Abstract
A thyroid incidentaloma is an unexpected, asymptomatic thyroid tumor fortuitously discovered during the investigation of an unrelated condition. The prevalence rate is 67% with ultrasonography (US) imaging, 15% with computed tomography (CT) or magnetic resonance imaging (MRI) of the neck, and 1-2% with fluorodeoxyglucose (FDG) positron emission tomography. In the absence of a history of external beam radiation or familial medullary thyroid cancer, the risk of malignancy ranges between 5 and 13% when discovered with US, CT or MRI, but is much higher if based on focal FDG uptake (30%). All patients with a thyroid incidentaloma, independent of the mode of detection, should undergo a dedicated neck US with risk stratification: US imaging allows a quantitative risk stratification of malignancy in thyroid nodules, named ‘reporting system’ or ‘TIRADs' (thyroid imaging reporting and data system). The reported sensitivity ranges from 87 to 95% for the detection of carcinomas and the negative predictive value from 88 to 99.8%. We suggest that the indications for fine-needle aspiration be based mainly on size and US risk stratification. However, the diagnosis and workup of thyroid incidentalomas leads to superfluous surgery for benign conditions, and excess diagnosis and treatment of papillary microcarcinomas, the vast majority of which would cause no harm. Recognizing this must form the basis of any decision as to supplementary investigations and whether to offer therapy, in a close dialogue between patient and physician. The current use of minimally invasive nonsurgical ablation options, as alternatives to surgery, is highlighted.
Key Words: Epidemiology, Thyroid, Thyroid nodule, Incidentaloma , Ultrasound, Malignancy rating scale, Fine-needle aspiration biopsy, Laser ablation, Radiofrequency ablation, Ethanol therapy
Introduction
Over the latest two decades the use of imaging procedures, especially ultrasonography (US) has been the culprit of an epidemic of thyroid incidentalomas [1]. Thus, the clinician is confronted with a situation that necessitates managing a condition that the patient did not complain of. In the first part, this paper outlines the magnitude of the problem and updates the concept of US risk stratification of thyroid nodules based on the TIRADS (thyroid imaging reporting and data system) classification. The second part deals with the indications for US fine-needle aspiration biopsy (FNA), addresses specifically the problem of subcentimetric incidentalomas and microcarcinomas, and finally discusses the potential of nonsurgical therapeutic alternatives.
Epidemiology
A thyroid incidentaloma is defined as an unexpected, asymptomatic thyroid tumor discovered during the investigation of an unrelated condition. Palpable thyroid nodules are known to be frequent, with a 5% prevalence rate in the general population [2,3]. There is a female preponderance and an increase in prevalence with age, reaching 30-40% [3] in individuals above the age of 50. Older studies report that neck US detects incidentalomas with a prevalence of 10-30% [4,5,6]. With more recent-generation US, which offers improved spatial resolution, the prevalence is 67%, comparable to that found at autopsy [7]. Prospective studies in the general population have shown a very high prevalence of small thyroid nodules detectable with US, measuring <10 mm in 70-83% of cases [5,6]. Employing computed tomography (CT) or magnetic resonance imaging (MRI) of the neck, prevalence is lower at around 15% [8,9], while it is 1-2% by fluorodeoxyglucose (FDG) positron emission tomography (PET) [10,11]. Using PET, a thyroid incidentaloma is defined as a focal uptake and should be clearly differentiated from bilateral and diffuse thyroid uptake linked to thyroiditis and with a much lower risk of malignancy.
Diagnosis and exploration of thyroid incidentalomas preferentially takes place in high-income countries where imaging is increasingly used for patients who have access to medical care [12]. For example, in the USA, the number of CTs performed between 1995 and 2005 increased more than threefold, and the number of MRIs more than doubled [13]. It follows, not surprisingly, that the number of FNAs has also increased correspondingly [13].
Risk of Malignancy according to the Way of Discovery
In the absence of a history of external beam radiation or familial medullary thyroid cancer, the risk of malignancy in thyroid incidentalomas diagnosed on neck US, CT or MRI is 5-13% [11,14]. In contrast, the risk of malignancy when diagnosed by focal FDG uptake on a PET scan is much higher, around 30% [10]. Importantly, although the FDG PET examinations are performed in the context of another malignancy, most FDG thyroid incidentalomas detected by PET scan are differentiated thyroid cancers and not intrathyroidal metastases [10]. Sixty-seven percent of thyroid cancers detected by imaging measure more than 10 mm and 38% measure more than 4 cm. Twenty-five percent are stage III or IV, and 30% have positive lymph nodes [15].
Risk Stratification of Thyroid Incidentalomas with Ultrasound
Can US be used as an accessible, simple and inexpensive tool to sort the wheat from the chaff among thyroid incidentalomas? Thyroid US was first used as a tool to measure, count and locate thyroid nodules. Since around 1998, specific US characteristics have been recognized as markers of thyroid carcinoma [16,17,18,19,20,21,22,23,24,25]. Tables 1 and 2 summarize the reported sensitivities, specificities, and positive and negative predictive values of each of these signs.
Table 1.
Reported sensitivities and specificities of US characteristics for the presence of malignancy in thyroid nodules
| Diagnostic value | Rago et al. [16] | Papini et al. [17] | Kim et al. [18] | Cappelli et al. [19] | Moon et al. [20] | Russ et al. [33] | Trimboli et al. [22] | Bojunga et al. [21] | Bojunga et al. [23] | Zhang et al. [24] | Reported range | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | 104 | 402 | 155 | 6,135 | 849 | 500 | 498 | 639 | 158 | 173 | 104 – 6,135 | |
| Carcinomas, % | 29 | 8 | 30 | 5 | 42 | 3.2 | 25 | 24 | 13 | 25 | 3 – 42 | |
| Study design | ? | P | P | R | R | R | P | meta-analysis | ? | ? | ||
| Absent halo | sensitivity | 67 | 87 | 90 | 91 | 67 – 91 | ||||||
| specificity | 77 | 34 | 37 | 50 | 34 – 77 | |||||||
| Macrocalcifications | sensitivity | 10 | 33 | 10 – 33 | ||||||||
| specificity | 96 | 87 | 87 – 96 | |||||||||
| Calcifications (type not specified) | sensitivity | 72 | 72 | |||||||||
| specificity | 71 | 71 | ||||||||||
| Microcalcifications | sensitivity | 54 | 29 | 59 | 44 | 38 | 31 | 71 | 52 | 29 – 71 | ||
| specificity | 76 | 95 | 86 | 91 | 99 | 98 | 67 | 89 | 67 – 99 | |||
| Hypoechogenicity | sensitivity | 67 | 87 | 81 | 87 | 67 | 72 | 62 | 98 | 67 – 98 | ||
| specificity | 49 | 43 | 47 | 58 | 87 | 60 | 65 | 46 | 43 – 87 | |||
| Marked | sensitivity | 27 | 41 | 17 | 17 – 41 | |||||||
| hypoechogenicity | specificity | 94 | 92 | 100 | 92 – 100 | |||||||
| Blurred margins | sensitivity | 78 | 53 | 41 | 41 – 78 | |||||||
| specificity | 85 | 81 | 89 | 81 – 89 | ||||||||
| Irregular margins | sensitivity | 55 | 48 | 26 | 25 | 52 | 25 – 55 | |||||
| specificity | 83 | 92 | 99 | 99 | 81 | 81 – 99 | ||||||
| Intranodular vascularization | sensitivity | 67 | 74 | 62 | 41 | 37 | 52 | 48 | 37 – 74 | |||
| specificity | 49 | 81 | 50 | 89 | 88 | 73 | 57 | 49 – 89 | ||||
| Taller than wide | sensitivity | 33 | 76 | 40 | 22 | 14 | 14 – 76 | |||||
| specificity | 93 | 60 | 91 | 97 | 99 | 60 – 99 | ||||||
| High stiffness | sensitivity | 74 | 81 | 82 – 100 | 48 – 76 | 63 – 75 | 48 – 100 | |||||
| specificity | 91 | 62 | 78 – 100 | 72 – 92 | 82 – 88 | 62 – 100 | ||||||
Values represent n unless otherwise indicated. R = Retrospective; P = prospective.
Table 2.
Reported negative (NPV) and positive predictive (PPV) values of US characteristics for the presence of malignancy in thyroid nodules
| Diagnostic value | Rago et al. [16] | Papini et al. [17] | Kim et al. [18] | Cappelli et al. [19] | Moon et al. [20] | Bonavita et al. [25] | Russ et al. [33] | Trimboli et al. [22] | Bojunga et al. [21] | Bojunga et al. [23] | Zhang et al. [24] | Reported range | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | 104 | 402 | 155 | 6,135 | 849 | 500 | 498 | 639 | 158 | 173 | 104 – 6,135 | ||
| Carcinomas, % | 29 | 8 | 30 | 5 | 42 | 3.2 | 25 | 24 | 13 | 25 | 3 – 42 | ||
| Study design | ? | P | P | R | R | R | P | M | ? | ? | |||
| Absent halo | PPV | 54 | 6 | 18 | 38 | 6 – 54 | |||||||
| Present halo | NPV | 85 | 93 | 94 | 85 – 94 | ||||||||
| Spongiform | NPV | 98 | 95 | 95 – 98 | |||||||||
| Macrocalcifications | PPV | 65 | 10 | 10 – 65 | |||||||||
| NPV | – | ||||||||||||
| Calcifications (type not specified) | PPV | 11 | 11 | ||||||||||
| NPV | |||||||||||||
| Microcalcifications | PPV | 56 | 33 | 80 | 78 | 60 | 87 | 25 | 62 | 25 – 87 | |||
| NPV | |||||||||||||
| Isoechogenicity | NPV | 93 | 85 | 85 – 93 | |||||||||
| Hypoechogenicity | PPV | 34 | 11 | 7 | 19 | 38 | 21 | 52 | 7 – 52 | ||||
| NPV | |||||||||||||
| Marked hypoechogenicity | PPV | 68 | 80 | 73 | 68 – 80 | ||||||||
| NPV | |||||||||||||
| Blurred margins | PPV | 30 | 12 | 16 | 16 – 30 | ||||||||
| NPV | |||||||||||||
| Irregular margins | PPV | 80 | 81 | 57 | 86 | 30 | 30 – 86 | ||||||
| NPV | |||||||||||||
| Intranodular vascularization | PPV | 24 | 6 | 16 | 52 | 23 | 27 | 6 – 52 | |||||
| Absent central vascularization | NPV | 96 | 94 | 97 | 81 | 81 – 96 | |||||||
| Taller than wide | PPV | 75 | 8 | 77 | 26 | 82 | 8 – 82 | ||||||
| NPV | |||||||||||||
| High stiffness | PPV | 37 | 42 | 46 – 100 | 38 | 59 | 37 – 100 | ||||||
| Low stiffness | NPV | 98 | 91 | 88 – 100 | 93 | 90 | 88 – 100 | ||||||
Values represent n unless otherwise indicated. R = Retrospective; P = prospective; M = meta-analysis.
Unfortunately, no single US sign has sufficient diagnostic value. Therefore, various combinations of signs have been studied for that purpose. Among these was a combination of four signs, first reported by Kim et al. [18] and then confirmed by other groups [19,20]. These included microcalcifications, a taller-than-wide shape, irregular borders and marked hypoechogenicity, and were considered capable of diagnosing 94% of thyroid carcinomas. Mild hypoechogenicity is often added to that list [16,17,19,22,23,24], and more recently low elasticity employing elastography [22,23,24]. At the other side of the spectrum, simple cysts and spongiform nodules have been classified as characteristic of benign lesions [25]. In a recent review and meta-analysis, Brito et al. [26] found that the US nodule features with the highest diagnostic odds ratio for malignancy was being ‘taller than wider’ and that spongiform appearance and cystic nodules were the best two features allowing avoidance of FNA.
In 2007, a qualitative risk assessment concept called the ‘grading system’ emerged. Thyroid nodules were classified into categories related to their US patterns. Indications for FNA were based on these categories [27,28]. In 2009, risk stratification shifted to quantitative assessment, linking US patterns to a quantitative risk of malignancy. In the six main reports on this subject, the authors named their work either ‘reporting systems’ or ‘TIRADS’ which is the acronym for ‘thyroid imaging-reporting and data system’.
In 2009, Horvath et al. [29] published the first study using TIRADS in 1,097 nodules (156 carcinomas). The grading concept is transposed in a way similar to BI-RADS (Breast Imaging-Reporting and Data System): score 1 denotes a normal examination, whereas scores 2, 3, 4 and 5 correspond to a risk of 0, <5, 5-80 and >80%, respectively. Ten US patterns (cumbersome to use in clinical practice) were defined. Sensitivity and specificity were 88 and 49%, respectively. However, among 1,097 nodules, 238 were classified as indeterminate/suspicious follicular lesions and only 12% were operated on, introducing a selection bias.
The same year, Park et al. [30], in a retrospective study, used TIRADS in a study that comprised 1,694 patients (364 carcinomas). The value of the 4 major signs of Kim et al. [18] was confirmed and 2 signs were added: solid and mildly hypoechoic, and the presence of suspicious lymph nodes. They established a mathematical equation with 12 parameters and a 5-point risk stratification scale. There were 390 nodules with a THY3 reading (indeterminate), and 256 were excluded from the analysis because they did not have thyroid surgery. FNA was recommended for scores 3 and 4, and surgery for score 5. The diagnostic value was not tested and, again, the process was too complex to be applied in daily practice.
In 2011, Kwak et al. [31] tried to simplify the system designed by Park et al. [30] in a multicenter retrospective study of 1,658 nodules >10 mm, 298 of which were surgically removed. The total number of cytologically indeterminate nodules is not available, but all the ones retained in the study were referred for surgery. The number of signs of suspicion could clearly be used to predict malignancy. However, as a main limitation, each suspicious US feature was assigned the same weight despite carrying a different probability of malignancy.
In 2013, to overcome this shortcoming, Kwak et al. [32] suggested a new model where each individual US sign was assigned a risk score according to its odds ratio for predicting malignancy. In their multicenter study of 2,000 nodules measuring at least 5 mm, all carcinomas (36.6%) were surgically confirmed. All benign nodules were characterized by at least 2 benign FNA examinations and evidence of lack of growth over the study period. The risk of malignancy in thyroid nodules increased in parallel with the calculated total score (sum of each score). Unfortunately, and intuitively, applying this 15-point scale is far too time-consuming.
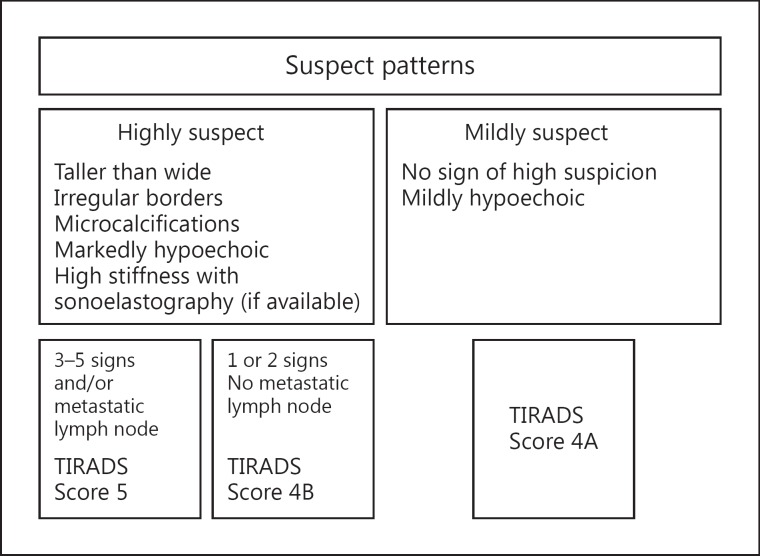
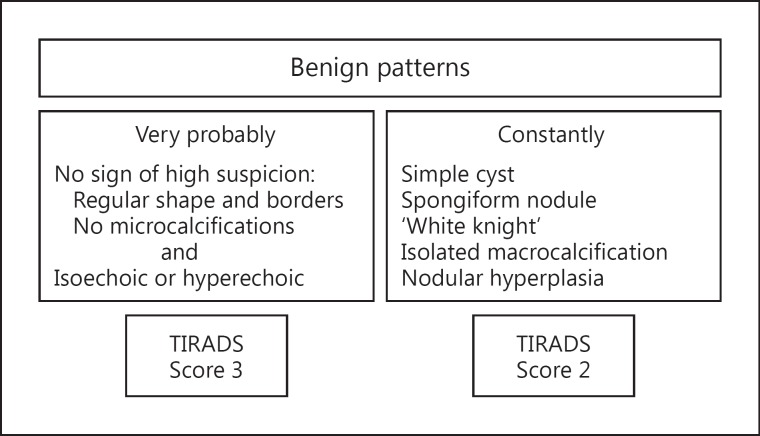
Given the above shortcomings, Russ et al. [33] constructed a system that is less cumbersome, reproducible and allows testing. First, a retrospective study of 500 nodules was performed. The sensitivity, specificity and odds ratio of each US sign were calculated, and a specific vocabulary and a standardized report were established. A flowchart was developed to easily define the score of a particular nodule. Sensitivity and specificity of this version of the TIRADS score were 95 and 68%, respectively. Feedback from the medical community led to simplification and subsequently a prospective study of 4,550 nodules over a 2-year period that included elastography [34]. There were 801 cytologically indeterminate results (17.6%), in which histological confirmation was available in 237 cases. The algorithm is shown in figures 1 and 2. Assessment categories corresponded to a 6-point scale: score 1 is for normal, 2 for benign, 3 for very probably benign, 4A for low suspicion, 4B for high suspicion and 5 for practically certainly malignant. The corresponding risk of malignancy, using this scale, was 0, 0, 0.25, 6, 69 and 100%, respectively. Sensitivity reached 98.5%; false-negative results corresponded, in most cases, to the encapsulated follicular variant of papillary carcinomas, which occasionally takes on the US appearance of a regular solid isoechoic nodule with or without central vascularization [35,36]. Specificity, negative predictive value and accuracy of this TIRADS score were 44.7, 99.8 and 48.3%, respectively. Nodules given a score of 2 or 3 represented 52% of all nodules referred for FNA, and 65% of all nodules detected by US. Interobserver reproducibility yielded a κ coefficient of 0.72, corresponding to substantial agreement. This figure is close to what was reported by Hambly et al. [37], who asked 7 radiologists to test a 5-point scale very similar to TIRADS and found that agreement was excellent for malignant nodules (κ, 0.88-1.00).
Fig. 1.
First part of the flowchart designed to score nodules with US. It defines the patterns of nodules suspicious for malignancy.
Fig. 2.
Second part of the flowchart designed to score nodules with US. It defines the patterns of benign nodules.
Most risk stratification systems are based on gray-scale US. Doppler US is frequently not taken into account. Its diagnostic value remains controversial, probably due to its entirely qualitative nature, poor interobserver agreement and dependence on the sensitivity of the US technology. However, predominantly central vascularization seems to increase the risk of malignancy and can be used to ascertain this risk in a more refined way [16,17,38]. Regarding US elastography, there is currently no clear superiority of one elastographic technique over another. Manual compression has the main advantage of widespread availability, but techniques based on the ultrasound radiation force, such as shear wave imaging, ought to be more reproducible. The main aim of elastography is to improve the sensitivity of gray-scale imaging, but it may also be used to enhance specificity in nodules with undetermined US patterns, such as TIRADS 4A or undetermined cytological patterns, such as follicular neoplasms [21,22,23,24].
All of the studies have two main shortcomings: (1) the lack of surgical confirmation of most nodules considered as cytologically benign, and (2) the exclusion of many cytologically indeterminate nodules. However, and importantly, we now have at our disposal a tool which can detect most thyroid carcinomas and classify more than half of all nodules as very probably benign with a <1/400 risk of missing a carcinoma [34]. We suggest using this tool when deciding which nodules to offer FNA and for managing the US follow-up.
Indications for US FNA in Thyroid Incidentalomas
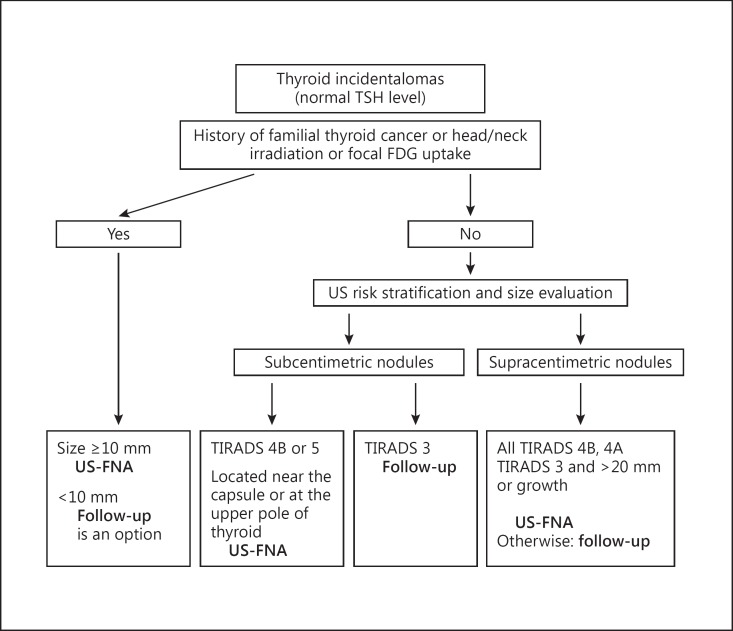
FNA is considered the most reliable test for the diagnosis of malignant thyroid nodules. Guidance regarding the indications for US FNA in case of incidentalomas does exist, especially in case of a history of familial thyroid cancer or previous head/neck irradiation, both of which increase the risk of thyroid cancer [39]. The nodule size at initial US, the US risk stratification score and the increase in size during follow-up may be accepted as the most reasonable criteria for deciding whether to proceed or not to US FNA (fig. 3).
Fig. 3.
Indications for FNA of thyroid incidentalomas.
Subcentimetric Nodules
As they are practically always asymptomatic, the sole question for these small nodules is what are the benefits and risks of overdiagnosis versus postponing diagnosis? Many (but far from all) thyroid incidentalomas are microcarcinomas. This helps to explain the rise in the incidence of papillary thyroid cancers, which has been observed in high-income countries for more than two decades [40]. However, among these, microcarcinomas fortuitously discovered after thyroidectomy for benign diseases represent 64% of all incidental microcarcinomas [41]. They do not correspond to the index tumor, which is primarily investigated by imaging, and should not be confused in the discussion of incidentalomas discovered with medical imaging.
The overall prognosis of papillary microcarcinomas (PTMCs) is excellent and evolution slow. The disease-specific mortality from microcarcinoma not diagnosed because of palpable lymph nodes is indeed <1% and some authors advocate follow-up of patients with thyroid cancer of <1 cm rather than surgery [42]. A wait-and-see policy is safe because the increase in size of microcarcinomas is low during follow-up. In the study by Ito et al. [43], which included PTMCs with a mean size of 6.9 mm, 6.4 and 15.9% of PTMCs followed up without any treatment showed increased size by 3 mm or more during a 5- and 10-year follow-up period, respectively. In the study by Sugitani et al. [44], which included PTMCs with a mean size of >8 mm, 7% of PTMCs increased in size during a mean 5-year follow-up period and 1% developed apparent lymph nodes. However, not all microcarcinomas represent indolent disease. Patients with follicular and Hürthle cell microcarcinomas have a much poorer prognosis [45], and in a report by Noguchi et al. [46] the recurrence rate at 35 years of treated carcinomas between 6 and 10 mm was 14%.
Not to be forgotten, several studies have reported that the proportion of adequate cytological material is significantly lower in small nodules [47] (85% in supracentimetric nodules and 69% in subcentimetric nodules) [48,49]. This was confirmed in 2009, in a report where the inadequacy rate was 20, 9 and 5% for nodules ≤5, >5 and ≤10, and >10 mm, respectively [50.]
The current guidelines on subcentimetric nodules give different recommendations. The ATA [51] recommends FNA for nodules >5 mm, in case of a high-risk history and if the nodule has suspicious sonographic features. The Society of Radiologists in Ultrasound [52] considers that there is no sufficient proof of any benefit of recommending FNA of subcentimetric nodules. Finally, in the guidelines of the AACE/AME/ETA [53], it is stated that suspicious lesions <10 mm should be assessed with FNA biopsy, especially in case of a suspicious history.
For these subcentimetric nodules, we suggest that routine FNA not be recommended in most cases, that it can be considered for nodules with a TIRADS score of 5 or 4B, and that it should – independent of the size of the nodule – be performed systematically on suspicious lymph nodes if one exists. Future guidelines should incorporate new US criteria to better define which nodules carry a risk of harboring aggressive characteristics and therefore warrant FNA, i.e. nodules located near the thyroid capsule or suspected of extending beyond it [54,55,56]. Since the risk of being a pT3 carcinoma and association with central and lateral lymph node extension is increased, nodules located at the upper pole of the thyroid also harbor a higher probability of lateral lymph node extension with an odds ratio of 10 [42]. Future guidelines should also take into account that for nodules measuring <10 mm and which have suspicious US signs but no signs of local or metastatic invasion, deferring from making the diagnosis of microcarcinoma by FNA and proceeding to US follow-up is an option. This is based on their overall good prognosis as emphasized above.
Supracentimetric Nodules
The selection of the nodules that should be referred for FNA is based mainly on US risk stratification and on the evolution in size. FNA can be suggested for all nodules scored TIRADS 4B and 4A. For nodules scored TIRADS 3, given the very high negative predictive value of these scores, FNA could be suggested for nodules >20 mm or in case of verified growth (+2 mm in 2 different axes) and the remainders could be monitored by periodic neck US, e.g. after 1 year initially and then after 2 or 3 years. Complete discharge of the patient could be advised in case the disease is stable.
Translating US Risk Stratification to Individualized Care
Independent of the means by which the thyroid incidentaloma is diagnosed, with the exception of a focal uptake on PET-CT, the risk of malignancy is thought to be low and the prognosis excellent. In these patients, overlooking thyroid malignancy, or more correctly postponing the diagnosis of malignancy, with few exceptions, is not likely to influence subsequent type of therapy or the life expectancy of the patient, although at present this remains unclarified [57,58]. Whether benign or malignant, there is no agreement on whether to offer therapy, and recommendations span from observation to total thyroidectomy. It is with this in mind, albeit difficult to maintain when the patient cannot be given a 100% assurance of the lesion being benign, that the management of thyroid incidentalomas should be considered. The available thyroid nodule guidelines give little guidance on how to manage incidentalomas [59]. Therefore, we believe that investigations should be based on risk stratification, including thyroid US and FNA, in order not to overdiagnose and overtreat the patients.
In the end, any decision concerning supplementary investigations and whether to offer therapy, and if so which one, is based on a dialogue between the patient and his/her physician. It follows that the statistical risk is of little help and may not influence the choice made, which is often based on factors that overrule the rationality of algorithms dealing with virtual patients [48,59,60].
Surgical and Nonsurgical Therapy of Thyroid Incidentalomas
Undoubtedly, many patients will accept conservative follow-up. However, a number, now burdened with a diagnosis, have become symptomatic and wish therapy. In case of large symptomatic multinodular goiters, the reference treatment remains surgery, total or near-total thyroidectomy, or radioiodine therapy, as dealt with elsewhere [2,60,61]. However, when the incidentaloma (1) has been proven to be benign by at least two US FNAs, (2) is a solitary or dominant nodule and (3) grows, alternative nonsurgical treatment options may be considered. Viewed in this way, and accepting that we have little evidence-based experience in this group of patients, it could be speculated that minimally invasive nonsurgical ablation could become an alternative to surgery and be performed similar to that published for symptomatic benign nodules over the past two decades and recently extensively reviewed [62,63,64]. There are several options, which include percutaneous ethanol injection therapy, interstitial laser photocoagulation and radiofrequency ablation.
Percutaneous Ethanol Injection Therapy
When used in solid nodules, whether functioning or not, volume is usually reduced by approximately 50-70%, depending on the number of sessions, with a concomitant improvement in symptomatology [62,63]. However, injecting small amounts of absolute ethanol can be painful, rarely leads to total ablation of the nodule, is associated with seepage of ethanol with the potential of causing extrathyroidal fibrosis and other potentially severe side effects, and is probably associated with a considerable recurrence risk [62,65]. For these reasons it has largely been abandoned, with the exception of dominantly cystic thyroid lesions where it performs excellently [59,66]. We have no reason to believe that incidentalomas would respond differently.
Interstitial Laser Photocoagulation
Increasingly used, and based on long-term follow-up studies, interstitial laser photocoagulation can achieve approximately the same results as percutaneous ethanol injection therapy, but with a more benign side-effect profile due to the ability to contain the energy intranodularly. Also here the best results are seen for cystic nodules [67], both as for remission of the cyst and for reduction of the solid portion. The feasibility and efficacy of ablating small unresectable thyroid malignancies, whether intrathyroidal microcarcinomas [68] or recurrent nodal metastases [69], have been documented in a few patients.
Radiofrequency Ablation
In 126 benign nonfunctioning thyroid nodules treated with radiofrequency ablation and followed up more than 3 years, a mean volume reduction of 93.4 ± 11.7% was obtained at final evaluation with an overall recurrence rate of 5.6% and a complication rate of 3.6% [70]. This technique could also be used to treat thyroid incidentalomas.
Other potential ablation techniques, such as microwaves and high-frequency US, have yet to be employed for this purpose.
Conclusions
Thyroid incidentalomas are overwhelmingly frequent and specific strategies to reduce the economic and psychological burden to patients and society alike are needed.
All patients with a thyroid incidentaloma, independent of mode of detection, should undergo a dedicated neck US with risk stratification. This can be used to decide which nodules should be offered FNA. However, all algorithms should be used as a supplement to clinical knowledge, and not as a substitute for clinical judgment and common sense. US imaging gives clues to the statistical risk of harboring carcinoma but cannot discern which of these nodules are aggressive and require treatment. Weighing the risks of overdiagnosis in the management of thyroid incidentalomas against the benefits of early discovery of some aggressive carcinomas in a dialogue with the patients is essential. A considerable number of patients with small nodules and no US signs of suspicion do not need medical follow-up.
Surgery and novel nonsurgical ablation techniques may offer the same benefits as in thyroid nodules diagnosed in any other way, but the use of these alternative techniques is not yet evidence-based, especially in the case of malignancy.
Additional studies primarily need to focus on increasing the number of patients who with negligible risk can be discharged from medical care. Unfortunately, we question whether long-term randomized studies focusing on risk of overlooking malignancy, overall cost and quality of life, which would provide the basis of evidence-based care, are feasible.
Disclosure Statement
The authors declare that no financial or other conflicts of interest exist in relation to the content of the article.
References
- 1.Ross DS. Nonpalpable thyroid nodules – managing an epidemic. J Clin Endoc Metab. 2002;87:1938–1940. doi: 10.1210/jcem.87.5.8552. [DOI] [PubMed] [Google Scholar]
- 2.Hegedüs L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev. 2003;24:102–132. doi: 10.1210/er.2002-0016. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–559. doi: 10.1056/NEJM199302253280807. [DOI] [PubMed] [Google Scholar]
- 4.Carroll BA. Asymptomatic thyroid nodules: incidental sonographic detection. AJR Am J Roentgenol. 1982;138:499–501. doi: 10.2214/ajr.138.3.499. [DOI] [PubMed] [Google Scholar]
- 5.Woestyn J, Afschrift M, Schelstraete K, Vermeulen A. Demonstration of nodules in the normal thyroid by echography. Br J Radiol. 1985;58:1179–1182. doi: 10.1259/0007-1285-58-696-1179. [DOI] [PubMed] [Google Scholar]
- 6.Brander AE, Viikinkoski VP, Nickels JI, Kivisaari LM. Importance of thyroid abnormalities detected at US screening: a 5-year follow-up. Radiology. 2000;215:801–806. doi: 10.1148/radiology.215.3.r00jn07801. [DOI] [PubMed] [Google Scholar]
- 7.Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154:1838–1840. doi: 10.1001/archinte.154.16.1838. [DOI] [PubMed] [Google Scholar]
- 8.Youserm DM, Huang T, Loevner LA, Langlotz CP. Clinical and economic impact of incidental thyroid lesions found with CT and MR. AJNR Am J Neuroradiol. 1997;18:1423–1428. [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon DY, Chang SK, Choi CS, Yun EJ, Seo YL, Nam ES, Cho SJ, Rho YS, Ahn HY. The prevalence and significance of incidental thyroid nodules identified on computed tomography. J Comput Assist Tomogr. 2008;32:810–815. doi: 10.1097/RCT.0b013e318157fd38. [DOI] [PubMed] [Google Scholar]
- 10.Soelberg KK, Bonnema SJ, Brix TH, Hegedus L. Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: a systematic review. Thyroid. 2012;22:918–925. doi: 10.1089/thy.2012.0005. [DOI] [PubMed] [Google Scholar]
- 11.Shetty SK, Maher MM, Hahn PF, Halpern EF, Aquino SL. Significance of incidental thyroid lesions detected on CT: correlation among CT, sonography, and pathology. AJR Am J Roentgenol. 2006;187:1349–1356. doi: 10.2214/AJR.05.0468. [DOI] [PubMed] [Google Scholar]
- 12.Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013;23:885–891. doi: 10.1089/thy.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross DS. Editorial: predicting thyroid malignancy. J Clin Endocrinol Metab. 2006;91:4253–4255. doi: 10.1210/jc.2006-1772. [DOI] [PubMed] [Google Scholar]
- 14.Leenhardt L, Hejblum G, Franc B, Fediaevsky LD, Delbot T, Le Guillouzic D, Menegaux F, Guillausseau C, Hoang C, Turpin G, Aurengo A. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J Clin Endocrinol Metab. 1999;84:24–28. doi: 10.1210/jcem.84.1.5418. [DOI] [PubMed] [Google Scholar]
- 15.Malone M, Zagzag J, Ogilvie JB, Patel KN, Heller KS. Thyroid cancers detected by imaging are not necessary small or early stage. Thyroid. 2014;24:314–318. doi: 10.1089/thy.2012.0651. [DOI] [PubMed] [Google Scholar]
- 16.Rago T, Vitti P, Chiovato L, Mazzeo S, De Liperi A, Miccoli P, Viacava P, Bogazzi F, Martino E, Pinchera A. Role of conventional ultrasonography and color flow-Doppler sonography in predicting malignancy in ‘cold’ thyroid nodules. Eur J Endocrinol. 1998;138:41–46. doi: 10.1530/eje.0.1380041. [DOI] [PubMed] [Google Scholar]
- 17.Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, Panunzi C, Rinaldi R, Toscano V, Pacella CM. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 18.Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, Yoo HS. New sonographic criteria for recommending fine needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 19.Cappelli C, Castellano M, Pirola I, Gandossi E, De Martino E, Cumetti D, Agosti B, Rosei EA. Thyroid nodule shape suggests malignancy. Eur J Endocrinol. 2006;155:27–31. doi: 10.1530/eje.1.02177. [DOI] [PubMed] [Google Scholar]
- 20.Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, Kim J, Kim HS, Byun JS, Lee DH. Benign and malignant thyroid nodules: US differentiation – multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 21.Bojunga J, Herrmann E, Meyer G, Weber S, Zeuzem S, Mireen FR. Real-time elastography for the differentiation of benign and malignant thyroid nodules: a meta-analysis. Thyroid. 2010;20:1145–1150. doi: 10.1089/thy.2010.0079. [DOI] [PubMed] [Google Scholar]
- 22.Trimboli P, Guglielmi R, Monti S, Misischi I, Graziano F, Nasrollah N, Amendola S, Morgante SN, Deiana MG, Valabrega S, et al. Ultrasound sensitivity for thyroid malignancy is increased by real-time elastography: a prospective multicenter study. J Clin Endocrinol Metab. 2012;97:4524–4530. doi: 10.1210/jc.2012-2951. [DOI] [PubMed] [Google Scholar]
- 23.Bojunga J, Dauth N, Berner C, Meyer G, Holzer K, Voelkl L, Herrmann E, Schroeter H, Zeuzem S, Friedrich-Rust M. Acoustic radiation force impulse imaging for differentiation of thyroid nodules. PLoS One. 2012;7:1–8. doi: 10.1371/journal.pone.0042735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang YF, Xu HX, He Y, Liu C, Guo LH, Liu LN, Xu JM. Virtual touch tissue quantification of acoustic radiation force impulse: a new ultrasound elastic imaging in the diagnosis of thyroid nodules. PLoS One. 2012;7:1–8. doi: 10.1371/journal.pone.0049094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonavita JA, Mayo J, Babb J, Bennett G, Oweity T, Macari M, Yee J. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? Am J Roentgenol. 2009;193:207–213. doi: 10.2214/AJR.08.1820. [DOI] [PubMed] [Google Scholar]
- 26.Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, Callstrom M, Elraiyah TA, Prokop LJ, Stan MN, Murad MH, Morris JC, Montori VM. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:1253–1263. doi: 10.1210/jc.2013-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito Y, Amino N, Yokozawa T, Ota H, Ohshita M, Murata N, Morita S, Kobayashi K, Miyauchi A. Ultrasonographic evaluation of thyroid nodules in 900 patients: comparison among ultrasonographic, cytological and histological findings. Thyroid. 2007;17:1269–1276. doi: 10.1089/thy.2007.0014. [DOI] [PubMed] [Google Scholar]
- 28.Tae HJ, Lim DJ, Baek KH, Park WC, Lee YS, Choi JE, Lee JM, Kang MI, Cha BY, Son HY, Lee KW, Kang SK. Diagnostic value of ultrasonography to distinguish between benign and malignant lesions in the management of thyroid nodules. Thyroid. 2007;17:461–466. doi: 10.1089/thy.2006.0337. [DOI] [PubMed] [Google Scholar]
- 29.Horvath E, Majilis S, Rossi R, Franco C, Niedmann J, Castro A, Dominguez M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;90:1748–1751. doi: 10.1210/jc.2008-1724. [DOI] [PubMed] [Google Scholar]
- 30.Park JY, Lee HJ, Jang HW, Kim HK, Yi JH, Lee W, Kim SH. A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid. 2009;19:1257–1264. doi: 10.1089/thy.2008.0021. [DOI] [PubMed] [Google Scholar]
- 31.Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, Jung HK, Choi JS, Kim BM, Kim EK. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260:892–899. doi: 10.1148/radiol.11110206. [DOI] [PubMed] [Google Scholar]
- 32.Kwak JY, Jung I, Baek JH, Baek SM, Choi N, Choi YJ, Jung SL, Kim EK, Kim JA, Kim JH, Kim KS, Lee JH, Lee JH, Moon HJ, Moon WJ, Park JS, Ryu JH, Shin JH, Son EJ, Sung JY, Na DG. Image reporting and characterization system for ultrasound features of thyroid nodules: multicentric Korean retrospective study. Korean J Radiol. 2013;14:110–117. doi: 10.3348/kjr.2013.14.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russ G, Bigorgne C, Royer B, Rouxel A, Bienvenu-Perrard M. Le système TIRADS en échographie thyroïdienne. J Radiol. 2011;92:701–713. doi: 10.1016/j.jradio.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Russ G, Royer B, Bigorgne C, Rouxel A, Bienvenu-Perrard M, Leenhardt L. Prospective evaluation of thyroid imaging reporting and data system on 4,550 nodules with and without elastography. Eur J Endocrinol. 2013;168:649–655. doi: 10.1530/EJE-12-0936. [DOI] [PubMed] [Google Scholar]
- 35.Kim DS, Kim JH, Na DG, Park SH, Kim E, Chang KH, Sohn CH, Choi YH. Sonographic features of follicular variant papillary thyroid carcinomas in comparison with conventional papillary thyroid carcinomas. J Ultrasound Med. 2009;28:1685–1692. doi: 10.7863/jum.2009.28.12.1685. [DOI] [PubMed] [Google Scholar]
- 36.Kim DW, Jung SJ, Eom JW, Kang T. Color Doppler features of solid, round, isoechoic thyroid nodules without malignant sonographic features: a prospective cytopathological study. Thyroid. 2013;23:472–476. doi: 10.1089/thy.2012.0238. [DOI] [PubMed] [Google Scholar]
- 37.Hambly NM, Gonen M, Gerst SR, Li D, Jia X, Mironov S, Sarasohn D, Fleming SE, Hann LE. Implementation of evidence-based guidelines for thyroid nodule biopsy: a model for establishment of practice standards. AJR Am J Roentgenol. 2011;196:655–660. doi: 10.2214/AJR.10.4577. [DOI] [PubMed] [Google Scholar]
- 38.Moon HJ, Kwak JY, Kim MJ, Son EJ, Kim EK. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology. 2010;255:260–269. doi: 10.1148/radiol.09091284. [DOI] [PubMed] [Google Scholar]
- 39.Schneider AB, Ron E, Lubin J, Stovall M, Gierlowski TC. Dose-response relationships for radiation-induced thyroid cancer and thyroid nodules: evidence for the prolonged effects of radiation on the thyroid. J Clin Endoc Metab. 1993;77:362–369. doi: 10.1210/jcem.77.2.8345040. [DOI] [PubMed] [Google Scholar]
- 40.McLeod DS, Sawka AM, Cooper DS. Controversies in primary treatment of low-risk papillary thyroid cancer. Lancet. 2013;381:1046–1057. doi: 10.1016/S0140-6736(12)62205-3. [DOI] [PubMed] [Google Scholar]
- 41.Sassolas G, Hafdi-Nejjari Z, Remontet L, Bossard N, Belot A, Berger-Dutrieux N, Decaussin-Petrucci M, Bournaud C, Peix JL, Orgiazzi J, Borson-Chazot F. Thyroid cancer: is the incidence rise abating? Eur J Endocrinol. 2009;160:71–79. doi: 10.1530/EJE-08-0624. [DOI] [PubMed] [Google Scholar]
- 42.Hay ID, Hutchinson ME, Gonzalez-Losada T, McIver B, Reinalda ME, Grant CS, Thompson GB, Sebo TJ, Goellner JR. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144:980–987. doi: 10.1016/j.surg.2008.08.035. discussion 987-988. [DOI] [PubMed] [Google Scholar]
- 43.Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, Tomoda C, Takamura Y, Kobayashi K, Miya A. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34:28–35. doi: 10.1007/s00268-009-0303-0. [DOI] [PubMed] [Google Scholar]
- 44.Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34:1222–1231. doi: 10.1007/s00268-009-0359-x. [DOI] [PubMed] [Google Scholar]
- 45.Kuo EJ, Roman SA, Sosa JA. Patients with follicular and Hürthle cell microcarcinomas have compromised survival: a population level study of 22,738 patients. Surgery. 2013;154:1246–1253. doi: 10.1016/j.surg.2013.04.033. discussion 1253-1254. [DOI] [PubMed] [Google Scholar]
- 46.Noguchi S, Yamashita H, Uchino S, Watanabe S. Papillary microcarcinoma. World J Surg. 2008;32:747–753. doi: 10.1007/s00268-007-9453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leenhardt L, Hejblum G, Franc B, Du Pasquier Fédiaevsky L, Delbot T, Le Guillouzic D, Ménégaux F, Guillausseau C, Hoang C, Turpin G, Aurengo A. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J Clin Endocrinol Metab. 1999;84:24–28. doi: 10.1210/jcem.84.1.5418. [DOI] [PubMed] [Google Scholar]
- 48.Moon HJ, Son E, Kim EK, Yoon JH, Kwak JY. The diagnostic values of ultrasound and ultrasound-guided fine needle aspiration in subcentimeter-sized thyroid nodules. Ann Surg Oncol. 2012;19:52–59. doi: 10.1245/s10434-011-1813-1. [DOI] [PubMed] [Google Scholar]
- 49.Mazzaferri EL, Sipos J. Should all patients with subcentimeter thyroid nodules undergo fine-needle aspiration biopsy and preoperative neck ultrasonography to define the extent of tumor invasion? Thyroid. 2008;18:597–602. doi: 10.1089/thy.2008.0100. [DOI] [PubMed] [Google Scholar]
- 50.Kim DW, Lee EJ, Kim SH, Kim TH, Lee SH, Kim DH, Rho MH. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules: comparison in efficacy according to nodule size. Thyroid. 2009;19:27–31. doi: 10.1089/thy.2008.0106. [DOI] [PubMed] [Google Scholar]
- 51.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 52.Frates MC, Benson CB, Charboneau JW, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 53.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedès L, Vitti P. AACE/AME/ETA Task Force on Thyroid Nodules American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. Endocr Pract. 2010;16 doi: 10.4158/EP.16.3.468. (suppl 1):1-43. [DOI] [PubMed] [Google Scholar]
- 54.Kwak JY, Kim EK, Youk JH, Kim MJ, Son EJ, Choi SH, Oh KK. Extrathyroid extension of well-differentiated papillary thyroid microcarcinoma on US. Thyroid. 2008;18:609–614. doi: 10.1089/thy.2007.0345. [DOI] [PubMed] [Google Scholar]
- 55.Qiu-Cheng W, Wen C, Xin W, Jie-Bing L, Hui J, Chun-Lei N. Shorter distance between the nodule and capsule has greater risk of cervical lymph node metastasis in papillary thyroid carcinoma. Asian Pac J Cancer Prev. 2014;15:855–860. doi: 10.7314/apjcp.2014.15.2.855. [DOI] [PubMed] [Google Scholar]
- 56.Kwak JY, Kim EK, Kim MJ, Son EJ, Chung WY, Park CS, Nam KH. Papillary microcarcinoma of the thyroid: predicting factors of lateral neck node metastasis. Ann Surg Oncol. 2009;16:1348–1355. doi: 10.1245/s10434-009-0384-x. [DOI] [PubMed] [Google Scholar]
- 57.Hay ID, Hutchinson ME, Gonzales-Losada T, McIver B, Reinalda ME, Grant CS, Thompson GB, Sebo TJ, Goellner JR. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144:980–987. doi: 10.1016/j.surg.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 58.Yu XM, Wan Y, Sippel RS, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254:653–660. doi: 10.1097/SLA.0b013e318230036d. [DOI] [PubMed] [Google Scholar]
- 59.Paschke R, Hegedüs L, Alexander E, Valcavi R, Papini E, Gharib H. Thyroid nodule guidelines: agreement, disagreement and need for future research. Nat Rev Endocrinol. 2011;7:354–361. doi: 10.1038/nrendo.2011.1. [DOI] [PubMed] [Google Scholar]
- 60.Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 61.Bonnema SJ, Hegedüs L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev. 2012;33:920–980. doi: 10.1210/er.2012-1030. [DOI] [PubMed] [Google Scholar]
- 62.Gharib H, Hegedüs L, Pacella CM, Baek JH, Papini E. Nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab. 2013;98:3949–3957. doi: 10.1210/jc.2013-1806. [DOI] [PubMed] [Google Scholar]
- 63.Papini E, Pacella CM, Hegedüs L. Thyroid ultrasound and ultrasound-assisted procedures. From the shadows into an array of applications. Eur J Endocrinol. 2014;170:133–146. doi: 10.1530/EJE-13-0917. [DOI] [PubMed] [Google Scholar]
- 64.Papini E, Pacella CM, Misischi I, Guglielmi R, Bizzarri G, Døssing H, Hegedüs L. The advent of US-guided ablation techniques in nodular thyroid disease. Toward a patient-tailored approach. Best Pract Res Clin Endocrinol Metab. 2014. DOI: http://dx.doi.org/10.1016/j.beem.2014.02.004. [DOI] [PubMed]
- 65.Bennedbaek FN, Karstrup S, Hegedüs L. Percutaneous ethanol injection therapy in the treatment of thyroid and parathyroid diseases. Eur J Endocrinol. 1997;136:240–250. doi: 10.1530/eje.0.1360240. [DOI] [PubMed] [Google Scholar]
- 66.Bennedbaek FN, Hegedüs L. Treatment of recurrent thyroid cysts with ethanol: a randomized double-blind controlled trial. J Clin Endocrinol Metab. 2003;88:5773–5777. doi: 10.1210/jc.2003-031000. [DOI] [PubMed] [Google Scholar]
- 67.Døssing H, Bennedbæk FN, Hegedüs L. Interstitial laser photocoagulation (ILP) of benign cystic thyroid nodules – a prospective randomized trial. J Clin Endocrinol Metab. 2013;98:E1213–E1217. doi: 10.1210/jc.2013-1503. [DOI] [PubMed] [Google Scholar]
- 68.Valcavi R, Piana S, Steconi Bortolan G, Lai R, Barbieri V, Negro R. Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: a feasibility study on three cases with pathological and immunohistochemical evaluation. Thyroid. 2013;23:1578–1582. doi: 10.1089/thy.2013.0279. [DOI] [PubMed] [Google Scholar]
- 69.Papini E, Bizzarri G, Bianchini A, Valle D, Misischi I, Guglielmi R, Salvatori M, Solbiati L, Crescenzi A, Pacella CM, Gharib H. Percutaneous ultrasound-guided laser ablation is effective for treating selected nodal metastases in papillary thyroid cancer. J Clin Endocrinol Metab. 2013;98:E92–E97. doi: 10.1210/jc.2012-2991. [DOI] [PubMed] [Google Scholar]
- 70.Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH. Radiofrequency ablation of benign nonfunctioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23:1044–1049. doi: 10.1007/s00330-012-2671-3. [DOI] [PubMed] [Google Scholar]