cognition to an external event (Daselaar et al. 2004; Huijbers et al. 2013).
Importantly, age-related differences in default mode activity are accompanied by analogous differences in negative subsequent memory effects. Several studies have reported that the effects are attenuated, or even reversed, in older subjects during the encoding of single items (Morcom et al. 2003, Gutchess et al. 2005; Duverne et al. 2009; Mormino et al. 2012) or item–item associations (Miller et al. 2008; de Chastelaine et al. 2011). In 4 of these studies (Miller et al. 2008; Duverne et al. 2009; de Chastelaine et al. 2011; Mormino et al. 2012), the magnitude of negative effects in older individuals was positively correlated with their memory performance, suggesting that the effects reflect the engagement of processes beneficial to memory encoding.
The primary motivation for the current study derives from prior reports of age-related attenuation in negative subsequent memory effects for the encoding of inter-item associations (face-name pairs in Miller et al. 2008; word pairs in de Chastelaine et al. 2011). Unlike recognition memory for single items, which can be supported both by retrieval of episodic information and a separate, acontextual sense of familiarity (Yonelinas 2002), memory for item–item associations is held to depend heavily on episodic retrieval (“recollection”). Thus, the findings from these studies suggest that the failure to appropriately modulate default mode activity during encoding plays an important role in age-related episodic memory impairment.
The present study builds on these prior findings by addressing the question of whether the age-related attenuation of negative subsequent memory effects reported for the encoding of item–item associations extends to the encoding of item–context associations (source memory). Like inter-item associations, memory for item–context associations is also held to depend heavily on the recollection of episodic information, with little contribution from familiarity (Mickes et al. 2010). Moreover, again like memory for inter-item associations (e.g. Naveh-Benjamin 2000), source memory is markedly affected by age (e.g. Spencer and Raz 1995; Glisky et al. 2001; but see Siedlecki et al. 2005). Thus, if the failure to disengage default mode activity plays a role in age-related episodic memory decline, as was proposed above, older subjects should demonstrate the same pattern of attenuated negative subsequent memory effects for the encoding of item–context associations as they do for the encoding of item–item associations.
Importantly, the present study incorporated a methodological refinement motivated by the fact that the distinction between successful and unsuccessful source memory tends to be confounded with the strength of item memory (accuracy and confidence of item recognition are higher for items that go on to elicit accurate rather than inaccurate source judgments; Squire et al. 2007; Kirwan et al. 2008; Wais et al. 2010). To control for the confounding effects of memory strength, subjects made confidence judgments for both item and source judgments. Items receiving a “confident old” judgment were segregated according to whether they went on to be given an accurate, highly confident source judgment (“source hit”) or an inaccurate/uncertain context judgment (“source miss”). Studied items that were recognized with low confidence or misclassified as new were assigned to a separate category. Thus, the contrast between source miss and source hit trials permitted identification of the neural correlates of strong item–context associations unconfounded by differences in item memory strength. Correspondingly, the contrast between “item miss” and source miss trials permitted the identification of the neural correlates of the encoding of memories, supporting high confidence item judgments in the absence of associated source information.
Materials and Methods
Subjects
Seventeen healthy young adults (11 females) aged between 18 and 27 years (mean age: 20 years), and 25 healthy older adults (16 females) aged between 63 and 74 years (mean age: 67 years), participated in the experiment. Young adults were recruited from the undergraduate and graduate student population of the University of California Irvine (UCI), and older adults were recruited from the Orange County community. All subjects were screened for histories of neurological, cardiovascular, or psychiatric illness, and contraindications for MR imaging. While none of the subjects were taking central nervous system-active medication, 2 older subjects were taking antihypertensive medication. All subjects had normal or corrected-to-normal vision, were right-handed, and were fluent English speakers. Subjects gave informed consent prior to participating and were remunerated for their participation in accordance with the human subjects procedures approved by the Institutional Review Board at UCI.
Potential subjects were excluded if they scored 1.5 standard deviations (SD) below their age-appropriate norm on the California Verbal Learning Test-II (CVLT), below 100 on the Full Scale Intelligence Quotient (FSIQ; estimated from the Wechsler Test of Adult Reading, WTAR), or more than 1.5 SD below the age-appropriate norm on any 2 of the other neuropsychological tests described below. Data collected from 1 younger adult and 2 additional older adults were excluded from all analyses because of excessive head movement (>3 degrees of rotation) during scanning. Data from one other older adult were excluded because of abnormal signal in subinsular regions in the structural scans.
Neuropsychological Testing
In a separate session prior to the fMRI procedure, a battery of standardized neuropsychological tests was administered to all subjects. The battery assessed a range of cognitive functions known to either decline or to be maintained with age. The mini-mental state examination was utilized as a dementia screening measure, where a cut-off score of 26 out of 30 was adopted. Long-term memory was assessed with the CVLT-II and the Wechsler Memory Scale-IV Logical Memory II. Short-term memory was assessed with the Digit Span Forward and Backward test of the Wechsler Adult Intelligence Scale-Revised (WAIS-R). General cognitive functions were further assessed with the Digit/Symbol Coding test of the WAIS-R, the Trail Making Test A and B, and letter fluency and category fluency tests.
An estimate of full scale IQ was obtained from the WTAR. The Geriatric Depression Scale was also administered to older subjects.
Stimulus Materials
Three hundred and thirty-two stimulus pictures were used in the experiment. The colored pictures depicting everyday objects were drawn from Hemera Photo Objects 50 000 Volume III. Of the 332 pictures, 12 served as buffers (2 at the beginning and end of each study list and test list) and 50 additional pictures were used as practice items before the study and test phases. Of the remaining 270 pictures, 180 were assigned to the “study” condition, while 90 were assigned as “new” items.
Two study lists were created from the 180 study pictures for each subject. Each picture list contained a pseudorandomized ordering of 90 pictures (45 “size” and 45 “where” judgments) and 30 null trials, with no more than 3 consecutive presentations of items belonging to the same encoding task. Test items comprised the 180 pictures from the study trials and 90 new pictures and were pseudorandomized, such that there were no more than 3 consecutive presentations of items belonging to the same experimental condition. All experimental stimulus display was implemented using the Cogent software package (http://www.vislab.ucl.ac.uk/cogent.php).
Experimental Tasks and Procedures
The experimental procedure consisted of a study task which took place during scanning, followed by a recognition memory test on a computer outside the scanner 20–25 min after the end of the scanning session. Prior to the scanning session, each subject was administered a 2-min practice study (24 pictures and 7 “null” events). Both a 7-min structural scan and 12-min diffusion tensor imaging (DTI) scan were conducted prior to the study phase. Immediately prior to the study phase, a second 1-min practice session was administered inside the scanner.
Study Phase
Two blocks of pictures (stimuli described above) were administered, separated by a 1-min break. The requirement was to make a size or location judgment depending on a 1-letter study cue preceding the stimulus picture: “S?” for a size judgment (bigger or smaller than a shoebox) and “W?” for a where judgment (indoors or outdoors). Instructions emphasized the need to respond quickly, but without sacrificing accuracy. Subjects were told their memories for the pictures would be tested later, but they were not informed of the source memory test (“size” vs. “where”).
During each study trial, the study cue was displayed for 500 ms (Helvetica, 30 point font), followed immediately by a study picture which was presented for 1500 ms. The study picture was replaced with a white fixation cross in the same font and size as the study cue for 1650 ms, which was switched to a red fixation cross for 500 ms signaling the end of the trial. The stimulus onset asynchrony of study trials was distributed stochastically with a minimum duration of 4150 ms modulated by the additional 60 null trials (Josephs and Henson 1999).
Study items were back-projected onto a screen and viewed via a mirror mounted on the scanner head coil. Pictures were presented in central vision and subtended a maximum visual angle of 9.5° × 9.5° in horizontal and vertical directions. Study task responses were made with the right index and middle fingers via a hand-held button. The assignment of each finger to the smaller/indoors or larger/outdoors response was counterbalanced across subjects.
Test Phase
After completion of the study session, subjects were removed from the scanner. They were then informed of the source memory test and given instructions and a short (34 item) practice test. The test requirement was to judge whether the item had been presented at study and, if so, to indicate which encoding task had been associated with the picture at study.
The test pictures were presented in central vision, and subtended 5.7° × 5.7° visual angles at the 1-m viewing distance. Instructions were to make an old/new judgment on a 5-point confident scale, the options for which appeared below the picture in white letters: “Conf-Old,” “Unconf-Old,” “Do-not-know,” “Unconf-New,” “Conf-New.” If one of the categories “Do-not-know”/“Unconf-New”/“Conf-New” was selected, the test advanced to the next item. If a “Conf-Old” or “Unconf-Old” response was made, an encoding context judgment was required: “Conf-S,” “Unconf-S,” “Do-not-know,” “Unconf-W,” or “Conf-W.”
The hands employed for old and don't know/new responses were counterbalanced across subjects, with the middle/index finger of one hand assigned to “Conf-Old”/“Unconf-Old,” respectively, and the index/middle/ring finger of the other hand assigned to “Do-not-know”/“Unconf-New”/“Conf-New,” respectively. Size/where responses were also counterbalanced with “Conf-S”/“Unconf-S” and “Do-not-know”/“Unconf-W”/“Conf-W” judgments assigned to separate hands. The test was self-paced, presented as a single block, and lasted approximately 25 min.
MRI Data Acquisition
A Philips Achieva 3-T MR scanner (Philips Medical Systems) equipped with a transmit/receive radio frequency head coil was used to acquire anatomical and functional images. Functional scans were acquired with a T2*-weighted, echo-planar image (EPI) sequence using the following parameters: time repetition (TR) 2 s, time echo (TE) 30 ms, flip angle 70°, field of view (FOV) 240 × 240, matrix size 80 × 79. Each EPI volume was acquired in ascending order and consisted of 30 slices (3 mm thick with a 1-mm interslice gap), oriented parallel to the line connecting anterior and posterior commissures, and positioned for full coverage of the cerebrum and most of the cerebellum. Functional data were acquired during each of the 2 study blocks (266 volumes per block) and concatenated across sessions prior to model estimation. The first 3 volumes were discarded to allow tissue magnetization to achieve a steady state.
T1-weighted anatomical images were acquired using a 3-dimensional magnetization-prepared rapid gradient-echo pulse sequence with the following parameters: FOV = 240 × 240, matrix size 220 × 193, voxel size 1 × 1 × 1 mm, 150 slices, sagittal acquisition. Although not reported here, diffusion tensor images were also acquired for each subject.
MRI Data Analysis
Data were preprocessed and analyzed with Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK; Friston et al. 1995) implemented under Matlab2008b (Mathworks, Inc., USA). Volumes were motion and slice-time corrected, realigned and then spatially normalized using a sample-specific template. The sample-specific template was created by first normalizing (Ashburner and Friston 1999) the initial volume of each subject's functional time series with reference to a standard EPI template based on the MNI reference brain (Cocosco et al. 1997). Normalized volumes were separately averaged within each age group, and the resulting 2 mean images were then averaged to generate a sample-specific template that was equally weighted with respect to each age group. Normalized volumes were resampled into 3-mm isotropic voxels, and smoothed with an isotropic 10-mm full-width half-maximum Gaussian kernel to accommodate residual anatomical variation between subjects. T1-weighted anatomical images were normalized with a procedure analogous to the functional images and resampled into 2-mm isotropic voxels.
Stimulus-elicited neural activity was modeled for each subject by convolving a delta function with 2 hemodynamic response functions (HRFs). The 2 functions consisted of a canonical (“early”) HRF as implemented in SPM (Friston et al. 1998) and delayed (“late”) HRF that was generated by temporally shifting the canonical HRF by one TR (2 s), and was included to capture possible delayed responses. The late function was orthogonalized with respect to the early function using the Gram-Schmidt procedure so as to give priority to the canonical function (Andrade et al. 1999). The findings for the late function did not add substantially to those obtained with the early function and are not reported here.
The design matrix of the general linear model included 5 early and 5 late covariates that modeled events defined by subjects' responses during the test phase. Three events of interest were identified for the fMRI analyses: (1) Studied items correctly and confidently endorsed as old that were associated with a confident correct context response (source hit); (2) items correctly and confidently endorsed as old followed by an incorrect or “don't know” context response (source miss); and (3) items that were either unrecognized or judged old with low confidence (item miss). A fourth category consisted of items correctly and confidently judged old followed by a correct source judgment of low confidence. These trials were modeled separately and not included in the fMRI analysis. The fifth category of trials comprised events of no interest, namely buffer trials and trials associated with omitted or multiple study responses. Six regressors modeling concatenated movement-related variance (3 rigid-body translations and 3 rotations determined from the realignment stage) and session-specific constant terms modeling the mean over scans in each session were also entered into the design matrix.
The functional time series for each voxel was high-pass filtered to 1/128 Hz and scaled within session to a grand mean of 100 across voxels and scans. Nonsphericity of the error covariance was accommodated by an auto-regressive (1) model, in which the temporal autocorrelation was estimated by pooling over suprathreshold voxels (Friston et al. 2002). The parameters for each covariate and the hyperparameters governing the error covariance were estimated using a Restricted Maximum Likelihood (ReML) approach. Parameter estimates derived from each covariate were taken forward to the second level of analysis.
To identify voxels that differentiated the 3 events of interest in an unbiased manner, the respective parameter estimates were subjected to a 2 × 3 mixed-design analysis of variance (ANOVA) with factors of age group (young and older subjects) and response category (source hit, source miss, and item miss) using the statistical methods implemented in SPM8. Pair-wise contrasts (t-maps) derived from the ANOVA model were thresholded at P < 0.001, 1-tailed. Interaction contrasts (F-maps) were threshold at P < 0.001, 2-tailed. Control of Type I error was effected by imposing a cluster-wise threshold of P < 0.05, corrected for multiple comparisons within a whole-brain mask. The threshold was set at 24 contiguous voxels on the basis of a Monte Carlo simulation implemented in the Alphasim routine of the AFNI analysis package (NIMH, Bethesda, MD, USA; http://afni.nimh.nih.gov/afni).
Results
Neuropsychological Data
Demographic and neuropsychological data for older and younger subjects are summarized in Table 1. As can be seen from the table, the groups were well matched on estimated IQ (WTAR) and performed comparably on tests of digit span and letter/category fluency. Older adults demonstrated significantly lower performance on some tests of long-term memory (CVLT composite recall and false positive scores), but not others (Wechsler Memory Scale-IV Logical Memory II), and demonstrated significantly lower performance on tests requiring speeded cognition (Trail Making A and B; Digit/Symbol Substitution).
Table 1.
Demographic and neuropsychological data (mean, SD, and range) for young and older subjects
| Young |
Old |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age | 19.7 | 2.2 | 18–27 | 67.1 | 3.6 | 62–74 |
| Years of education* | 14.2 | 1.8 | 13–20 | 16.6 | 2.3 | 12–22 |
| Mini-mental state examination | 29.5 | 1.0 | 26–30 | 28.8 | 1.2 | 27–30 |
| CVLT composite recall score* | 13.6 | 1.8 | 10–16 | 12.0 | 2.3 | 8–16 |
| CVLT recognition hits | 15.2 | 0.8 | 14–16 | 14.8 | 1.3 | 12–16 |
| CVLT recognition false positives* | 0.6 | 1.1 | 0–4 | 1.7 | 1.0 | 0–7 |
| WMS Logical Memory II composite score | 28.6 | 6.0 | 20–39 | 26.4 | 5.0 | 15–36 |
| Forward/Backward Digit Span | 18.4 | 3.8 | 12–25 | 17.8 | 3.5 | 11–28 |
| Digit/Symbol Substitution Test** | 69.1 | 11.3 | 54–86 | 50.6 | 8.0 | 35–68 |
| Trail Making Test A** | 18.9 | 3.8 | 13–26 | 24.7 | 5.0 | 15–37 |
| Trail Making Test B* | 46.4 | 14.5 | 26–83 | 65.7 | 20.2 | 37–98 |
| Letter Fluency | 42.2 | 10.2 | 31–69 | 44.6 | 11.4 | 22–64 |
| Category Fluency | 24.2 | 4.1 | 16–30 | 21.8 | 5.3 | 11–36 |
| WTAR FSIQ | 110.4 | 4.9 | 101–119 | 111.2 | 4.3 | 101–117 |
CVLT: California Verbal Learning Test; CVLT composite recall score: averaged scores from CVLT cued and free recall for both short and long delay;
WMS: Wechsler Memory Scale; FSIQ: Full Scale Intelligence Quotient.
*P < 0.01, **P < 0.001, 2-tailed t-tests.
Behavioral Results
Study Phase
Reaction times (RTs) to study items are given in Table 2 segregated according to the subsequent memory condition. To assess whether RT varied with age group or later memory, ANOVA with factors of memory condition for the fMRI analysis (source hit, source miss, and item miss) and age group (young and older subjects) was performed. There was no main effect of group (F1,40 < 1), nor was there a significant interaction between group and subsequent memory condition (F1.9,74.0 < 1). There was, however, a main effect of subsequent memory condition (F1.9,74.0 = 13.26, P < 0.001). Follow-up analyses revealed that the effect reflected significantly shorter study RTs for source hit than source miss trials (F1,40 = 7.70, P < 0.01) and significantly longer RTs for source miss relative to item miss trials (F1,40 = 27.62, P < 0.001). Additionally, RTs for source hit trials were significantly longer than those for item miss trials (F1,40 = 5.03, P < 0.05). RTs thus followed the pattern of item miss < source hit < source miss.
Table 2.
Mean study RTs (ms) segregated by subsequent memory response (±SD)
| CC | UC | DK | UI | CI | |
|---|---|---|---|---|---|
| Item | |||||
| Young | 1737 (276) | 1717 (304) | 1687 (370) | 1641 (309) | 1736 (310) |
| Old | 1799 (360) | 1801 (474) | 1779 (407) | 1710 (383) | 1750 (396) |
| Source | |||||
| Young | 1734 (273) | 1746 (299) | 1767 (265) | 1771 (351) | 1751 (347) |
| Old | 1790 (364) | 1818 (408) | 1798 (550) | 1798 (362) | 1848 (419) |
Note: Study RTs for source memory restricted to encoding trials later endorsed as confidently old. Response abbreviations correspond to the following: CC: confident correct; UC: unconfident correct; UI: unconfident incorrect; CI: confident incorrect.
Test Phase
Item Memory
Table 3 summarizes performance on the later memory test. Mean item hit rates (correct “old” judgments to old items, collapsed across confidence and source accuracy) were 0.83 (SD = 0.06) for young and 0.76 (SD = 0.11) for older subjects. Mean item false alarm rates (incorrect old judgments to new items, collapsed across confidence and source accuracy) were 0.03 (SD = 0.03) and 0.04 (SD = 0.05) for young and older subjects, respectively. Item memory performance (pHit–pFalse alarms) was significantly lower for the older group compared with the younger group (t40 = 2.97, P = 0.005), with values of 0.81 (SD = 0.08) and 0.72 (SD = 0.13) for the young and older subjects, respectively. When subjects made a “confident old” response to an item, however, there was no difference in the accuracy of the judgments (as measured by pConfident Hits/[pConfident Hits + p(Confident False Alarms)]) between young and older subjects. The accuracy of these high confidence recognition judgments approached ceiling in both groups, with values of 0.99 (SD = 0.02) and 0.98 (SD = 0.02) for young and older subjects, respectively.
Table 3.
Mean proportions of item memory judgments for old and new trials by response type (±SD)
| CO | UO | DK | UN | CN | |
|---|---|---|---|---|---|
| Young | |||||
| Old | 0.76 (0.09) | 0.08 (0.05) | 0.03 (0.03) | 0.07 (0.05) | 0.06 (0.06) |
| New | 0.01 (0.02) | 0.01 (0.02) | 0.04 (0.06) | 0.15 (0.18) | 0.78 (0.23) |
| Old | |||||
| Old | 0.68 (0.15) | 0.07 (0.07) | 0.03 (0.06) | 0.08 (0.08) | 0.13 (0.10) |
| New | 0.02 (0.02) | 0.02 (0.04) | 0.03 (0.05) | 0.18 (0.24) | 0.75 (0.27) |
Note: Response abbreviations correspond to the following: CO: confident old; UO: unconfident old; DK: don't know; UN: unconfident new; CN: confident new.
Source Memory
Source memory performance is summarized in Table 4. To allow comparison with prior studies of the effects of age on source memory performance, which typically did not include either confidence ratings or a don't know response option, an overall measure of source recollection (pSr) was estimated. The measure was derived from a single high-threshold model (Snodgrass and Corwin 1988; for example, see Gottlieb et al. 2010). To correct for the influence of guesses, source hit rates (collapsed over item and source confidence) were adjusted according to the formula, p(corrected source hit) = [p(source hit) − 0.5(1 − p(source don't know)]/[1−0.5(1 − p(source don't know))]. Replicating prior reports of age-related decrements in source accuracy, the adjusted source hit rate was significantly greater for the young group compared with the older group (t40 = 2.83, P < 0.01), with values of 0.65 (SD = 0.13) and 0.51 (SD = 0.19), respectively.
Table 4.
Mean proportions of source judgments for confidently and unconfidently recognized study items
| Confident source correct | Unconfident source correct | Don't know source | Unconfident source incorrect | Confident source incorrect | |
|---|---|---|---|---|---|
| Young | |||||
| Confident Old | 0.67 (0.13) | 0.17 (0.13) | 0.03 (0.04) | 0.06 (0.04) | 0.07 (0.06) |
| Unconfident Old | 0.20 (0.18) | 0.41 (0.27) | 0.11 (0.14) | 0.20 (0.13) | 0.08 (0.14) |
| Old | |||||
| Confident Old | 0.63 (0.16) | 0.14 (0.12) | 0.03 (0.04) | 0.06 (0.07) | 0.15 (0.09) |
| Unconfident Old | 0.15 (0.15) | 0.47 (0.22) | 0.06 (0.10) | 0.26 (0.22) | 0.07 (0.13) |
As described below, to avoid confounding the variable of source accuracy with strength of item memory, we restricted analysis of fMRI subsequent source memory effects to those study items that went on to receive accurate, high confidence old judgments. In addition, to minimize possible confounds between age group and source memory confidence, we further restricted the analyses to those source judgments that were both correct and made with high confidence. The source accuracies (the proportion of items receiving a confident old response that also received a correct, confident source judgment) associated with these critical trials were 0.67 (SD = 0.13) and 0.63 (SD = 0.16) for young and older subjects, respectively; these means did not significantly differ.
fMRI Results
The subsequent memory analyses described below were based on contrasts derived from a mixed-effects 2 × 3 ANOVA model that incorporated factors of age group and subsequent memory condition (see Materials and Methods section).
We first present an analysis of the data from the young subjects alone, demonstrating that negative subsequent memory effects for item and source memory are localized to largely nonoverlapping cortical regions. We then go on to describe the analyses of the data derived from both age groups. In these analyses, we sought evidence for both age-invariant negative subsequent memory effects, and effects that differed according to age.
Negative Subsequent Memory Effects in the Young Group
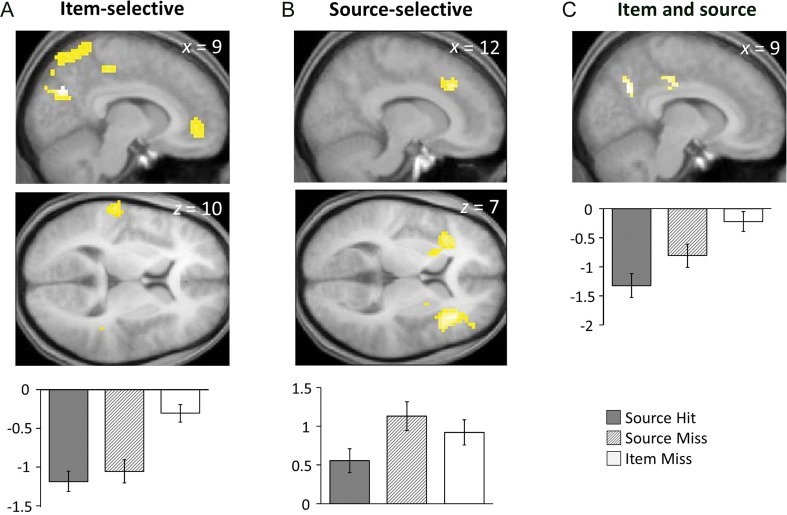
In this preliminary set of analyses, we investigated whether negative subsequent memory effects for source and item encoding could be dissociated. This was accomplished by identifying each effect using the appropriate pair-wise contrast (source hit < source miss, and source miss < item miss, each thresholded at P < 0.001 1-sided with a cluster extent threshold of 24 voxels), and exclusively masking it with the alternate contrast, thresholded at P < 0.05 (note that the more liberal the threshold of an exclusive mask, the more conservative is the procedure). As is evident in Figure 1B, and documented in Table 5, effects selective for the source contrast were localized to medial and bilateral PFC, bilateral anterior insula/frontal operculum, the putamen, and right temporo-parietal junction. In contrast, selective item effects were identified in medial parietal cortex, parts of the posterior cingulate, left temporo-parietal junction, and ventromedial PFC (Fig. 1A and Table 5). Thus, whereas the source effects were localized primarily to frontal regions, item effects were evident primarily in posterior regions of the cortex.
Figure 1.
Negative subsequent memory effects in young subjects. (Top) Regions demonstrating (A) negative subsequent memory effects for item, but not source, memory (left supramarginal gyrus, and right superior temporal gyrus not shown) (B) negative subsequent memory effects for source, but not item, memory (right superior temporal gyrus not shown), and (C) negative subsequent memory effects for both item and source memory (averaged across item and source peaks) in young subjects. Effects are shown on sections of young subjects' mean normalized structural image. (Bottom) Average parameter estimates (arbitrary units) for each response category.
Table 5.
Young subjects: negative subsequent memory effects
| Coordinates |
Peak Z | Number of above-threshold voxels | Region | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Item | −63 | −25 | 10 | 4.00 | 56 | L temporoparietal junction |
| −63 | −43 | 37 | 4.41 | 154 | L supramarginal gyrus | |
| 9 | 47 | 1 | 3.90 | 73 | R middle orbital gyrus | |
| 3 | −28 | 49 | 4.18 | 86 | R middle/posterior cingulate cortex | |
| 45 | −40 | 34 | 3.72 | 47 | R temporoparietal junction | |
| 9 | −70 | 31 | 5.04 | 443 | R precuneus | |
| Source | −33 | 23 | 7 | 4.04 | 169 | L insula |
| 36 | 29 | 7 | 4.72 | 253 | R insula | |
| 12 | 26 | 37 | 4.11 | 138 | R anterior cingulate cortex | |
| 57 | −43 | 28 | 4.57 | 57 | R temporoparietal junction | |
| Item and source (item peaks) | 6 | −22 | 40 | 3.87 | 24 | R middle cingulate cortex |
| 12 | −64 | 37 | 4.12 | 50 | R precuneus | |
| Item and source (source peaks) | 3 | −25 | 31 | 3.19 | 24 | R middle cingulate cortex |
| 9 | −58 | 31 | 3.55 | 50 | R precuneus | |
We also addressed the question of whether there were any regions where negative subsequent memory effects for item and source memory overlapped. When the contrasts were each thresholded at P < 0.001, no voxels survived the masking procedure. When the thresholds were lowered to <0.005 (preserving the 24 voxel extent threshold; note that as the contrasts are nonorthogonal, it is not possible to estimate the conjoint significance level), 2 clusters were identified in the right medial posterior cortex (Fig. 1C).
Age-Invariant Negative Subsequent Memory Effects
Negative subsequent memory effects for item and source memory common to the 2 age groups were identified by separate contrasts for the item (item miss > item only) and source (source miss > source hit) effects, respectively. Each contrast was thresholded at P < 0.001 with a cluster extent threshold of 24 contiguous voxels. The contrasts were exclusively masked by the appropriate group × subsequent memory interaction effect (P < 0.05, 2-sided) to remove voxels, where effects differed reliably according to age (cf. Morcom et al. 2003; Duverne et al. 2009; de Chastelaine et al. 2011). The masked contrasts identified the age-invariant negative subsequent memory effects are summarized in Table 6 and briefly described below.
Table 6.
Age-invariant negative subsequent memory effects
| Coordinates |
Peak Z (young; old) | Number of above-threshold voxels | Region | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Item | 3 | −19 | 37 | 3.96 (3.56; 2.01) | 33 | R middle cingulate cortex |
| Source | −33 | 44 | 7 | 3.47 (2.52; 2.46) | 25 | L middle frontal gyrus |
| −36 | 41 | 25 | 3.95 (3.60; 1.94) | 71 | L middle frontal gyrus | |
| −30 | 29 | 7 | 4.44 (3.81; 2.45) | 207 | L insula/frontal operculum | |
| 33 | 32 | 7 | 5.08 (4.24; 3.05) | 480 | R insula/frontal operculum | |
| 6 | 14 | 46 | 4.75 (3.69; 3.17) | 355 | R anterior cingulate cortex | |
| 12 | −22 | 40 | 4.20 (3.43; 2.56) | 137 | R middle/posterior cingulate cortex | |
| 63 | −43 | 31 | 4.26 (3.98; 1.99) | 105 | R supramarginal gyrus | |
| 12 | −76 | 40 | 4.25 (2.85; 3.33) | 504 | R precuneus/posterior cingulate cortex | |
Item Memory
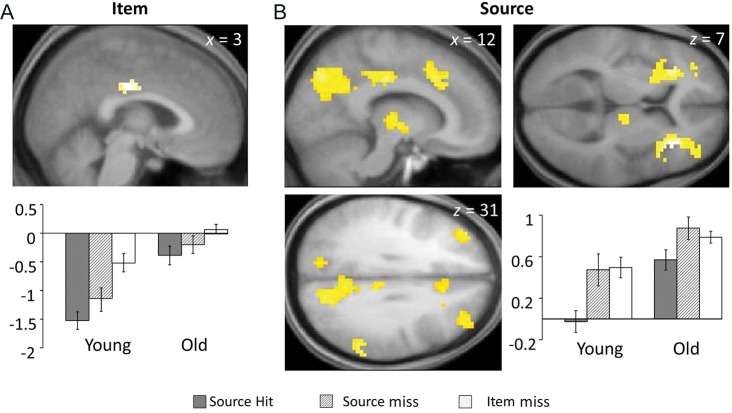
An age-invariant item effect was identified in a small region of the right middle cingulate cortex (Fig. 2A and Table 6).
Figure 2.
Regions demonstrating age-invariant negative subsequent memory effects for item memory (A) and source memory (B) effects. Bar charts depict mean across-region parameter estimates (arbitrary units).
Source Memory
Age-invariant negative source memory effects were identified in several regions, including left middle frontal gyrus, right middle cingulate cortex, bilateral insula/frontal operculum, and right supramarginal gyrus (Fig. 2B and Table 6). As is evident from the figure, the pattern of these effects across the brain resembles the pattern of selective negative subsequent memory effects for source memory illustrated in Figure 1B, with the exception of the inclusion of 2 posterior midline regions (that overlap the regions where item- and source effects coexisted in the young subjects). The mean across-region parameter effects for source hit and source miss study trials (along with the estimates for item miss trials, for illustrative purposes) are shown in Figure 2B.
Age-Related Differences in Negative Subsequent Memory Effects
We searched for regions where subsequent memory effects differed according to age by first computing separate group × subsequent memory interaction effects for item and source memory (thresholded at P < 0.001, 2-sided). These contrasts identified regions where item or source effects differed with respect to age. To identify the voxels where reliable interaction effects were associated with negative subsequent memory effects in the young subjects, each F-contrast was inclusively masked with the respective negative subsequent memory contrast conducted on the young subjects' data only (thresholded at P < 0.05, 1-sided).
Item Memory
Age-sensitive negative subsequent memory effects for item memory were identified in bilateral precuneus (overlapping one of the regions that demonstrated age-invariant negative source effects) and the left middle temporal gyrus (Fig. 3 and Table 7). To characterize these effects, we subjected the parameter estimates derived from these 4 regions to separate within-group ANOVAs, employing factors of region and subsequent memory condition (item only vs. item miss). As would be expected in light of how the regions had been selected, the ANOVA for the young group revealed a reliable negative subsequent memory effect (F1,16 = 14.44, P < 0.005) that did not vary across region (P > 0.1). In contrast, and consistent with the impression given by Figure 3, the ANOVA of the data from the older group revealed a significant “positive” effect (F1,24 = 6.28, P < 0.05) that also did not vary significantly across region (F < 1).
Figure 3.
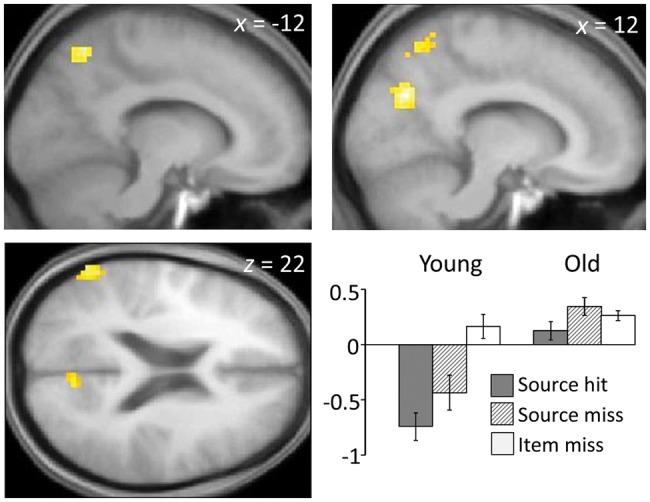
Regions demonstrating age-related differences in negative item subsequent memory effects. Bar chart depicts mean across-region parameter estimates (arbitrary units).
Table 7.
Age-dependent negative subsequent memory effects
| Coordinates |
Peak Z | Number of above-threshold voxels | Region | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Item | −60 | −61 | 22 | 3.84 | 41 | L temporoparietal junction |
| −12 | −61 | 58 | 3.88 | 46 | L precuneus | |
| 15 | −58 | 55 | 3.56 | 30 | R precuneus | |
| 12 | −67 | 31 | 4.24 | 168 | R precuneus | |
Source Memory
No voxels were identified where negative subsequent memory effects for source memory differed significantly with age at the pre-experimentally determined threshold.
Association of Negative Subsequent Memory Effects with Memory Performance
As noted in the Introduction, it has consistently been reported that the magnitude of negative subsequent memory effects in older subjects is positively correlated with memory performance (Miller et al. 2008; Duverne et al. 2009; de Chastelaine et al. 2011; Mormino et al. 2012). We therefore computed correlations between memory performance and the magnitude of negative subsequent memory effects in the regions identified in the foregoing analyses.
Item Memory
Regardless of whether item memory was estimated across confidence ratings (pHit–pFalse Alarms) or was restricted to those items given a confident recognition response, we were unable to identify any relationship, in either age group or across the combined groups, between performance and the magnitude of negative subsequent memory effects for items. This was the case for both the age-invariant effect in the right middle cingulate cortex (for pHit–pFA, r = 0.217, P = 0.297, and r = 0.029, P = 0.856 in the older and young subjects, respectively), and with respect to the mean effects across the 4 regions demonstrating age-dependent effects (for pHit–pFA, r = 0.372, P = 0.067, and r = −0.181, P = 0.488 for older and young subjects, respectively).
Source Memory
For the purposes of these analyses, we used as a measure of source performance the proportion of items receiving confident old judgments that also received a correct, confident source judgment (see Behavioral Findings above). Thus, the measure of source performance was derived from the same trials employed to estimate the fMRI effects. Results similar to those reported below, but with weaker correlations, were obtained when either pSr or the simple probability of a correct source judgment (collapsed across item and source confidence) was used to index source performance.
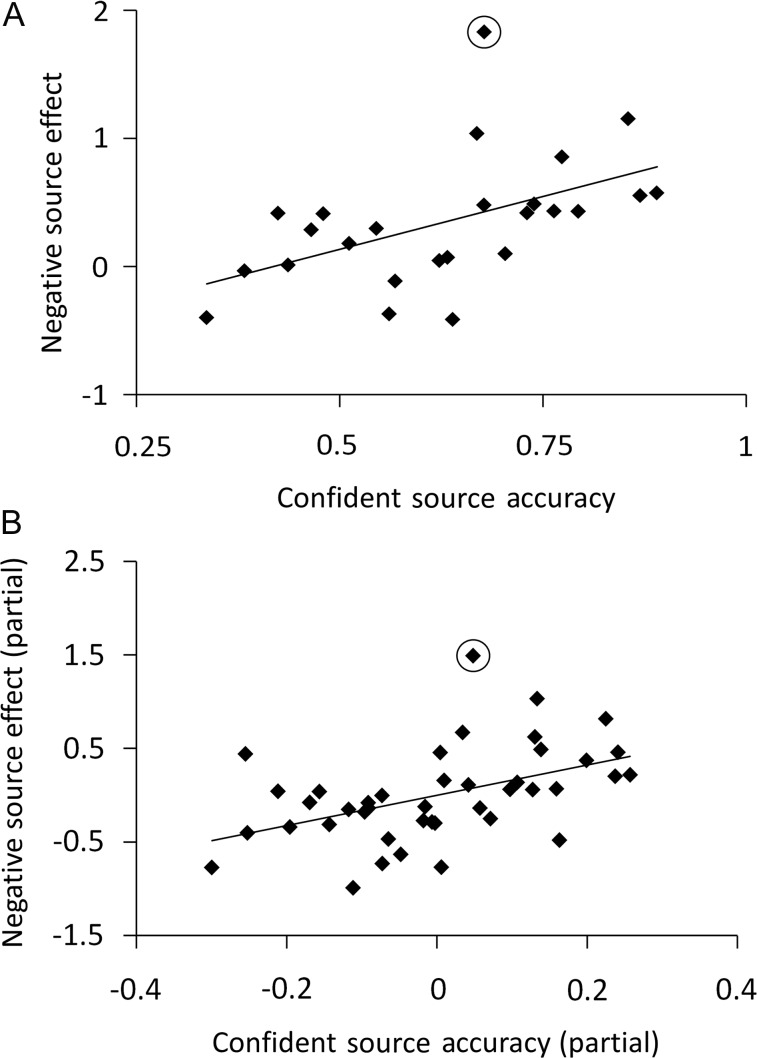
Figure 4A illustrates the relationship between source memory performance and the across-region mean of the older subjects' negative subsequent source memory effects and source memory performance. Across the entire older sample, the correlation was 0.507 (P < 0.005, 1-tailed). When an outlying subject (who had an across-region negative subsequent memory effect >2 SDs above that of the across-region group mean) was omitted, the correlation increased substantially (r = 0.592, P < 0.001, 1-tailed). We went on to compute separate correlations for each of the 8 regions listed in Table 6, omitting the outlier in each case. Significant correlations were evident in all but left and right insula regions (r = 0.364–0.564, max P < 0.05, 1-tailed). These correlations remained significant when the outlier subject was retained (r = 0.355–0.541).
Figure 4.
Scatterplots illustrating the relationship between negative subsequent memory effects (in arbitrary units) for source memory and the proportion of confidently recognized old items given an accurate, confident source judgment. The subsequent memory effects are averaged across the 8 regions listed in Table 6 that demonstrate age-invariant negative effects in older subjects. (A) Older, (B) young, and older subjects (residual scores after controlling for age). Outlying older subject circled (see text).
We repeated these analyses in the young group. The correlation between confident source accuracy and the negative source effects collapsed across regions approached significance (r = 0.398, P < 0.06, 1-tailed). One region in the left dorsolateral PFC (Fig. 2B) independently correlated significantly with performance (r = 0.534, P < 0.05, 1-tailed).
In a final analysis, we employed partial correlation to assess whether source performance was associated with the magnitude of negative subsequent memory effects for source memory (collapsed across region) in the combined samples after controlling for the effects of age. The partial r was 0.471 (P < 0.005, 1-tailed; r = 0.545, P < 0.001, with the outlier removed) indicating that, as would be expected on the basis of the findings reported above, the relationship between negative subsequent source memory effects and source performance was independent of age (Fig. 4B).
Discussion
We investigated the effects of age on negative subsequent memory effects for item and source memory using a subsequent memory procedure that allowed item memory strength and source accuracy to be unconfounded, and the confidence of source memory judgments to be equated across age. In a preliminary analysis, restricted to data from the young subjects, negative subsequent memory effects for item and source memory were found to be largely nonoverlapping. Analyses of the data from both age groups identified 3 classes of negative subsequent memory effect: Age-invariant and age-dependent item effects, and age-invariant source effects. We did not identify any regions where there was a reliable age-related reduction in source effects, and the magnitude of the effects correlated with source memory performance in both age groups. Below, we discuss the implications of these findings and their possible relationship to previous reports of age-related differences in negative subsequent memory effects.
Behavioral Findings
There was no difference between age groups in study RTs, or in how study RT interacted with subsequent memory performance. Between-group differences in fMRI subsequent memory effects are therefore unlikely to be attributable to gross differences between the age groups in how efficiently the different classes of study items were processed. Study RTs did differ, however, as a function of subsequent memory condition, with both young and older subjects demonstrating the same pattern (item miss < source hit < source miss). The explanation for these RT effects is unclear. It is unlikely though that they contributed to any of the fMRI subsequent memory findings discussed below, since a subsidiary fMRI analysis conducted on study trials that had been matched for RT across the 3 subsequent memory conditions RT yielded qualitatively similar results (The analysis equated study RTs in each subject by modeling a randomly selected 5% of the slowest source miss trials and 10% of the fastest item miss trials as events of no interest.).
Consistent with numerous prior findings (e.g. McIntyre and Craik 1987; Schacter et al. 1991), young subjects demonstrated higher item and source memory performance than older subjects. As we discuss below, it is unlikely that these differences in memory performance can account for the age-related differences that were identified in fMRI subsequent memory effects. That said, it is important to note that recognition of items for which source memory failed (source misses) may have received differential support from recollection and familiarity in the 2 age groups. This possibility arises because recollection is more vulnerable to advancing age than familiarity (Howard et al. 2006; Prull et al. 2006; see Yonelinas 2002 for a review of early studies). Thus, although item memory strength (as indexed by response confidence and accuracy) was equated between the age groups, it is possible that source miss judgments in young subjects were more likely to be supported by recollection of episodic information from the study trial, albeit information that was nondiagnostic of source. Evidence in support of this possibility emerges from a study by Toth and Parks (2006) in which it was demonstrated that “noncriterial recollection” is, indeed, lower in older than in young subjects. This has implications for the interpretation of age-related differences in fMRI negative subsequent memory effects for item memory, as is discussed below.
fMRI Findings
Negative Subsequent Memory Effects in the Young Subjects
Before turning to the effects of age on negative subsequent memory effects, we briefly discuss the findings from the analysis of the young group alone. This revealed a clear dissociation between a largely posterior set of regions where only negative item effects were evident, and a second set, localized mainly in the PFC, anterior insula, and putamen, where negative source, but not item, effects were identified. Two medial posterior regions demonstrated conjoint effects, such that negative item effects were enhanced when source encoding was successful.
As was noted in the Introduction, it is commonly held that negative subsequent memory effects reflect the benefit to encoding that ensues when default mode processes are disengaged and cognitive resources are reallocated to the study event (e.g. Daselaar et al. 2004; Miller et al. 2008; Duverne et al. 2009; de Chastelaine et al. 2011). This account appears to work well in respect of the present negative subsequent memory effects for item memory. The effects were localized to regions typically considered to belong to the default mode network (see Buckner et al. 2008; Andrews-Hanna 2012 for reviews) and demonstrated “task-negative” stimulus-related activity—that is, below-baseline parameter estimates—characteristic of the network (Raichle et al. 2001; Buckner et al. 2008).
In contrast, with the exception of the medial posterior regions where they overlapped with item effects, the present negative subsequent memory effects for source memory do not appear to reflect modulation of default mode activity. Several of the regions manifesting these effects, most notably, anterior insula and frontal operculum, are not considered components of the default mode network and, as is evident in Figure 2B, stimulus-related activity in these regions was task-positive (see Daselaar et al. 2004 for a prior report of negative subsequent memory effects in a task-positive region of the insula). Interestingly, the anterior insula and adjacent inferior frontal cortex are components of the “salience network”, which is held to be important for the initiation of cognitive control in response to behaviorally salient events (Seeley et al. 2007; Ham et al. 2013). This invites the speculation that the negative subsequent source memory effects in this region reflect the allocation of resources to salient aspects of a study episode that do not include information relevant to the later source judgment (see Otten and Rugg 2001; Wagner and Davachi 2001, for earlier accounts of negative subsequent memory effects along similar lines).
To our knowledge, the present study is the first in which negative subsequent memory effects associated with successful item and source memory have been contrasted. Therefore, it remains to be seen how far the findings generalize beyond the specific experimental procedures adopted here, for example, whether they extend to source memory tests for extrinsic rather than intrinsic contextual features.
Effects of Age on Negative Subsequent Memory Effects
Item Memory Effects
Age-invariant negative subsequent memory effects for item memory were limited to a relatively small region of the right middle cingulate cortex. This finding is reminiscent of a result from the study of Duverne et al. (2009), who also reported an age-invariant negative subsequent memory effect in posterior midline cortex, albeit in the precuneus rather than the cingulate. The present finding is mitigated, however, by the clear trend toward a larger middle cingulate effect in the young subjects (Fig. 2A). Moreover, this small age-invariant effect is overshadowed by the finding that negative item effects in medial posterior and left lateral temporal cortex were present in young subjects only, the effects in these regions demonstrating a statistically significant reversal in the older group (Fig. 3). These findings are consistent with prior reports of age-related attenuation or reversal of negative subsequent memory effects for item memory (Morcom et al. 2003; Gutchess et al. 2005; Duverne et al. 2009; Mormino et al. 2012).
Although negative subsequent memory effects for item memory were largely absent in older subjects, this appears to have had little impact on their recognition memory performance. Not only was there no relationship between the size of these effects and recognition performance, but also a subsidiary analysis revealed that negative effects were absent even in a subset of 16 older subjects selected so that their mean item memory did not differ significantly from that of the young group (pHits-FA of 0.80 in both groups). In the older group as a whole, the effects in these high-performing subjects were not only attenuated, but reversed (P < 0.05, 2-tailed). A similar result was reported by Duverne et al. (2009), who described nonsignificant (rather than reversed) negative subsequent memory effects in an older subgroup whose recognition memory performance was matched with that of a young sample.
Clearly, reliable negative subsequent memory effects for item memory are not necessary for older individuals to attain levels of memory performance equivalent to those in young subjects. What, then, is the functional significance of these effects? As was noted above, the effects in the young group were localized mainly to regions belonging to the default mode network. Thus, the attenuation of negative item effects in the older group might be a reflection of a more general failure to disengage the network in response to a stimulus event to the same extent as young individuals (e.g. Lustig et al. 2003; Grady et al. 2006). This does not, however, explain why failure to disengage default mode processes seemingly had no impact on item memory performance.
One possible account of the dissociation between negative item effects and item memory performance in older subjects arises from the possibility, discussed previously, that older and young subjects differed in the likelihood that they recollected details of the study episode that were nondiagnostic of source information (noncriterial recollection). The account is predicated on 2 assumptions. First, that in older subjects, recognition memory for source miss items was largely familiarity-driven, but that recognition of these items in young subjects was supported by a combination of familiarity and noncriterial recollection. Secondly, that negative subsequent memory effects for item memory reflect encoding operations that benefit subsequent recollection, but do not impact familiarity. Given these assumptions, the age-related dissociation in negative subsequent memory effects for item memory can be attributed to the failure of older subjects to encode noncriterial information and, concomitantly, to disengage default processes that facilitate the encoding of such information.
An alternative explanation for the lack of correspondence in older subjects between negative subsequent memory effects for item memory and performance is suggested by the findings of a recent event-related potential (ERP) study. Wang et al. (2012; see Duarte et al. 2006 for similar findings) reported that a putative ERP correlate of familiarity-driven recognition memory was undetectable in older subjects, including a subgroup in whom familiarity-strength was matched with that of a group of young subjects in whom the ERP correlate was highly reliable. Wang et al. (2012) proposed that their findings might indicate that familiarity-driven recognition memory is supported by multiple memory signals, not all of which are reflected in the ERP effect. They further proposed that the different familiarity signals decline differentially with advancing age, causing the dissociation they reported between a putative neural correlate of familiarity and familiarity-driven recognition memory. Applying the same argument here, the proposal would be that whereas source misses in both older and young individuals were supported largely by familiarity, the familiarity signal that depended on encoding processes reflected in negative item subsequent memory effects was minimal in older subjects, in whom familiarity was supported by encoding processes uncorrelated with negative subsequent memory effects.
Arbitrating between these (and other) accounts will not be possible until more evidence is available about the relationship between negative subsequent memory effects and familiarity- and recollection-based recognition memory. It should also be noted that while these accounts might explain why older subjects can demonstrate seemingly intact memory performance in the face of attenuated or absent negative subsequent memory effects, they do not explain why the effects sometimes reverse, as in the present case and some previous reports (Morcom et al. 2003; Duverne et al. 2009). We can offer no explanation for this puzzling finding.
Source Memory Effects
In striking contrast to negative subsequent memory effects for item memory, we did not identify any regions where negative source effects varied according to age. Furthermore, reminiscent of prior findings (Miller et al. 2008; de Chastelaine et al. 2011), the magnitude of negative source effects correlated with source memory performance both in older subjects and in the combined older and young samples. The findings from the separate analysis of the young subjects indicate that negative source memory effects dissociate according to whether or not they coexist with item memory effects. We discuss the finding of age-invariant negative subsequent memory effects for source memory in light of this dissociation.
The negative source effects identified in the posterior midline cortex (Fig. 2B) are in the same default mode regions where source and item effects overlapped in the analyses of the data from the young subjects (cf. Fig. 1C). The more posterior of these regions also overlaps with where negative item effects dissociated with age (Fig. 1A). Therefore, the absence in older subjects of negative subsequent memory effects for item memory in this region does not reflect an incapacity to disengage the region in service of episodic encoding: Relative to source miss study trials, source hit trials were associated with equivalent levels of disengagement in the 2 age groups. This finding is consistent with the proposal that, in at least some components of the default mode network, negative subsequent memory effects reflect processes that facilitate later recollection of the study episode. The proposal receives further support from the finding that the magnitude of negative source effects in the aforementioned medial posterior regions correlated with source memory performance across age groups (controlling for age, partial r = 0.368; P < 0.01, 1-tailed). The proposal is also consistent with prior findings that negative subsequent memory effects in older subjects correlate with performance on other memory tests that likely depend heavily on recollection (Miller et al. 2008; de Chastelaine et al. 2011). In short, despite the more restricted modulation of default mode activity that is apparent in older individuals relative to their young counterparts (Lustig et al. 2003; Grady et al. 2006), the relationship between successful episodic encoding and disengagement of default processing does not appear to change with age.
If the foregoing account is correct, why have prior studies that employed subsequent memory tests that were dependent on recollection reported age-related attenuation of negative subsequent memory effects? We conjecture that this is because, unlike in the present study, the memory tests employed in previous studies did not allow strength of recollection to be equated across the age groups. For example, in the study by de Chastelaine et al. (2011), associative recognition performance in older subjects was markedly lower than that in the young, indicative of an age-related difference in the strength of the memory signal supporting the associative recognition judgments. Thus, the subsequent memory effects were likely associated with the encoding of information that (on average) supported strong and weak recollection-based judgments in younger and older subjects, respectively. According to this account, had de Chastelaine et al. (2011) been able to restrict negative subsequent memory effects to study pairs that went on to receive highly confident recognition judgments, age-related differences in the magnitude of the effects would have been much diminished.
As was noted previously, age-invariant negative subsequent memory effects for source memory were mainly localized to frontal “task-positive” regions that would usually be considered to fall outside of the default mode network. It has been suggested that enhanced task-positive responses to “forgotten” items are indicative of the engagement of processes that interfere or compete for resources with processes that support effective encoding (Daselaar et al. 2004). Regardless of the validity of this proposal, the frontal negative source effects appear to be as closely associated with successful episodic encoding as those in the putative default mode regions that were discussed above: Collapsed across regions and age group, the partial correlation (controlling for age) between the magnitude of these effects and source memory performance was 0.446 (P < 0.005, 1-tailed). As we have already discussed, it is possible that these frontal effects, or at least those localized to the anterior insula and adjacent PFC, reflect the allocation of cognitive resources to salient, but mnemonically unhelpful, aspects of the study episode. Whether or not this turns out to be a valid account of these effects, it is clear they are as closely linked to the successful encoding of contextual information in older individuals as they are in young subjects. Thus, as in the case of negative source effects localized to putative default mode regions, the present findings suggest that the relationship between negative frontal effects and source memory performance does not vary with age.
Concluding Comments
Age-related attenuation of negative subsequent memory effects is one of the most consistent findings in studies investigating the effects of age on the neural correlates of episodic encoding. The present study is no exception: Relative to study items that failed to be recognized on a later memory test, negative subsequent memory effects in regions of the default mode network were attenuated in older subjects both for confidently recognized items that attracted a correct source memory judgment, and items that did not. Crucially, though, the negative effects that predicted whether memory for an item's encoding context would be strong and accurate, or would fail, did not differ with age and demonstrated the same relationship with memory performance in the 2 age groups. We conclude that the negative subsequent memory effects for source memory identified here reflect modulations of stimulus-related activity that support successful episodic encoding and that, when the strength of subsequent source memory is matched, these effects are age-invariant. It remains to be seen whether, when strength of item and contextual memory are controlled as here, the present findings generalize to other study materials and subsequent memory tests.
Funding
This work was supported by NIH (grants 5P50AG16573, 1RO1AG039103, and MH074528).
Notes
Conflict of Interest: None declared.
References
- Andrade A, Paradis AL, Rouquette S, Poline JB. Ambiguous results in functional neuroimaging data analysis due to covariate correlation. Neuroimage. 1999;10:483–486. doi: 10.1006/nimg.1999.0479. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain's default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Clark D, Wagner AD. Assembling and encoding word representations: fMRI subsequent memory effects implicate a role for phonological control. Neuropsychologia. 2003;41:304–317. doi: 10.1016/s0028-3932(02)00163-x. [DOI] [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RS, Evans AC. BrainWeb: online interface to a 3D MRI simulated brain database. Proceedings of the third international conference on function mapping of the human brain; Copenhagen, Denmark. 1997. p. S235. [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- de Chastelaine M, Wang TH, Minton B, Muftuler LT, Rugg MD. The effects of age, memory performance, and callosal integrity on the neural correlates of successful associative encoding. Cereb Cortex. 2011;21:2166–2176. doi: 10.1093/cercor/bhq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, Knight RT. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. J Cogn Neurosci. 2006;18:33–47. doi: 10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb Cortex. 2009;19:733–744. doi: 10.1093/cercor/bhn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Friston KJ, Penny W, Phillips C, Kiebel S, Hinton G, Ashburner J. Classical and Bayesian inference in neuroimaging: theory. Neuroimage. 2002;16:465–483. doi: 10.1006/nimg.2002.1090. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Rubin SR, Davidson PS. Source memory in older adults: an encoding or retrieval problem? J Exp Psychol Learn Mem Cogn. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Gottlieb LJ, Uncapher MR, Rugg MD. Dissociation of the neural correlates of visual and auditory contextual encoding. Neuropsychologia. 2010;48:137–144. doi: 10.1016/j.neuropsychologia.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Ham T, Leff A, de Boissezon X, Joffe A, Sharp DJ. Cognitive control and the salience network: an investigation of error processing and effective connectivity. J Neurosci. 2013;33:7091–7098. doi: 10.1523/JNEUROSCI.4692-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang Y, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures: evidence from modeling and receiver operating characteristic curves. Psychol Aging. 2006;21:96–106. doi: 10.1037/0882-7974.21.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Schultz AP, Vannini P, McLaren DG, Wigman SE, Ward AM, Hedden T, Spering RA. The encoding/retrieval flip: interactions between memory performance and memory stage and relationship to intrinsic cortical networks. J Cogn Neurosci. 2013;25:1163–1179. doi: 10.1162/jocn_a_00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs O, Henson RN. Event-related functional magnetic resonance imaging: modelling, inference and optimization. Philos Trans R Soc Lond B Biol Sci. 1999;354:1215–1228. doi: 10.1098/rstb.1999.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Wixted JT, Squire LR. Activity in the medial temporal lobe predicts memory strength, whereas activity in the prefrontal cortex predicts recollection. J Neurosci. 2008;28:10541–10548. doi: 10.1523/JNEUROSCI.3456-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre JS, Craik FIM. Age differences in memory for item and source information. Can J Psychol. 1987;41:175–192. doi: 10.1037/h0084154. [DOI] [PubMed] [Google Scholar]
- Mickes L, Johnson EM, Wixted JT. Continuous recollection versus unitized familiarity in associative recognition. J Exp Psychol Learn Mem Cogn. 2010;36:843–863. doi: 10.1037/a0019755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamäki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Brandel MG, Madison CM, Marks S, Baker SL, Jagust WJ. Aβ deposition in aging is associated with increases in brain activation during successful memory encoding. Cereb Cortex. 2012;22:1813–1823. doi: 10.1093/cercor/bhr255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: tests of an associative deficit hypothesis. J Exp Psychol Learn Mem Cogn. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Nilsson LG. Memory function in normal aging. Acta Neurol Scand Suppl. 2003;179:7–13. doi: 10.1034/j.1600-0404.107.s179.5.x. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Curr Biol. 2001;11:1528–1530. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Park H, Rugg MD. Neural correlates of successful encoding of semantically and phonologically mediated inter-item associations. Neuroimage. 2008;43:165–172. doi: 10.1016/j.neuroimage.2008.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Prull MW, Dawes LL, Martin AM, 3rd, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: adult age differences and neuropsychological test correlates. Psychol Aging. 2006;21:107–118. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR, Donaldson DI, Wagner AD, Braver TS. Item- and task-level processes in the left inferior prefrontal cortex: positive and negative correlates of encoding. Neuroimage. 2004;21:1472–1483. doi: 10.1016/j.neuroimage.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging. 2010;31:839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Kaszniak AW, Kihlstrom JF, Valdiserri M. The relation between source memory and aging. Psychol Aging. 1991;6:559–568. doi: 10.1037//0882-7974.6.4.559. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2458. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Kirwan CB, Squire LR. Activity in both hippocampus and perirhinal cortex predicts the memory strength of subsequently remembered information. Neuron. 2008;59:547–553. doi: 10.1016/j.neuron.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedlecki KL, Salthouse TA, Berish DE. Is there anything special about the aging of source memory? Psychol Aging. 2005;20:19–32. doi: 10.1037/0882-7974.20.1.19. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychol Aging. 1995;10:527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth WP, Parks CM. Effects of age on estimated familiarity in the process dissociation procedure: the role of noncriterial recollection. Mem Cognit. 2006;34:527–537. doi: 10.3758/bf03193576. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Yi DJ, Chun MM. Linking implicit and explicit memory: common encoding factors and shared representations. Neuron. 2006;49:917–927. doi: 10.1016/j.neuron.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Davachi L. Cognitive neuroscience: forgetting of things past. Curr Biol. 2001;11:R964–R967. doi: 10.1016/s0960-9822(01)00575-9. [DOI] [PubMed] [Google Scholar]
- Wais PE, Squire LR, Wixted JT. In search of recollection and familiarity signals in the hippocampus. J Cogn Neurosci. 2010;22:109–123. doi: 10.1162/jocn.2009.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TH, de Chastelaine M, Minton B, Rugg MD. Effects of age on the neural correlates of familiarity as indexed by ERPs. J Cogn Neurosci. 2012;24:1055–1068. doi: 10.1162/jocn_a_00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]