Abstract
BACKGROUND
Pulmonary vein isolation (PVI) is recognized as a potentially curative treatment for atrial fibrillation (AF). Ablation of complex fractionated atrial electrograms (CFAEs) in addition to PVI has been advocated as a means to improve procedural outcomes, but the benefit remains unclear.
OBJECTIVE
To synthesize the available data testing the incremental benefit of adding CFAE ablation to PVI.
METHODS
We performed a meta-analysis of controlled studies comparing the effect of PVI with CFAE ablation versus PVI alone in patients with paroxysmal and nonparoxysmal AF.
RESULTS
Of the 481 reports identified, 8 studies met our inclusion criteria. There was a statistically significant increase in freedom from atrial tachyarrhythmia (AT) with the addition of CFAE ablation (RR 1.15, p=0.03). In the 5 reports of nonparoxsymal AF (3 randomized controlled trials, one controlled clinical trial, and one trial using matched historical controls), addition of CFAE ablation resulted in a statistically significant increase in freedom from AT (n=112/181 [62%] for PVI+CFAE versus n=84/179 [47%] for PVI alone; RR 1.32, p=0.02). In trials of paroxysmal AF (3 randomized controlled trials and one trial using matched historical controls), addition of CFAE ablation did not result in a statistically significant increase in freedom from AT (n=131/166 [79%] for PVI+CFAE versus n=122/164 [74%] for PVI alone; RR 1.04, p=0.52).
CONCLUSIONS
In these studies of patients with nonparoxysmal AF, addition of CFAE ablation to PVI results in greater improvement in freedom from AF. No additional benefit of this combined approach was observed in patients with paroxysmal AF.
Keywords: atrial fibrillation, catheter ablation, complex fractionated atrial electrogram, pulmonary vein isolation
Introduction
Atrial fibrillation affects more than 2 million Americans1 and is associated with increased risk of stroke and death despite optimal medical therapy.2, 3 The observation that ectopic beats from the pulmonary veins play a major role in the initiation of atrial fibrillation4 has led to the development of percutaneous isolation of the pulmonary veins (PVI) as an accepted treatment for atrial fibrillation. Pulmonary vein isolation has resulted in high single procedural success rates for patients with paroxysmal atrial fibrillation,5–8 but success rates for PVI alone in patients with nonparoxysmal atrial fibrillation are often lower, and additional ablation has been advocated by some to increase procedural success rates in this population.
More recently, the targeting of specific sites within the atria that contain electrogram fractionation has been described as a technique for terminating AF.9 These sites, termed complex fractionated atrial electrograms (CFAEs), are areas where electrograms are rapid and multiphasic or continuous and are thought to be important drivers for the maintenance of AF. Ablation of CFAEs has been proposed as a way to improve the success of PVI,10–18 but the added benefit remains unclear. We conducted a meta-analysis to determine the effect of adding ablation of complex fractionated atrial electrograms to pulmonary vein isolation for patients with paroxysmal and nonparoxysmal atrial fibrillation.
Methods
Search strategy
We performed an electronic literature search of MEDLINE (1950 to March 27, 2010), MEDLINE In-Process and other Non-Indexed Citations, EMBASE (all years, searched March 28, 2010), the Cochrane Database of Systematic Reviews (February 2010), and the Cochrane Central Register of Controlled Trials (First Quarter 2010). Search strategies were developed with the help of a research librarian and are published as an online data supplement. We also manually searched the bibliographies of the selected publications, our personal archives, and review articles published in the last 5 years on catheter ablation for atrial fibrillation.
Eligibility
Randomized trials or controlled clinical trials that compared electrical isolation of the pulmonary veins to the same procedure with additional ablation of complex fractionated atrial electrograms were included. To be included, studies were required to report on the endpoint of freedom from atrial tachyarrhythmia or atrial fibrillation at least 3 months post-procedure. There were no language restrictions and incomplete information (abstracts, brief reports) could be included as long as it met all other inclusion criteria. Ablation could be performed as a first-line therapy or after failure of antiarrhythmic drugs. Any method of isolation of the pulmonary veins (including pulmonary vein isolation or pulmonary vein antrum isolation [PVAI]) was accepted as long as electrical isolation of the pulmonary veins was confirmed. Additional anatomical ablation lines performed after PVI were allowed as long as these were performed for both groups. CFAEs could be defined, identified, and ablated in any manner and this could be performed before or after pulmonary vein isolation.
Endpoints
The primary endpoint was freedom from atrial arrhythmia off antiarrhythmic drugs. If the endpoint was unclear, the authors were contacted directly for clarification. Repeat procedures were not allowed (and were considered failure to reach the primary endpoint if they occurred). Secondary endpoints were procedure time, fluoroscopy time, all-cause mortality, pericardial tamponade, and thromboembolic events. For the purpose of this analysis, pericardial tamponade was defined as any pericardial effusion requiring percutaneous or surgical drainage.
Data extraction
Data extraction was performed independently by two investigators and results were recorded on a standardized data extraction form. Disagreements were resolved by consensus.
Data analysis
Dichotomous outcomes were analyzed using the DerSimonian and Laird random effects model. We explored heterogeneity by visually inspecting the outcome plots and then calculating the I2 statistic as well as the χ2 statistic; I2 > 50% indicated significant heterogeneity. Statistical significance was set at α = 0.05. If significant heterogeneity was observed, we explored this by repeating the analysis while excluding studies with methodological differences from the rest. All statistical analysis was performed using Review Manager 5.0.21 (The Cochrane Collaboration, Copenhagen, Denmark).
Procedure time and fluoroscopy time, both continuous variables, were also analyzed using a random-effects model. Between-study variance was estimated using the DerSimonian and Laird method.
RESULTS
Search results
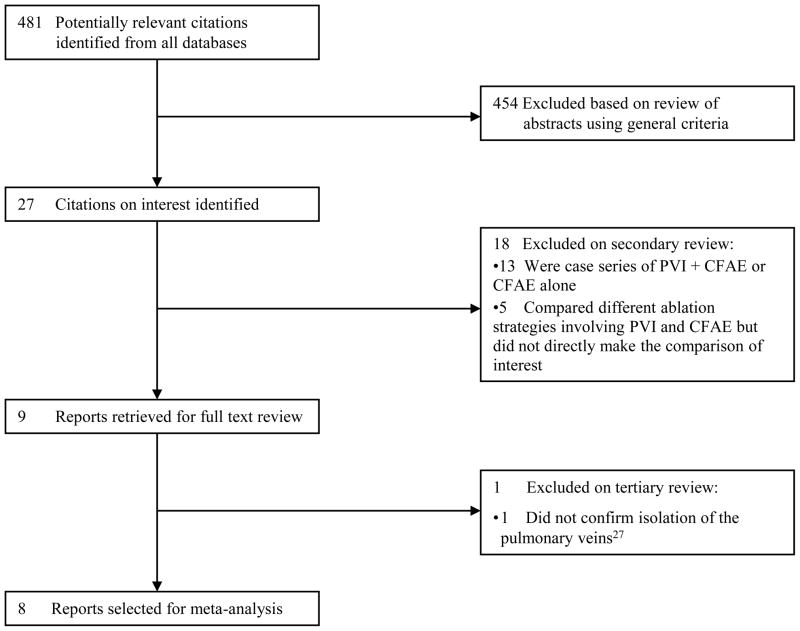
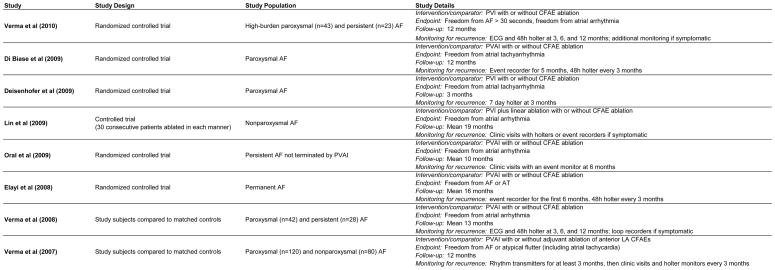
Our electronic search identified 481 potentially eligible references; 8 of these met our inclusion criteria (Figure 1). Of these, 5 were randomized controlled trials,19–23 two used matched historical controls24, 25 and one study performed PVI and CFAE in 30 consecutive patients followed by PVI alone in 30 consecutive patients.26 There were 2 studies of patients with paroxysmal AF, 3 studies of patients with nonparoxysmal AF, and 3 studies of patients with both paroxysmal and nonparoxysmal AF (Figure 2). Table 1 summarizes the baseline characteristics of the patients in the included studies. These 8 studies represent a total of 760 patients. 372 had paroxysmal AF and 388 had nonparoxysmal AF. The mean age of the patients was 57 years and 74% were male. The mean left atrial dimension was 43 mm and the average duration of AF was 5.6 years. 378 patients underwent PVI alone and 382 underwent PVI+CFAE.
Figure 1.
Search Flow Diagram for Reports Included in the Meta-Analysis
Figure 2.
Individual Study Characteristics
RCT = randomized controlled trial
LA = left atrium
Table 1.
Baseline Characteristics of Patients Included in Meta-Analysis
| Verma (2010) | Di Biase (2009) | Deisenhofer (2009) | Lin (2009) | Oral (2009) | Elayi (2008) | Verma (2008) | Verma (2007) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PVI+CFAE | PVI Alone | PVI+CFAE | PVI Alone | PVI+CFAE | PVI Alone | PVI+CFAE | PVI Alone | PVI+CFAE | PVI Alone | PVI+CFAE | PVI Alone | PVI+CFAE | PVI Alone | PVI+CFAE | PVI Alone | |
| Number of Patients | 34 | 32 | 34 | 35 | 50 | 48 | 30 | 30 | 50 | 50 | 49 | 48 | 35 | 35 | 100 | 100 |
| Age, mean+/−SD (years) | 59±10 | 55±11 | 58±8 | 57±8 | 55±10 | 58±10 | 49±10 | 49±12 | 62±8 | 58±10 | 59±12 | 58±10 | 61±9 | 60±11 | 56±9 | 57±12 |
| Male Gender (%) | 74 | 75 | 88 | 83 | 82 | 69 | 80 | 87 | 82 | 82 | 65 | 69 | 74 | 77 | 63 | 63 |
| LA Size, mean +/−SD (mm) | 41±6 | 43±5 | 44±6 | 43±6 | 44±5 | 43±6 | 41±8 | 40±5 | 46±6 | 47±6 | 46±6 | 45±7 | 43±9 | 41±10 | 43±10 | 42±9 |
| LVEF, mean+/−SD (%) | 59±12 | 62±7 | 55±6 | 55±8 | NA | NA | 54±8 | 56±8 | 54±9 | 53±12 | 55 | 52 | 53±7 | 53±8 | 53±11 | 53±12 |
| Duration of AF (years) | 7.6±9.4 | 6.4±6.6 | 5.3±5.0 | 5.3±5.7 | 4±4 | 4±3 | 8.4±7.2 | 5.4±6.4 | 5±4 | 6±5 | 6.3±2.5 | 5.5±3.5 | 5.5±4.0 | 4.9±4.5 | 5.1±2.0 | 5.3±3.0 |
PVI = pulmonary vein isolation; CFAE = complex fractionated atrial electrogram ablation; SD = standard deviation; LA = left atrium; LVEF = left ventricular ejection fraction; AF = atrial fibrillation; NA = not available
Of note, a single investigator (A. Verma) was the lead author on three of the included trials. However, the subjects in these trials did not overlap and two of these trials were multicenter studies.
Primary Outcomes
Paroxysmal and Nonparoxysmal AF studies combined
For all studies combined, there was a statistically significant benefit to the addition of CFAE ablation to electrical isolation of the pulmonary veins in terms of freedom from atrial tachyarrhythmia at follow-up (RR 1.15, p=0.03, Figure 3). There was no evidence of heterogeneity between included studies (I2=36%, p=0.14). The meta-analysis was repeated with the 5 studies that were randomized controlled trials and the effect size was similar, although the results were no longer statistically significant (RR 1.17, p=0.13). To further explore the results, the analysis was repeated for paroxysmal and nonparoxysmal atrial fibrillation individually.
Figure 3.
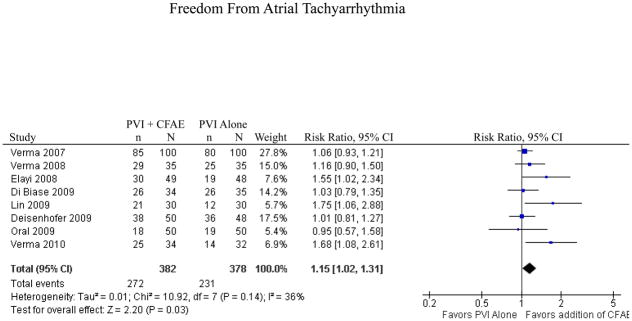
Freedom from Atrial Tachyarrhythmia for All Studies
CI = confidence interval
Paroxysmal atrial fibrillation
4 studies (3 randomized controlled trials and one trial using matched historical controls) representing 330 patients reported results for patients with paroxysmal atrial fibrillation. One study that included patients with paroxysmal atrial fibrillation was not included as results were not reported for the subgroup of patients with paroxysmal atrial fibrillation.25 The addition of CFAE ablation for patients with paroxysmal atrial fibrillation did not increase rates of freedom from atrial tachyarrhythmia (n=131/166 [79%] for PVI+CFAE versus n=122/164 [74%] for PVI alone; RR 1.04, p=0.52, Figure 4). There was no heterogeneity in this study population (I2=0%, p=0.52). The analysis was repeated again using only the 3 randomized controlled trials and the results were unchanged (RR 1.06, p=0.50).
Figure 4.
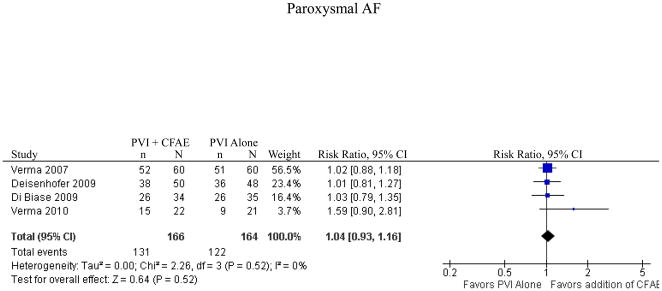
Freedom from Atrial Tachyarrhythmia for Paroxysmal AF
CI = confidence interval
Nonparoxysmal atrial fibrillation
5 studies reported results for patients with nonparoxysmal atrial fibrillation. These studies represented 360 patients and included 3 randomized controlled trials, one controlled clinical trial, and one trial using matched historical controls. Addition of CFAE ablation resulted in a statistically significant increase in freedom from atrial tachyarrhythmia (n=112/181 [62%] for PVI+CFAE versus n=84/179 [47%] for PVI alone; RR 1.32, p=0.02, Figure 5). There was no evidence of significant heterogeneity between included studies (I2=29%, p=0.23). The analysis was repeated using only the 3 randomized controlled trials and the effect size was similar, although no longer statistically significant (RR 1.36, p=0.10).
Figure 5.
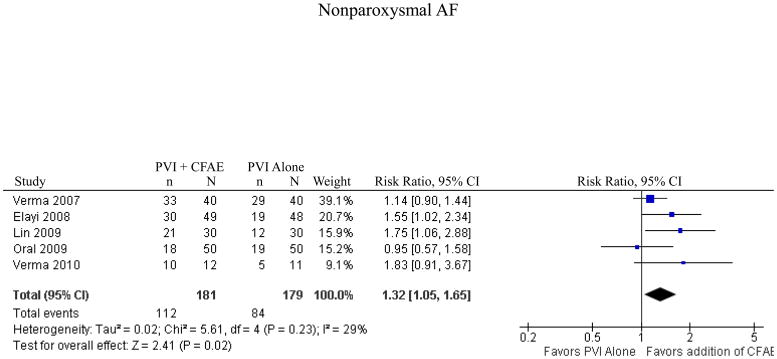
Freedom from Atrial Tachyarrhythmia for Nonparoxysmal AF
CI = confidence interval
Secondary Outcomes
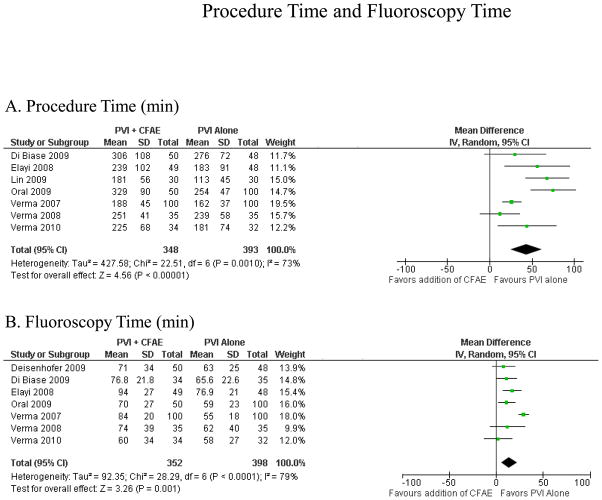
Fluoroscopy Time and Procedure Time
Seven studies reported data for procedure time and seven reported on fluoroscopy time (Figure 6). The addition of CFAE ablation resulted in increased procedure time (43 ± 19 minutes, p<0.001) and fluoroscopy time (14 ± 8 minutes, p=0.001).
Figure 6.
Procedure Time and Fluoroscopy Time. Procedure time (A) and fluoroscopy time (B), both in minutes.
Complications and Mortality
6 studies reported rates of cardiac tamponade. The rate of tamponade in the PVI alone group was 0.6% (2/308) and in the PVI with CFAE ablation group was 1.0% (3/313). This difference was not statistically significant (p=1.0, Fisher’s exact test, two-tailed). Thromboembolic complications were reported by 6 studies representing 592 patients. No thromboembolic complications were observed in either the PVI alone arm or the PVI with CFAE ablation arm. No deaths were reported by any of the included studies (although not all studies reported on deaths at follow-up).
DISCUSSION
Main Findings
In this meta-analysis of controlled trials comparing PVI alone versus PVI with CFAE ablation for atrial fibrillation, we found that the addition of CFAE ablation to PVI results in a statistically significant increase in freedom from atrial tachyarrhythmia at follow-up for subjects with nonparoxysmal, but not paroxysmal, atrial fibrillation. The added benefit of CFAE ablation comes at the cost of increased procedure time and increased fluoroscopy time. There was no statistically significant difference in the rate of tamponade and the rate of other complications was low enough to prohibit statistical analysis. To our knowledge, this is the first meta-analysis addressing these questions.
Pulmonary vein isolation aims to electrically isolate the pulmonary veins and thus the triggers of atrial fibrillation. This procedure has resulted in high success rates for paroxysmal atrial fibrillation, but is inadequate for many patients with nonparoxysmal atrial fibrillation. As atrial fibrillation moves from the paroxysmal to the nonparoxysmal state, the atria undergo both structural and electrical remodeling, resulting in areas of fibrosis and slowed conduction. These structural changes facilitate anisotropy, and the associated pivoting and colliding of wavelets, which result in regions with fractionated electrograms. It was first proposed by Konings and colleagues27 that these areas are critical to the persistence of atrial fibrillation. Nademanee et al.9 were the first to show that substrate modification through ablation of CFAEs could be used to terminate atrial fibrillation. As atrial fibrillation moves from the paroxysmal to the persistent state, abnormal atrial substrate plays a larger role in the persistence of AF. As a result, simply isolating the pulmonary vein triggers of AF may not be sufficient to prevent recurrence of nonparoxysmal atrial fibrillation. In this meta-analysis, targeted substrate ablation was shown to improve procedural outcomes for patients with nonparoxysmal atrial fibrillation, possibly by eliminating the specific substrate responsible for perpetuation of AF in these patients.
In the pooled analysis of studies of nonparoxysmal atrial fibrillation, the use of CFAE ablation as an adjunct to pulmonary vein isolation resulted in an increase in freedom from atrial tachyarrhythmia at follow-up (relative risk ratio 1.32, 95% CI 1.05–1.65). This analysis included 5 studies, four of which showed improved long-term efficacy over anatomical ablation alone. Oral et al.22 found no improvement in outcome with the addition of CFAE ablation. This study differed from the others in the group in several ways. First, the study population had larger left atrial diameters (4.65 cm on average versus 4.29 cm for the other studies) and the success rates of the procedure were lower. Given this, the patient population may have had more advanced atrial fibrillation that was less suitable for ablation in general, biasing against an effect. Finally, the investigators limited CFAE ablation to 2 hours or stopped ablating CFAEs when AF terminated.
In the pooled analysis of studies of paroxysmal atrial fibrillation, the use of CFAE ablation as an adjunct to PVI did not result in an increase in freedom from atrial tachyarrhythmia at follow-up (relative risk ratio 1.04, 95% CI 0.93–1.16). One of the four studies, Verma et al. (2010),19 showed a non-significant trend toward benefit from the combined approach (p=0.14). Interestingly, this study included patients with high-burden paroxysmal atrial fibrillation, possibly accounting for this trend.
Study Limitations
Meta-analysis was developed for application to randomized trials. However, there has been a recent trend the use of meta-analysis for nonrandomized studies.28 To address the fact that we included nonrandomized data, we performed a sensitivity analysis by repeating each part of the meta-analysis using only the data from RCTs. The effect sizes remained remarkably consistent, although the statistically significant findings were no longer significant with the smaller number of included studies.
The second limitation of this study was the variation in the technique of ablation between centers. Different studies used different methods to electrically isolate the pulmonary veins (pulmonary vein isolation or pulmonary vein antrum isolation). One study, Lin et al.,26 performed linear ablation by an anatomic approach after PVI. In addition, the definition of CFAEs, the method of identification of CFAEs (visual identification or automated mapping), and the method of CFAE ablation differed between studies. For example, the study by Verma et al.24 limited CFAE ablation for the anterior left atrium and the anterior septum while the study by Deisenhofer et al.21 performed a more extensive CFAE ablation involving both the left and right atria. Oral et al.22 limited CFAE ablation to 2 hours. However, the average additional procedure time for CFAE ablation was only 75 minutes, which was similar to the other included studies and comfortably below their 2 hour limit.
Another limitation of this study was the variation on the length of follow-up. Deisenhofer et al.21 had only 3 months of follow-up. In their study, two subjects who were classified as procedural successes at 3 months later developed atrial tachycardias that required ablation. These patients would have been considered treatment failures in any of the other studies that had a longer period of follow-up. The study by Lin et al26 had different follow-up durations for the intervention and control groups. By chance, this did not affect the analysis as both curves reached a plateau by 15 months.
A final limitation is that Verma et al. (2010)19 had a small percentage of patients still on antiarrhythmic drugs at follow-up. This was allowed in this analysis for three reasons: the study protocol specified that patients should be taken off antiarrhythmic drugs after 2 months, a high percentage (96%) of subjects considered to have a successful post-ablation outcome were off antiarrhythmic drugs, and the small number of subjects still on antiarrhythmic drugs were evenly distributed between the groups.
CONCLUSION
In this meta-analysis of randomized controlled trials and controlled clinical trials, addition of CFAE ablation to PVI results in greater improvement in freedom from atrial arrhythmia for patients with nonparoxysmal atrial fibrillation, but not for patients with paroxysmal atrial fibrillation. This improvement comes at the cost of longer fluoroscopy time and procedure time. This analysis provides another piece of evidence that CFAE ablation may be a useful adjunct to PVI for patients with nonparoxysmal, but not paroxysmal, atrial fibrillation. The analysis was limited by the retrospective nature of some of the studies in addition to variation in the technique of ablation between centers. More randomized trials are needed to fully evaluate the benefits of CFAE ablation.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
We thank Eric A. Macklin, PhD, for his assistance in creating a random-effects model to analyze procedure time and fluoroscopy time.
ABBREVIATIONS
- PVI
Pulmonary vein isolation
- AF
atrial fibrillation
- CFAE
complex fractionated atrial electrogram
- AT
atrial tachyarrhythmia
- PVAI
pulmonary vein antrum isolation
Footnotes
Disclosures
Dr. Mela is a consultant to Biotronik and has lectured for Medtronic, St. Jude Medical, and Biotronik. Dr. Heist is a consultant to St. Jude Medical; has received research support from Boston Scientific and St. Jude Medical; and has lectured for Biotronik, Boston Scientific, Medtronic, St. Jude Medical, and Sorin. Dr. Verma is a consultant to Biosense Webster, Medtronic, Sanofi Aventis, Astra Zeneca, and Servier; has lectured for Biosense Webster, Medtronic, and St. Jude Medical; and has received research grants from Biosense Webster, Medtronic, and St. Jude Medical. Dr. Singh is a consultant to Biotronik, Boston Scientific, Biosense Webster, CardioInsight, Medtronic, St. Jude Medical, CardioInsight, Thoratec, and the Sorin Group; has lectured for Biotronik, Boston Scientific, Medtronic, St. Jude Medical, and the Sorin Group; and has received research grants from Biotronik, Boston Scientific, Medtronic, and St. Jude Medical.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA: the journal of the American Medical Association. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke; a journal of cerebral circulation. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. The New England journal of medicine. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 5.Hocini M, Sanders P, Jais P, et al. Techniques for curative treatment of atrial fibrillation. Journal of cardiovascular electrophysiology. 2004;15:1467–1471. doi: 10.1046/j.1540-8167.2004.04524.x. [DOI] [PubMed] [Google Scholar]
- 6.Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. Journal of the American College of Cardiology. 2006;48:2340–2347. doi: 10.1016/j.jacc.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Jais P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–2505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 8.Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA: the journal of the American Medical Association. 2005;293:2634–2640. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 9.Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. Journal of the American College of Cardiology. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 10.Estner HL, Hessling G, Ndrepepa G, et al. Acute effects and long-term outcome of pulmonary vein isolation in combination with electrogram-guided substrate ablation for persistent atrial fibrillation. American Journal of Cardiology. 2008;101:332–337. doi: 10.1016/j.amjcard.2007.08.053. [DOI] [PubMed] [Google Scholar]
- 11.Mansour M, Forleo GB, Pappalardo A, et al. Combined use of cryoballoon and focal open-irrigation radiofrequency ablation for treatment of persistent atrial fibrillation: Results from a pilot study. Heart Rhythm. 2010;7:452–458. doi: 10.1016/j.hrthm.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Nair M, Nayyar S, Rajagopal S, Balachander J, Kumar M. Results of radiofrequency ablation of permanent atrial fibrillation of >2 years duration and left atrial size >5 cm using 2-mm irrigated tip ablation catheter and targeting areas of complex fractionated atrial electrograms. American Journal of Cardiology. 2009;104:683–688. doi: 10.1016/j.amjcard.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 13.Oral H, Chugh A, Good E, et al. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation. 2006;113:1824–1831. doi: 10.1161/CIRCULATIONAHA.105.601898. [DOI] [PubMed] [Google Scholar]
- 14.Porter M, Spear W, Akar JG, et al. Prospective study of atrial fibrillation termination during ablation guided by automated detection of fractionated electrograms. Journal of cardiovascular electrophysiology. 2008;19:613–620. doi: 10.1111/j.1540-8167.2008.01189.x. [DOI] [PubMed] [Google Scholar]
- 15.Scharf C, Boersma L, Davies W, et al. Ablation of persistent atrial fibrillation using multielectrode catheters and duty-cycled radiofrequency energy. Journal of the American College of Cardiology. 2009;54:1450–1456. doi: 10.1016/j.jacc.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Y, O’Neill MD, Hocini M, et al. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. Journal of the American College of Cardiology. 2008;51:1003–1010. doi: 10.1016/j.jacc.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 17.Tondo C, Forleo GB, Pappalardo A, et al. A combined cryoenergy and radiofrequency approach to catheter ablation of persistent atrial fibrillation: Preliminary results from a pilot study. Journal of cardiovascular electrophysiology. 2009;20:S27–S28. [Google Scholar]
- 18.Wang X, Liu X, Shi H, et al. Heart rhythm disorders and pacemakers: Pulmonary vein isolation combined with substrate modification for persistent atrial fibrillation treatment in patients with valvular heart diseases. Heart. 2009;95:1773–1783. doi: 10.1136/hrt.2007.124594. [DOI] [PubMed] [Google Scholar]
- 19.Verma A, Mantovan R, Macle L, et al. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF): a randomized, multicentre, international trial. European heart journal. 2010 doi: 10.1093/eurheartj/ehq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Biase L, Elayi CS, Fahmy TS, et al. Atrial fibrillation ablation strategies for paroxysmal patients: randomized comparison between different techniques. Circulation: Arrhythmia and Electrophysiology. 2009;2:113–119. doi: 10.1161/CIRCEP.108.798447. [DOI] [PubMed] [Google Scholar]
- 21.Deisenhofer I, Estner H, Reents T, et al. Does electrogram guided substrate ablation add to the success of pulmonary vein isolation in patients with paroxysmal atrial fibrillation? A prospective, randomized study. Journal of cardiovascular electrophysiology. 2009;20:514–521. doi: 10.1111/j.1540-8167.2008.01379.x. [DOI] [PubMed] [Google Scholar]
- 22.Oral H, Chugh A, Yoshida K, et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. Journal of the American College of Cardiology. 2009;53:782–789. doi: 10.1016/j.jacc.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 23.Elayi CS, Verma A, Di Biase L, et al. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm. 2008;5:1658–1664. doi: 10.1016/j.hrthm.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Verma A, Patel D, Famy T, et al. Efficacy of adjuvant anterior left atrial ablation during intracardiac echocardiography-guided pulmonary vein antrum isolation for atrial fibrillation. Journal of cardiovascular electrophysiology. 2007;18:151–156. doi: 10.1111/j.1540-8167.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 25.Verma A, Novak P, Macle L, et al. A prospective, multicenter evaluation of ablating complex fractionated electrograms (CFEs) during atrial fibrillation (AF) identified by an automated mapping algorithm: acute effects on AF and efficacy as an adjuvant strategy. Heart Rhythm. 2008;5:198–205. doi: 10.1016/j.hrthm.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Lin YJ, Tai CT, Chang SL, et al. Efficacy of additional ablation of complex fractionated atrial electrograms for catheter ablation of nonparoxysmal atrial fibrillation. Journal of cardiovascular electrophysiology. 2009;20:607–615. doi: 10.1111/j.1540-8167.2008.01393.x. [DOI] [PubMed] [Google Scholar]
- 27.Konings KT, Smeets JL, Penn OC, Wellens HJ, Allessie MA. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation. 1997;95:1231–1241. doi: 10.1161/01.cir.95.5.1231. [DOI] [PubMed] [Google Scholar]
- 28.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA: the journal of the American Medical Association. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.