Abstract
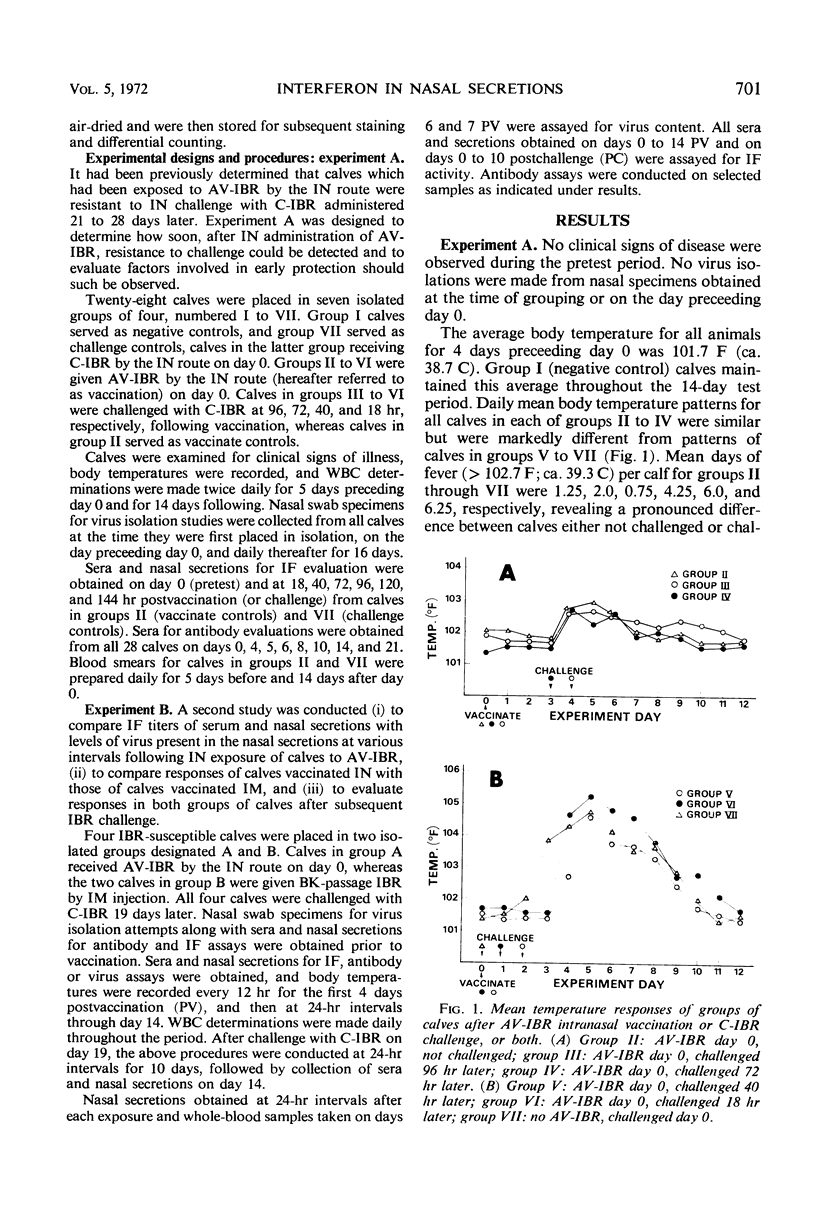
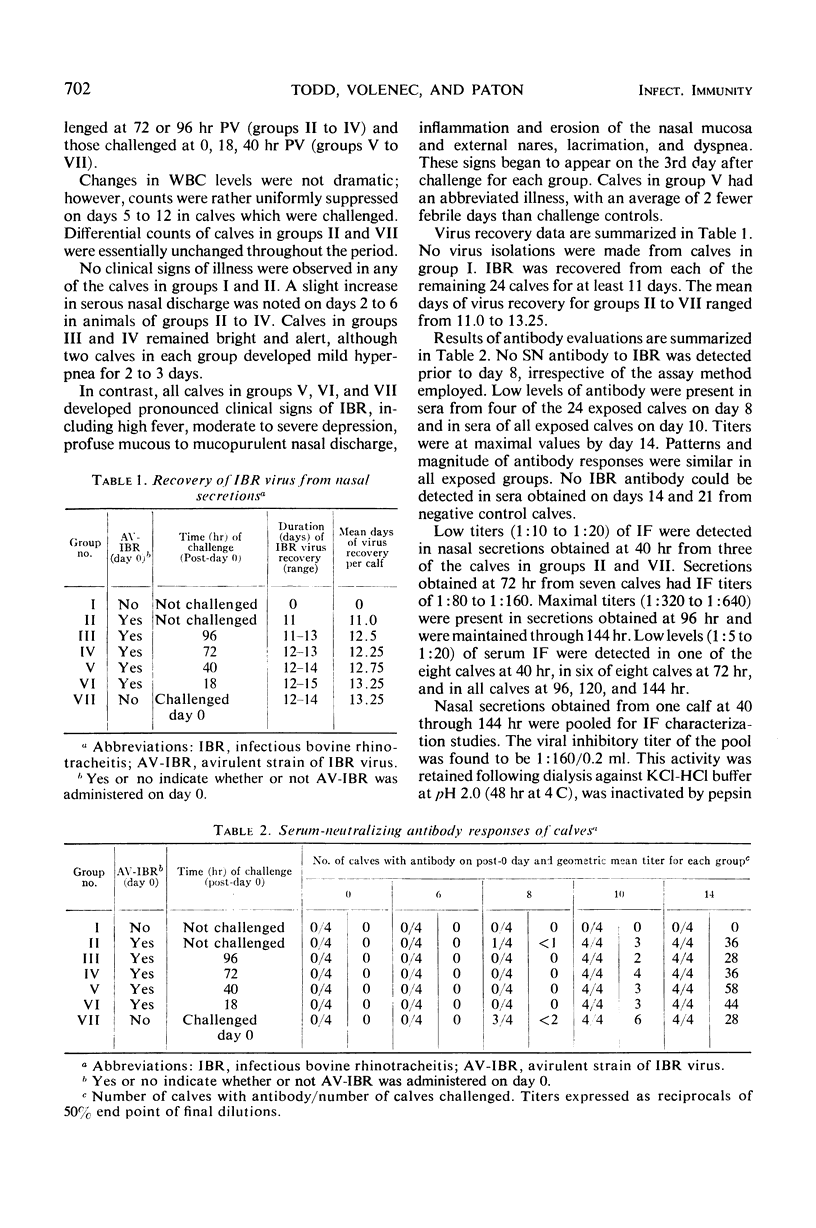
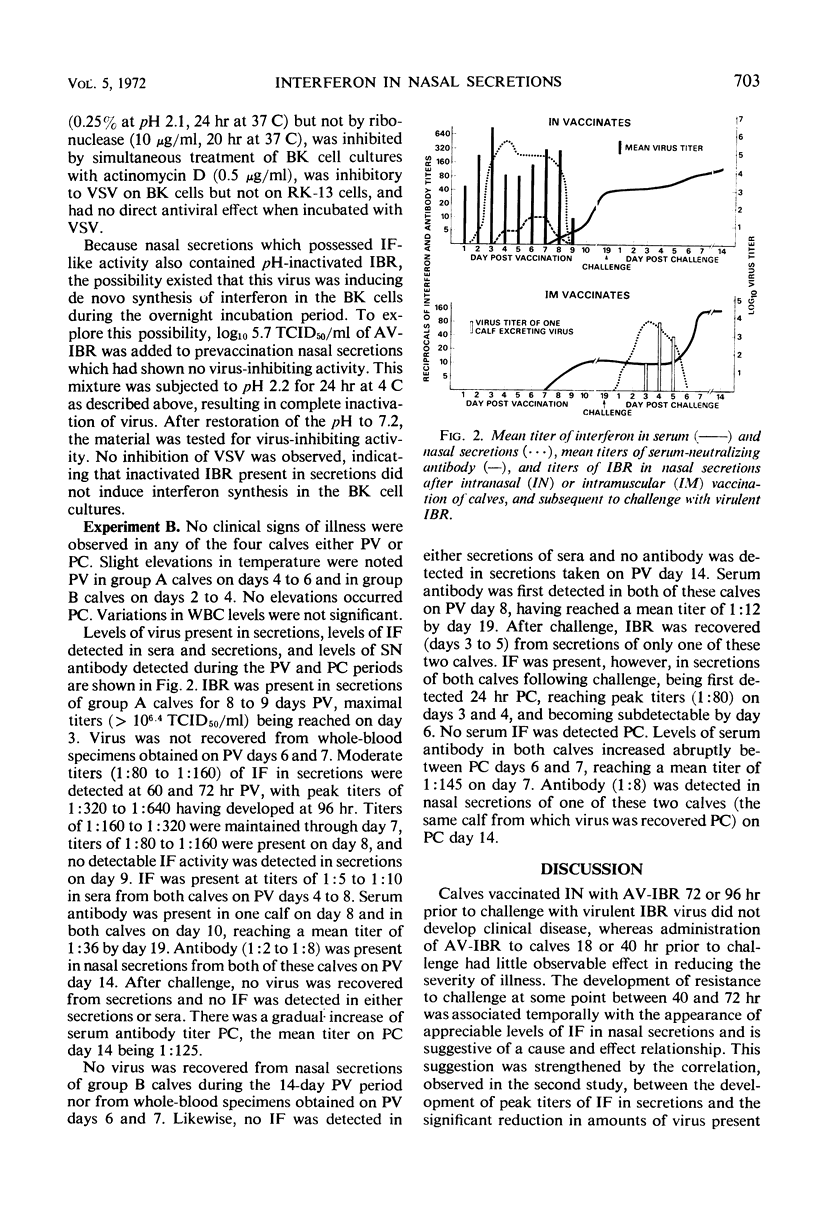
Calves which had received avirulent infectious bovine rhinotracheitis virus (AV-IBR) by intranasal (IN) administration developed detectable levels of interferon (IF) in nasal secretions as early as 40 hr later. Peak titers (1:640) of IF appeared in secretions 72 to 96 hr after administration of virus, and titers of 1:80 to 1:320 were maintained through the 8th day. Lower titers (1:5 to 1:10) of IF were detected in sera obtained on the 4th to 8th days after administration of virus. Peak titers of IF in respiratory tract secretions were accompanied by a 100- to > 1,000-fold reduction in the levels of AV-IBR present in the secretions. Serum antibody was not detected prior to the 8th day after administration of AV-IBR. Calves which received AV-IBR by the IN route 72 or 96 hr earlier were refractory to challenge with virulent infectious bovine rhinotracheitis virus (IBR), whereas calves receiving AV-IBR 18 or 40 hr earlier became clinically ill following challenge. The temporal association between appearance of IF in respiratory tract secretions and onset of protection against challenge suggests a cause and effect relationship. No IF was detected in either nasal secretions or sera of calves receiving modified IBR virus by intramuscular injection. Following subsequent IN challenge of these calves, IF was detected in nasal secretions as early as 24 hr postchallenge and was maintained at titers of 1:40 to 1:80 for approximately 4 days, even in the absence of virus recovery. Greater ease of local IF induction with IBR virus in calves previously sensitized with that virus is suggested.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. W., Gurwith M. J., Waddell D., Merigan T. C. Cutaneous interferon production in patients with Hodgkin's disease and other cancers infected with varicella or vaccinia. N Engl J Med. 1970 Nov 26;283(22):1182–1187. doi: 10.1056/NEJM197011262832202. [DOI] [PubMed] [Google Scholar]
- Ash R. J., Bubel H. C. Temporal relationship of interferon production and resistance to experimentally induced virus infection. J Infect Dis. 1966 Feb;116(1):1–7. doi: 10.1093/infdis/116.1.1. [DOI] [PubMed] [Google Scholar]
- BARON S. MECHANISM OF RECOVERY FROM VIRAL INFECTION. Adv Virus Res. 1963;10:39–64. doi: 10.1016/s0065-3527(08)60696-x. [DOI] [PubMed] [Google Scholar]
- Catalano L. W., Jr, Moossy J., Sell S. Viral interference in the brains of mice infected subcutaneously with herpes simplex virus. Proc Soc Exp Biol Med. 1970 Nov;135(2):509–514. doi: 10.3181/00379727-135-35085. [DOI] [PubMed] [Google Scholar]
- Cate T. R., Douglas R. G., Jr, Couch R. B. Interferon and resistance to upper respiratory virus illness. Proc Soc Exp Biol Med. 1969 Jun;131(2):631–636. doi: 10.3181/00379727-131-33941. [DOI] [PubMed] [Google Scholar]
- DINTER Z. An inhibitor of viral activity in cell cultures infected with the virus of foot-and-mouth disease. Acta Pathol Microbiol Scand. 1960;49:270–271. doi: 10.1111/j.1699-0463.1960.tb01138.x. [DOI] [PubMed] [Google Scholar]
- FORCE E. E., STEWART R. C., HAFF R. F. DEVELOPMENT OF INTERFERON IN RABBIT DERMIS AFTER INFECTION WITH HERPES SIMPLEX VIRUS. Virology. 1965 Feb;25:322–325. doi: 10.1016/0042-6822(65)90210-2. [DOI] [PubMed] [Google Scholar]
- Finter N. B. A comparison of the amounts of mouse interferon obtained from different sources. Nature. 1965 May 8;206(984):597–599. doi: 10.1038/206597a0. [DOI] [PubMed] [Google Scholar]
- Fruitstone M. J., Michaels B. S., Rudloff D. A., Sigel M. M. Role of the spleen in interferon production in mice. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1008–1011. doi: 10.3181/00379727-122-31311. [DOI] [PubMed] [Google Scholar]
- GILLESPIE J. H., MADIN S. H., DARBY N. B., Jr Cellular resistance in tissue culture, induced by noncytopathogenic strains, to a cytopathogenic strain of virus diarrhea virus of cattle. Proc Soc Exp Biol Med. 1962 Jun;110:248–250. doi: 10.3181/00379727-110-27481. [DOI] [PubMed] [Google Scholar]
- GRESSER I., DULL H. B. A VIRUS INHIBITOR IN PHARYNGEAL WASHINGS FROM PATIENTS WITH INFLUENZA. Proc Soc Exp Biol Med. 1964 Jan;115:192–196. doi: 10.3181/00379727-115-28867. [DOI] [PubMed] [Google Scholar]
- Glasgow L. A. Leukocytes and interferon in the host response to viral infections. II. Enhanced interferon response of leukocytes from immune animals. J Bacteriol. 1966 Jun;91(6):2185–2191. doi: 10.1128/jb.91.6.2185-2191.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. H., McVicar J. W., Sutmoller P., Trautman R., Wagner G. G. Latent viral infection in transmission of foot-ana-mouth disease by contact between infected and susceptible cattle. J Infect Dis. 1971 Sep;124(3):270–276. doi: 10.1093/infdis/124.3.270. [DOI] [PubMed] [Google Scholar]
- Green J. A., Cooperband S. R., Kibrick S. Immune specific induction of interferon production in cultures of human blood lymphocytes. Science. 1969 Jun 20;164(3886):1415–1417. doi: 10.1126/science.164.3886.1415. [DOI] [PubMed] [Google Scholar]
- HERMODSSON S. ACTION OF PARAINFLUENZA VIRUS TYPE 3 ON SYNTHESIS OF INTERFERON AND MULTIPLICATION OF HETEROLOGOUS VIRUSES. Acta Pathol Microbiol Scand. 1964;62:224–238. doi: 10.1111/apm.1964.62.2.224. [DOI] [PubMed] [Google Scholar]
- HITCHCOCK G., PORTERFIELD J. S. The production of interferon in brains of mice infected with an arthropod-borne virus. Virology. 1961 Mar;13:363–365. doi: 10.1016/0042-6822(61)90155-6. [DOI] [PubMed] [Google Scholar]
- ISAACS A. INTERFERON. Adv Virus Res. 1963;10:1–38. [PubMed] [Google Scholar]
- Jao R. L., Wheelock E. F., Jackson G. G. Production of interferon in volunteers infected with Asian influenza. J Infect Dis. 1970 Apr;121(4):419–426. doi: 10.1093/infdis/121.4.419. [DOI] [PubMed] [Google Scholar]
- KONO Y., HO M. THE ROLE OF THE RETICULOENDOTHELIAL SYSTEM IN INTERFERON FORMATION IN THE RABBIT. Virology. 1965 Jan;25:163–166. doi: 10.1016/0042-6822(65)90268-0. [DOI] [PubMed] [Google Scholar]
- Paccaud M. F., Jacquier C. A respiratory syncytial virus of bovine origin. Arch Gesamte Virusforsch. 1970;30(4):327–342. doi: 10.1007/BF01258363. [DOI] [PubMed] [Google Scholar]
- Rosenquist B. D., Loan R. W. Interferon induction in the bovine species by infectious bovine rhinotracheitis virus. Am J Vet Res. 1969 Aug;30(8):1305–1312. [PubMed] [Google Scholar]
- Rosenquist B. D., Loan R. W. Interferon production with strain SF-4 of parainfluenza-3 virus. Am J Vet Res. 1967 May;28(124):619–628. [PubMed] [Google Scholar]
- Rosenquist B. D., Loan R. W. Production of circulating interferon in the bovine species. Am J Vet Res. 1969 Aug;30(8):1293–1303. [PubMed] [Google Scholar]
- Rosenquist B. D. Polyriboinosinic-polyribocytidylic acid-induced interferons in calves. Am J Vet Res. 1971 Jan;32(1):35–39. [PubMed] [Google Scholar]
- SHELOKOV A., VOGEL J. E., CHI L. Hemadsorption (adsorption-hemagglutination) test for viral agents in tissue culture with special reference to influenza. Proc Soc Exp Biol Med. 1958 Apr;97(4):802–809. doi: 10.3181/00379727-97-23884. [DOI] [PubMed] [Google Scholar]
- TYRRELL D. A. Interferon produced by cultures of calf kidney cells. Nature. 1959 Aug 8;184(Suppl 7):452–453. doi: 10.1038/184452a0. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R. Biological studies of interferon. II. Temporal relationships of virus and interferon production by cells infected with Eastern equine encephalomyelitis and influenza viruses. Virology. 1963 Feb;19:215–224. doi: 10.1016/0042-6822(63)90011-4. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R. INTERFERON. A REVIEW AND ANALYSIS OF RECENT OBSERVATIONS. Am J Med. 1965 May;38:726–737. doi: 10.1016/0002-9343(65)90193-2. [DOI] [PubMed] [Google Scholar]
- Yamada M., Azuma M., Nishioka R., Togashi T. Relationship between immunity and interferon production in macrophages. I. Effect of immunity on interferon production. Jpn J Microbiol. 1970 Jul;14(4):311–318. doi: 10.1111/j.1348-0421.1970.tb00529.x. [DOI] [PubMed] [Google Scholar]


