Abstract
BACKGROUND
The HeartMate II is the most frequently used left ventricular assist device (LVAD) in patients with end-stage heart failure. There is a paucity of data regarding its longitudinal cardiac effects, particularly that on diastole.
METHODS
This retrospective study was an evaluation of echocardiograms pre-operatively, post-operatively and at 3, 6 and 12 month intervals in patients with a HeartMate II. Measurements included left ventricle (LV) dimensions, ejection fraction (EF), right ventricle (RV) size and function, parameters of diastolic function, analysis of mitral regurgitation (MR), tricuspid regurgitation (TR), aortic insufficiency (AI) and aortic valve thickening.
RESULTS
Forty-seven patients were evaluated. LV size decreased but EF, RV size and RV function were unchanged. Filling improved with a decrease in central venous pressure (CVP). Right ventricular systolic pressure (RVSP) and diastolic parameters including E/A, deceleration time (DT), pulmonary vein inflow, left atrial size and E/e′ all improved. Ventricular relaxation measured by tissue Doppler (e′) was unchanged. Regarding valve function, MR decreased, TR was unchanged and the aortic valve became increasingly thickened with increased AI severity.
CONCLUSIONS
The HeartMate II unloads the left ventricle shown by decreased LV size, decreased MR and improved filling. Neither systolic function nor diastolic relaxation improves in this cohort of mostly ischemic cardiomyopathy. RV size and function also remained unchanged. The aortic valve shows deterioration with increased thickening and AI likely from valve fusion. These results improve our understanding regarding the effects of the HeartMate II, particularly that on diastole.
Keywords: Left ventricular assist device, heart failure, echocardiography, aortic valve
Second generation continuous-flow left ventricular assist devices (LVADs) are now considered standard of care for patients with end-stage heart failure. Although heart transplantation is typically the goal, organs are often not readily available. Additionally, some patients may fail to meet the requirements necessary to receive this scarce commodity. The HeartMate II (Thoratec Corporation, Pleasanton, CA) is currently the only surgical device which is approved by the United States Food and Drug Administration to be used as a bridge to transplantation or for destination therapy. Increasing usage of these devices has lead not only to improved survival but also improvement in heart failure symptoms and quality of life.1,2 The serial evaluation of the effects on cardiac function, specifically of the HeartMate II, has recently been under review. Echocardiography is the imaging modality most often used as it is non-invasive, widely available and generally produces adequate image quality. Expected changes in ventricular geometry, systolic and diastolic function, hemodynamics and valvular function are critically important to understand, not only to maximize potential benefits of this therapy but also to avoid complications.
It is generally agreed upon that the HeartMate II is a ventricular unloading device. Left ventricular (LV) dimensions have been reported to decrease and both mitral regurgitation and measures of ventricular filling are noted to improve.3-5 Reverse remodeling to the point of systolic or diastolic LV recovery is more controversial and likely depends on the cohort of patients being evaluated. Recovery to the point of explant of an LVAD remains rare although it has been documented that there are notable shifts at the myocyte and gene level towards more normal ventricular function.6 In general, those with ischemic cardiomyopathy that is end-stage and non-ischemic cardiomyopathy that is significantly dilated have shown little in the way of improvements in left ventricular ejection fraction (LVEF) with continuous-flow LVADs.4 Improvements in right ventricular (RV) size and function also remains controversial and depends on the echocardiographic parameter used. Right sided hemodynamics, however, have shown to improve.4,7 Finally, evaluation of the aortic valve and its notable structural and functional changes over time has been of increasing interest. Several published case series, reviews and mock circulatory systems have demonstrated a worsening of aortic insufficiency (AI), enlargement in aortic root dimensions, commissural aortic valve fusion and aortic stenosis. A better understanding of the pathophysiology behind these aortic valve changes is still desired in efforts to prevent potential clinical adverse effects.8-11
Several more recent publications have focused on the utility of transthoraic echocardiography to provide a comprehensive assessment of LVAD function.3,12 The primary aim of this study was to use echocardiography to serially evaluate all patients with a HeartMate II implanted at our institution out to 12 months to help establish the expected cardiac changes in this cohort. Some data has now been published establishing normal values for different echocardiographic measurements in stable outpatients out to 6 months but the total number of patients assessed with a HeartMate II remains small.4 Our goal is to not only further enrich the data available describing these effects but to also help draw conclusions about how best to monitor these patients and manage their devices. An additional goal is to supplement what is known regarding the effects of the HeartMate II on diastolic function, as little has been previously published.
Methods
Patients and Study Design
This was a retrospective chart and echocardiographic review of subjects in whom a HeartMate II left ventricular assist device was implanted between May 2006 and June 2010 at the University of Wisconsin Hospital and Clinics or William S. Middleton Memorial Veterans Hospital in Madison, WI. This study was approved and complied with all regulations of the University of Wisconsin Institutional Review Board and the Veterans Affairs Research & Development Committee. Baseline and demographic data was collected including age, gender, New York Heart Association (NYHA) class, history of diabetes (DM), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD) and history of cardiac surgery or percutaneous intervention. Etiology of heart failure was also collected as well as clinical characteristics assessing the urgency of LVAD placement. Subjects were categorized whether the LVAD was placed as a bridge to transplantation, candidacy or as destination therapy. Finally, LVAD speed, flow, pulsatility index and power were recorded at implantation. The subjects were then followed out to 12 months or until transplant, explant or death. Complications including infection, bleeding, thrombosis, hemolysis, hospitalizations, acute coronary syndrome, arrhythmias and device malfunction were also collected.
Each subject underwent serial comprehensive transthoracic echocardiographic examinations pre-operatively, post-operatively and at 3, 6 and 12 month intervals until transplant, explant or death occurred. Standard two-dimensional and Doppler measurements were performed. In the parasternal long axis view the RV internal dimension, septal wall thickness, LV internal dimension and posterior wall thickness were obtained at end-diastole. At end-systole the LV internal dimension was measured and percent fractional shortening was calculated. In the apical 2 and 4 chamber views LVEF was determined by the Simpson’s area biplane method.13 RV size and function was assessed using all available views and graded semi-quantitatively, 0 (normal), 1 (mild), 2 (moderate) or 3 (severe) which correlates with degrees of dilation or dysfunction respectively. Assessment of right heart hemodynamics was accomplished by estimating central venous pressure (CVP) from the subcostal view of the inferior vena cava and estimating a right ventricular systolic pressure (RVSP) from the maximal tricuspid valve regurgitation (TR) gradient plus the CVP.14
Diastolic parameters were also assessed and included pulsed-wave Doppler measurements of the peak E and A wave mitral inflow velocities measured at the mitral valve tips, early diastolic DT, peak tissue Doppler velocity (e′) measured at the lateral mitral valve annulus and calculated E/A and E/e′. Biplane left atrial volume averaged from the apical 2 and 4 chamber views was collected as was the pattern of pulmonary vein inflow categorized as 0 (normal), 1 (S wave>D wave), 2 (systolic blunting), 3 (systolic reversal). From the above parameters an estimate of diastolic grade was made and classified as 0 (normal), 1 (mild), 2 (moderate), 3 (severe).15
Finally, valvular function of the aortic, mitral and tricuspid valves was investigated. The aortic valve was visualized primarily in the parasternal long and short axis views and assessed semi-quantitatively by the following parameters: systolic opening 0 (none), 1 (intermittent), 2 (mild), 3 (moderate), 4 (normal), degree of insufficiency 0 (none), 1 (mild), 2 (moderate), 3 (severe), degree of thickening 0 (none), 1 (mild), 2 (moderate), 3 (severe) and regurgitant jet orifice 0 (central), 1 (commissural). The vena contracta of AI was also measured in the parasternal long axis view. The mitral and tricuspid valves were imaged in multiple views and assessed semi-quantitatively only for severity of regurgitation 0 (none), 1 (mild), 2 (moderate), 3 (severe).
Statistical Analysis
Categorical, non-parametric variables were analyzed using Kruskal-Wallis analysis of variance and continuous variables were analyzed using ANOVA for repeated measures to detect significant changes during the follow-up period. Outcomes were reviewed with the goal to detect which echocardiographic parameters fail to improve or worsen and to identify those subjects who may do poorly post LVAD implantation. T-test comparisons were made using the last collected echocardiographic parameter between those who died and those who survived.
Results
There were a total of 47 subjects included. One subject received two HeartMate II devices and 3 subjects had received an XVE pulsatile left ventricular assist device prior to their HeartMate II implantation. Four subjects in whom a right ventricular assist device was also implanted were excluded so as not to confound which changes were due to the LVAD alone. Baseline and demographic data are displayed in Table 1.
Table 1.
Baseline Data
| N=47 | ||
|---|---|---|
| Demographics | Male Gender | 39 (83%) |
| Age | 53.9 (+/− 10.4) | |
| Body Mass Index (kg/m2) | 29.2 (+/−6.5) | |
| Chronic Kidney Disease | 22 (47%) | |
| Average Serum Creatinine | 1.6 (+/−0.7) | |
| Chronic Obstructive Pulmonary Disease | 4 (9%) | |
| Diabetes Mellitus | 23 (49%) | |
| Insulin Dependent Diabetes | 12 (26%) | |
| History of Cardiac Surgery | 13 (28%) | |
| Previous Percutaneous Coronary Intervention |
23 (49%) | |
| History of Smoking | 27 (57%) | |
| Clinical Characteristics | Urgent or Emergent | 32 (68%) |
| Acute Coronary Syndrome | 5 (11%) | |
| Balloon Pump | 15 (32%) | |
| Inotrope Use | 25 (53%) | |
| Average NYHA Class | 3.5 (+/− 0.5) | |
| Etiology of Heart Failure | Ischemic Cardiomyopathy | 23 (49%) |
| Non−Ischemic Cardiomyopathy | 23 (49%) | |
| Myocarditis | 1 (2%) | |
| Biventricular Failure | 12 (26%) | |
| Indication for LVAD | Destination Therapy | 6 (13%) |
| Bridge to Transplant | 35 (74%) | |
| Bridge to Recovery | 1 (2%) | |
| Bridge to Candidacy | 5 (11%) | |
| Median Device Readings | Speed (RPM) | 9040 (+/−383) |
| Flow (L/min) | 5 (+/− 0.7) | |
| Pulsatility Index | 4.8 (+/−0.7) | |
| Power (watts) | 6 (+/− 0.7) |
Changes in Ventricle Dimensions and Systolic Function
Serial changes observed after HeartMate II implantation of the left and right ventricle are displayed in Table 2. The changes in chamber dimensions, systolic function and right sided hemodynamics are listed. There was a significant change in LV end-systolic and end-diastolic dimension which was on average decreased by 7.7 mm and 8.4 mm immediately post-operatively respectively and which continued to decrease further throughout the 12 months of follow-up. There was no change noted in LV septal or posterior wall thickness and there was no change noted in the calculated fractional shortening or LVEF. Regarding the right ventricle, there was no observed change in the RV end-diastolic dimension and no change in RV size or function. Hemodynamics of the RV was estimated by CVP which decreased post-operatively but this did not reach significance. The RVSP also decreased and this was significant.
Table 2.
Changes in ventricular dimensions and function.
| Pre-op (46) | Post-op (47) | 3 months (33) | 6 months (25) | 12 months (12) | p-value | |
|---|---|---|---|---|---|---|
|
LV End-
Systolic Dimension (mm) |
62.2 (13.3) | 54.5 (12.7) | 54.3 (13.7) | 54.1 (14.5) | 47.1 (13.5) | 0.003 |
|
LV End-
Diastolic Dimension (mm) |
69.7 (11.5) | 61.4 (12.1) | 61.0 (12.2) | 60.3 (14.6) | 55.2 (10.5) | <0.001 |
|
Septal
Thickness (mm) |
8.0 (2.5) | 8.3 (2.0) | 8.1 (2.0) | 8.9 (2.2) | 7.8 (1.8) | 0.568 |
|
Posterior
Wall Thickness (mm) |
9.1 (2.2) | 9.6 (1.9) | 8.8 (1.5) | 9.0 (1.4) | 9.3 (1.2) | 0.391 |
|
Fractional
Shortening (%) |
0.1 (0.1) | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.1) | 0.2 (0.1) | 0.39 |
|
Biplane
LVEF (%) |
0.2 (0.1) | 0.2 (0.1) | 0. 2 (0.1) | 0.2 (0.1) | 0.2 (0.1) | 0.819 |
|
RV End-
Diastolic Dimension (mm) |
35.5 (7.4) | 34.7 (5.2) | 33.9 (4.8) | 34.1 (5.0) | 37.6 (5.9) | 0.351 |
| RV size | 1.5 (0.7) | 1.7 (0.7) | 1.5 (0.7) | 1.4 (0.8) | 1.7 (0.8) | 0.653 |
| RV function | 1.5 (0.7) | 1.8 (0.8) | 1.6 (0.7) | 1.6 (0.6) | 1.8 (0.7) | 0.342 |
|
CVP
(mmHg) |
11.7 (5.4) | 9.0 (4.5) | 8.9 (5.0) | 8.8 (4.9) | 8.6 (4.5) | 0.096 |
|
RVSP
(mmHg) |
45.6 (11.8) | 30.4 (9.2) | 26.5 (6.4) | 27.1 (8.0) | 32.6 (10.4) | <0.001 |
Data is mean (standard deviation)
At the top of each column (n) = number of subjects available for analysis.
LV = left ventricle, RV = right ventricle, LVEF = left ventricular ejection fraction, CVP = central venous pressure, RVSP = right ventricular systolic pressure
Changes in Measures of Diastole
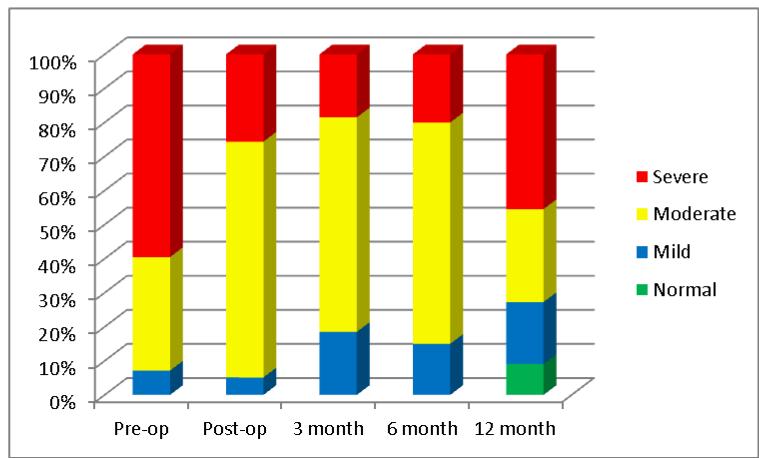
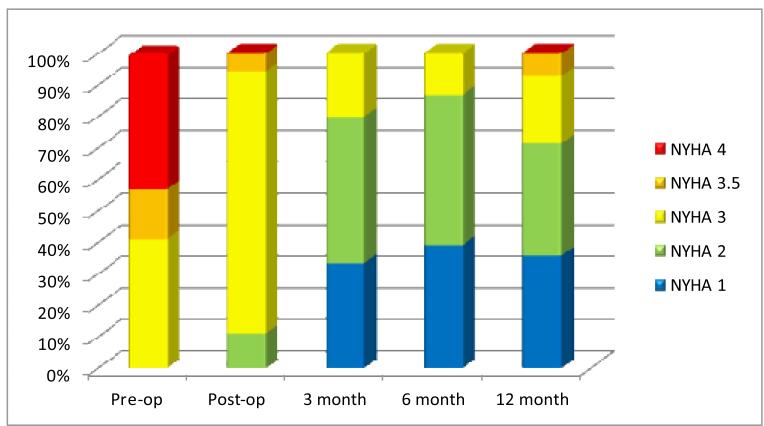
The LV was further assessed by measures of diastole which is depicted in Table 3. Of the 163 total echocardiograms performed, 139 (85%) had measures of diastole performed. The average transmitral Doppler peak E wave velocity decreased post-operatively and the A wave increased; however, neither reached significance. The E/A ratio decreased and the early mitral deceleration time prolonged, both significantly, demonstrating an improvement in overall diastolic filling characteristics. The absolute e′ wave velocity measured by tissue Doppler at the lateral mitral valve annulus which is a correlate of ventricular relaxation did not change throughout the follow-up period. The calculated average E/e′ which is a correlate of ventricular filling pressure decreased initially but this trend was lost over time; therefore, significance was not reached. The pulmonary vein inflow pattern significantly changed from that of systolic blunting pre-operatively to that of an S wave greater then D wave post-operatively, and the left atrial volume decreased significantly throughout the follow-up period, both consistent with improved left sided filling and reduced filling pressures. Finally, an estimate of the severity of diastolic dysfunction from the above parameters was estimated to be between moderate and severe pre-operatively and improved significantly to moderate post-operatively. Thirty eight percent of subjects changed from severe to moderate diastolic grade and this trend was sustained throughout at least 6 months of follow-up with some decline back to severe by 12 months (Figure 1). These measurement findings correlated well with improvements noted in NYHA class as most subjects improved from class 3 or 4 initially to class 2 or better by 3 months post-operatively (Figure 2).
Table 3.
Changes in measures of diastole and grade of diastolic dysfunction.
| Pre-op (46) | Post-op (47) | 3 months (33) | 6 months (25) | 12 months (12) |
p-value | |
|---|---|---|---|---|---|---|
|
E wave
(cm/s) |
87.3 (26.3) | 78.0 (28.0) | 76.6 (32.0) | 79.2 (37.3) | 85.4 (35.2) | 0.543 |
|
A wave
(cm/s) |
40.4 (18.1) | 44.8 (15.9) | 53.3 (22.5) | 56.3 (26.1) | 55.3 (31.0) | 0.056 |
| DT (ms) | 146.3 (46.3) | 181.2 (55.0) | 199.6 (73.0) | 195.2 (83.6) | 166.5 (71.4) | 0.004 |
| E/A ratio | 2.5 (1.4) | 1.7 (0.7) | 1.6 (0.7) | 1.5 (0.9) | 1.9 (0.9) | 0.003 |
|
Lateral e′
(cm/s) |
8.3 (4.0) | 8.2 (3.9) | 7.7 (2.8) | 7.9 (3.2) | 8.0 (2.5) | 0.976 |
| E/e′ ratio | 13.3 (7.6) | 9.5 (3.5) | 11.8 (8.4) | 11.4 (7.7) | 12.2 (7.7) | 0.317 |
|
Pulmonary
Vein Pattern |
2.1 (0.5) | 1.9 (0.5) | 1.6 (0.8) | 1.7 (0.8) | 2.0 (0.5) | 0.039 |
|
Left Atrial
Volume (ml) |
94.7 (33.3) | 62.4 (19.9) | 73.1 (31.9) | 64.9 (24.4) | 63.8 (28.5) | <0.001 |
|
Diastolic
Grade |
2.5 (0.6) | 2.2 (0.5) | 2.0 (0.6) | 2.1 (0.6) | 2.1 (1.0) | 0.005 |
E = peak early diastolic velocity of mitral valve inflow
A = peak late (atrial) diastolic velocity of mitral valve inflow
DT = early deceleration time of mitral valve inflow
e′ = early diastolic tissue Doppler longitudinal velocity of lateral mitral annular motion
Figure 1.
Stacked percentage graph showing progressive changes in the severity of diastolic performance over 12 months. The grade of severity is based on American Society of Echocardiography guidelines.15
Figure 2.
Serial changes in New York Heart Association functional class using a stacked percentage graph over 12 months.
Changes in Valvular Function
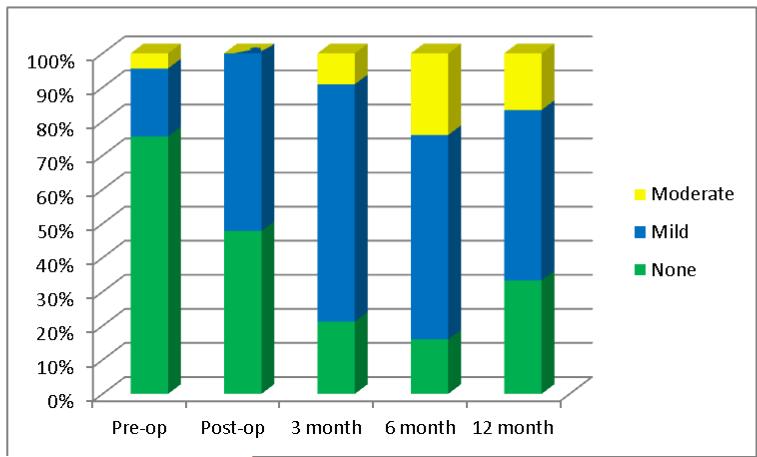
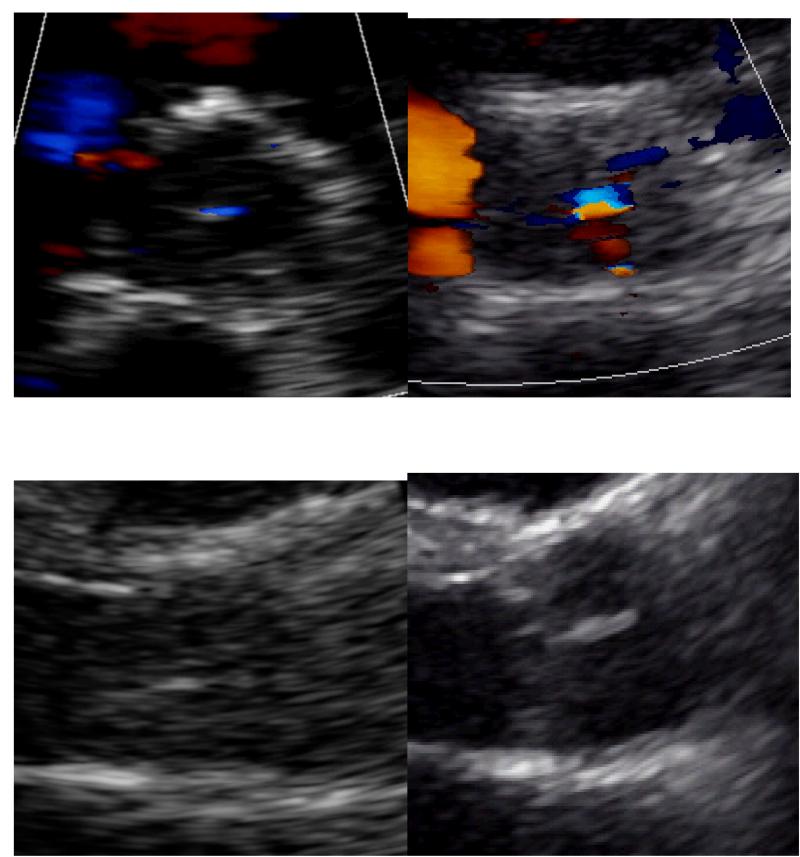
The effects of the HeartMate II on the aortic, mitral and tricuspid valves were also investigated and this is presented in Table 4. The systolic opening motion of the aortic valve significantly decreases from normal to essentially none to intermittent every few beats post implantation. The degree of AI measured semi-quantitatively and quantitatively by vena contracta both increased significantly post-operatively and continued to worsen over time from essentially none to mild. This change in AI during the follow-up period is depicted in Figure 3. AI developed in 25 subjects (53%), worsened in 4 (9%), remained unchanged in 15 (32%) and improved in 3 (6%). The average speed of the LVAD at each interval was recorded and did not change, remaining around 9000 RPMs. As the AI worsened, the degree of aortic valve thickening also increased; however, this trend did not meet significance. It developed in 15 subjects (32%), worsened in 1 (2%) and remained unchanged in 31 (66%). Finally, the regurgitant jet orifice was observed to change from being central to being spread across the commissures in 11 subjects and this was considered significant. An example of the changes observed in the aortic valve is pictured in Figure 4. The degree of mitral and tricuspid valve regurgitation was also assessed semi-quantitatively after HeartMate II implantation. MR improved from moderate to mild post-operatively which was sustained. The degree of TR did not change.
Table 4.
Serial changes in heart valve performance.
| Pre-op (46) |
Post-op (47) | 3 months (33) |
6 months (25) |
12 months (12) |
p- value |
|
|---|---|---|---|---|---|---|
|
Aortic Valve Opening
Motion |
3.6 (1.1) | 0.5 (1.0) | 0.6 (0.8) | 0.5 (0.7) | 0.5 (0.5) | <0.001 |
| Aortic Insufficiency Grade | 0.3 (0.6) | 0.5 (0.5) | 0.9 (0.6) | 1.1 (0.6) | 0.8 (0.7) | <0.001 |
|
Average LVAD Speed
(RPM) |
9040 (383) | 9107 (378) | 9075 (358) | 9043 (369) | ||
|
Vena Contracta of AI
(mm) |
0.7 (1.7) | 1.4 (1.7) | 2.1 (1.6) | 2.8 (1.7) | 2.2 (2.3) | <0.001 |
| Aortic Valve Thickening | 0.5 (0.7) | 0.5 (0.7) | 0.6 (0.7) | 0.7 (0.7) | 0.8 (0.6) | 0.090 |
| Jet Orifice of AI | 0.04 (0.2) | 0.1 (0.3) | 0.2 (0.4) | 0.3 (0.5) | 0.3 (0. 5) | 0.020 |
|
Mitral Insufficiency
Grade |
1.9 (0.9) | 1.0 (0.7) | 1.0 (0.7) | 1.2 (0.8) | 1.2 (0.8) | <0.001 |
|
Tricuspid Insufficiency
Grade |
1.5 (0.7) | 1.4 (0.7) | 1.2 (0.6) | 1.3 (0.8) | 1.7 (0.8) | 0.162 |
LVAD = left ventricular assist device
AI = aortic insufficiency
Figure 3.
Serial changes in severity of aortic valve insufficiency over time using a stacked percentage bar graph.
Figure 4.
Aortic valve pre-operatively and at 6 months. Short-axis image demonstrating the increase in AI and widening of the jet orifice (top panel). Parasternal long-axis image demonstrating the increase in valve thickness (bottom panel).
Complications
Of the 47 patients followed, 30 were transplanted, 9 died, 1 had their device explanted and 1 had their device replaced. Complications encountered within the follow-up period are listed in Table 5. Some patients developed multiple complications. Specifically, the echocardiography parameters of the 9 subjects who died were examined more closely for associations. Three of the 9 subjects died after one year of follow-up and were designated destination therapy at implantation. In the remaining 6 subjects, death occurred early at 3 months or sooner. RV function was worse in those that died early and was on average moderately reduced as compared to between mild and moderately reduced in those who survived (p=0.06). Diastolic function was also worse in those who died early and was on average between moderate and severely abnormal as compared to moderately abnormal in those that survived (p=0.06). Neither of these trends met significance. The only significantly different parameter was interestingly less AI in those that died early (p=0.01), There were no other notable valvular differences between groups.
Table 5.
Outcomes and complications during the follow-up period.
| Outcomes | Transplanted | 30 |
| Death | 9 | |
| Explanted due to Malfunction | 1 | |
| Explanted due to Recovery | 0 | |
| Replaced | 1 | |
| Complications | Infection | 35 |
| Bleeding | 26 | |
| Thrombotic | 11 | |
| Hemolysis | 4 | |
| Hospitalizations | 75 | |
| ACS | 3 | |
| Arrhythmias | 26 | |
| Device Malfunction | 4 |
Discussion
Our serial echocardiographic assessment of HeartMate II recipients corroborates that continuous-flow ventricular assist devices provide excellent ventricular unloading. This is seen by the reduction in end-systolic and end-diastolic LV dimensions, improvement in Doppler parameters of diastole, improvement in left atrial volume, CVP, RVSP and reduction in the degree of MR. What this study further concludes is that the HeartMate II, in primarily end-stage ischemic heart disease patients, unlikely allows for significant reverse remodeling in ventricular function as there were no improvements noted in left ventricular systolic function or relaxation. The lack of improvement in diastolic function, specifically, has not previously been reported in any detail in the literature. Furthermore, the HeartMate II has little effect on the size or function of the RV despite improved right sided hemodynamics. Regarding the effects most notable on the aortic valve, it is apparent that there is a significant reduction in systolic aortic valve opening with subsequent deterioration of the valve leaflets leading to visible commissural thickening and increasing AI. These observed findings help predict the expected cardiac changes after HeartMate II implantation.
Reverse remodeling is defined as improvement in ventricular function after remote myocyte injury. In this cohort of patients reverse remodeling was not observed despite appropriate ventricular unloading of the LV as evidenced by a reduction in LV size and MR. The wall thickness remained unchanged as did the fractional shortening and LVEF. The effects of the HeartMate II on the RV also show improved hemodynamics and unloading with an improved CVP and RVSP; however, unlike the LV both the size and function of the right ventricle remained unchanged. Furthermore, there is no improvement in the degree of TR, unlike the mitral valve which showed improved leaflet coaptation from shrinking of the LV volume. These findings are all consistent with the idea that the LV gets unloaded by the HeartMate II likely to a greater extent than the RV.
There is little prior literature examining the effects of the HeartMate II on parameters of diastole or diastolic function. In this study the Doppler parameters of diastole that improved significantly included E/A, DT and the pulmonary vein inflow pattern; however, the E wave, A wave and E/e′ also trended towards improvement. Each of these changes occur with reduced ventricular filling pressures or unloading. The absolute lateral e′, however, remained virtually unchanged at less than 10, which has been shown to be consistent with abnormal relaxation. Therefore, the improvement in overall diastolic grade from between severe and moderate to moderate is most likely due to the unloading of the left ventricle, not improvement in true diastolic function or relaxation. It is also the unloading of the left ventricle which likely contributes to the notable improvement in NYHA class which correlates with improved diastolic grade (Figures 2 and 3).
The deterioration of the aortic valve observed after HeartMate II implantation has recently been described with multiple postulated mechanisms. The lack of systolic aortic valve motion post implantation likely leads to fibrosis of the aortic valve leaflets which is observed as increased thickening by echocardiography. This fibrosis leads to progressive AI and theoretically if allowed to continue could cause significant clinical effects due to volume overload of the LV. The continuous proximal aortic flow of blood directed towards the aortic valve is also thought to contribute to this deterioration. The speed of the LVAD at each interval did not change and therefore was not felt to be a factor. Few of our subjects were implanted with a HeartMate II as destination therapy; however, the effects on the aortic valve should be considered in anyone who may have a device implanted for a protracted period of time. One might also consider allowing for at least mild opening of the aortic valve leaflets when adjusting the LVAD settings postoperatively so as to avoid the fibrosis cascade.
One final goal of this study was to look for abnormal echocardiographic parameters which might indicate a bad outcome. Of those who died early, RV function and diastolic grade were more abnormal. Both could therefore indicate individuals who might do poorly. These conclusions have to be taken carefully, however, for several reasons. First, there were few patients who died in this study; therefore, these were trends and did not meet significance. Second, there was an unexpected finding of less AI present in those who died, which may indicate that these results were spurious.
Limitations
There are several limitations to this study worth discussing. First, this was a retrospective chart and echocardiographic review of data with a relatively small sample size, which reduces the strength of our conclusions. Few patients remained with an assist device after 12 months of follow-up; therefore, some of the statistical trends appeared to decrease over time. Analysis of those 12 patients alone did not show any new significant findings. Regarding specific echocardiographic measures, diastole with the use of transmitral or tissue Doppler has not been previously validated in subjects with a LVAD. Finally, the assessment of valvular function and right ventricular function in this study was primarily qualitative. Despite these limitations, it should be noted that there are few larger studies with available serial echocardiographic data demonstrating the effects of the HeartMate II on cardiac dimensions, systolic function, relaxation, filling, hemodynamics or valve function. The echocardiographic measures, despite some being qualitative, are current standards of practice and imaging.
Conclusions
In conclusion, the HeartMate II appropriately unloads the LV as evidenced by reduced LV size, MR and improved parameters of diastole. Reverse remodeling does not occur as there are no noted improvements in either LV or RV systolic function. Diastolic function measured by tissue Doppler as a correlate for LV relaxation also does not improve. Furthermore, RV size does not improve despite improved hemodynamics. Regarding the aortic valve, there is a deterioration which may become clinically relevant in patients implanted as destination therapy or in whom may take a long time to transplant. Finally, those with worsening RV function and diastolic grade may have worse outcomes. These observed findings provide a useful documentation of expected changes one should note after HeartMate II implantation and the lack of changes specifically in systolic function and diastolic relaxation.
Reference List
- 1.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Rasalingam R, Johnson SN, Bilhorn KR, Huang PH, Makan M, Moazami N, et al. Transthoracic echocardiographic assessment of continuous-flow left ventricular assist devices. J Am Soc Echocardiogr. 2011;24:135–48. doi: 10.1016/j.echo.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Topilsky Y, Oh JK, Atchison FW, Shah DK, Bichara VM, Schirger JA, et al. Echocardiographic findings in stable outpatients with properly functioning HeartMate II left ventricular assist devices. J Am Soc Echocardiogr. 2011;24:157–69. doi: 10.1016/j.echo.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Weiss RM, Kerber RE, Goerbig-Campbell JL, Davis MK, Cabuay BM, Ashrith G, et al. The impact of prolonged rotary ventricular assist device support upon ventricular geometry and flow kinetics. J Am Soc Echocardiogr. 2011;24:149–56. doi: 10.1016/j.echo.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Burkhoff D, Klotz S, Mancini DM. LVAD-induced reverse remodeling: basic and clinical implications for myocardial recovery. J Card Fail. 2006;12:227–39. doi: 10.1016/j.cardfail.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Lam KM, Ennis S, O’Driscoll G, Solis JM, Macgillivray T, Picard MH. Observations from non-invasive measures of right heart hemodynamics in left ventricular assist device patients. J Am Soc Echocardiogr. 2009;22:1055–62. doi: 10.1016/j.echo.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Cowger J, Pagani FD, Haft JW, Romano MA, Aaronson KD, Kolias TJ. The development of aortic insufficiency in left ventricular assist device-supported patients. Circ Heart Fail. 2010;3:668–74. doi: 10.1161/CIRCHEARTFAILURE.109.917765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pak SW, Uriel N, Takayama H, Cappleman S, Song R, Colombo PC, et al. Prevalence of de novo aortic insufficiency during long-term support with left ventricular assist devices. J Heart Lung Transplant. 2010;29:1172–76. doi: 10.1016/j.healun.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 10.John R, Mantz K, Eckman P, Rose A, May-Newman K. Aortic valve pathophysiology during left ventricular assist device support. J Heart Lung Transplant. 2010;29:1321–29. doi: 10.1016/j.healun.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Tuzun E, Rutten M, Dat M, van d V, Kadipasaoglu C, de Mol B. Continuous-Flow Cardiac Assistance: Effects on Aortic Valve Function in a Mock Loop. J Surg Res. 2010 doi: 10.1016/j.jss.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 12.Estep JD, Stainback RF, Little SH, Torre G, Zoghbi WA. The role of echocardiography and other imaging modalities in patients with left ventricular assist devices. JACC Cardiovasc Imaging. 2010;3:1049–64. doi: 10.1016/j.jcmg.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]