Abstract
A plaque assay system for pathogenic rickettsiae, which utilizes primary chick embryo tissue cultures, is described. It proved to be a highly reproducible measure of infectiousness for Rickettsia rickettsi and R. typhi, which were employed in most studies; as well as for R. canada, R. prowazeki, R. sibirica, R. akari, R. conori, and Coxiella burneti. Plaque-forming units (PFU) were compared to direct rickettsial counts and to 50% infectious dose (ID50) values for embryonated eggs, mice, and guinea pigs. Plaque size, appearance, and number were influenced by diluent, incubation temperature after nutrient overlay, centrifugation of inoculated tissue cultures, and number of host cells planted initially in each flask. The most critical factors in plaque formation were diluent used in making rickettsial suspensions and incubation temperature (32 C) after nutrient overlay. Brain Heart Infusion was the only diluent capable of preventing significant delay in plaque formation and decreases in PFU and mouse ID50. Plaque formation was unaffected by genetic background of host cells, volume of inoculum, temperature and length of incubation period before nutrient overlay, and rapid freezing and thawing of rickettsial seed. Centrifugation of inoculated cultures at 600 × g resulted in 100% irreversible absorption of rickettsiae to host cells within 5 min, whereas without centrifugation at least 4 hr was required to achieve the same effect.
Full text
PDF


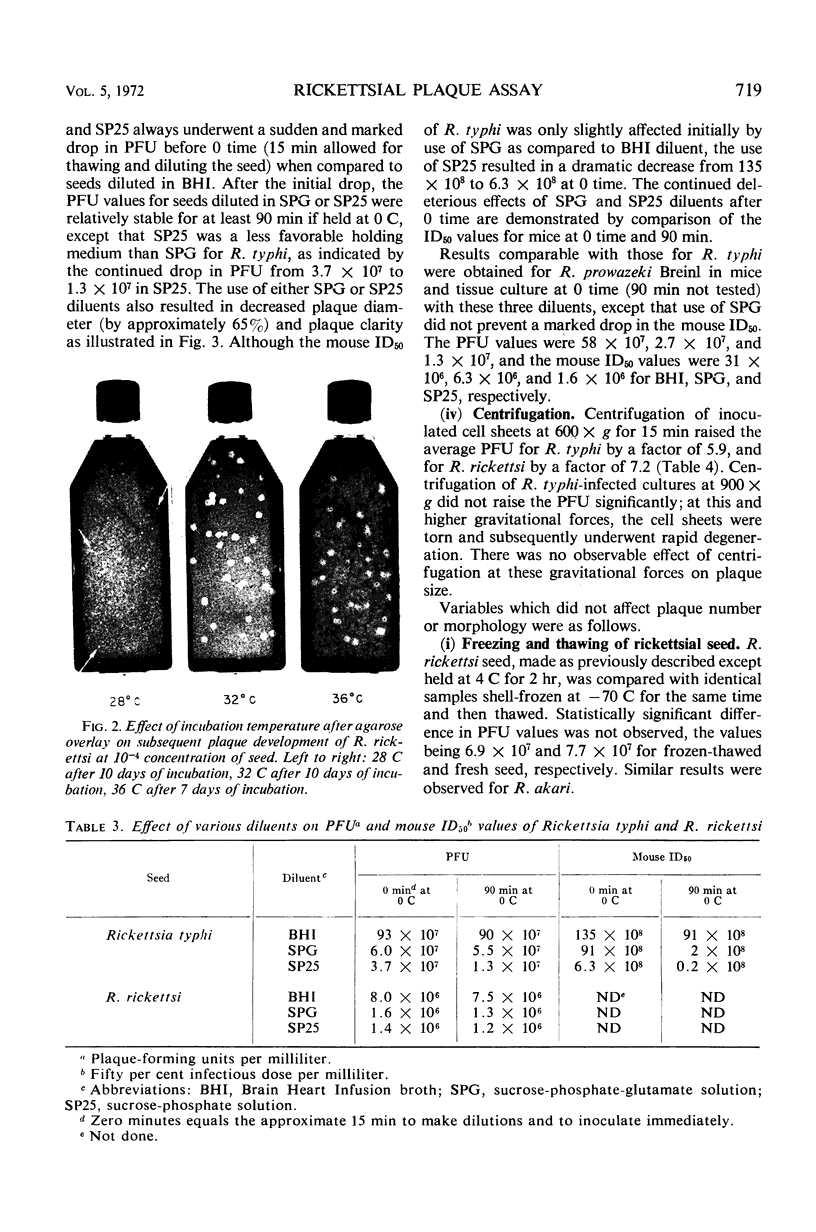
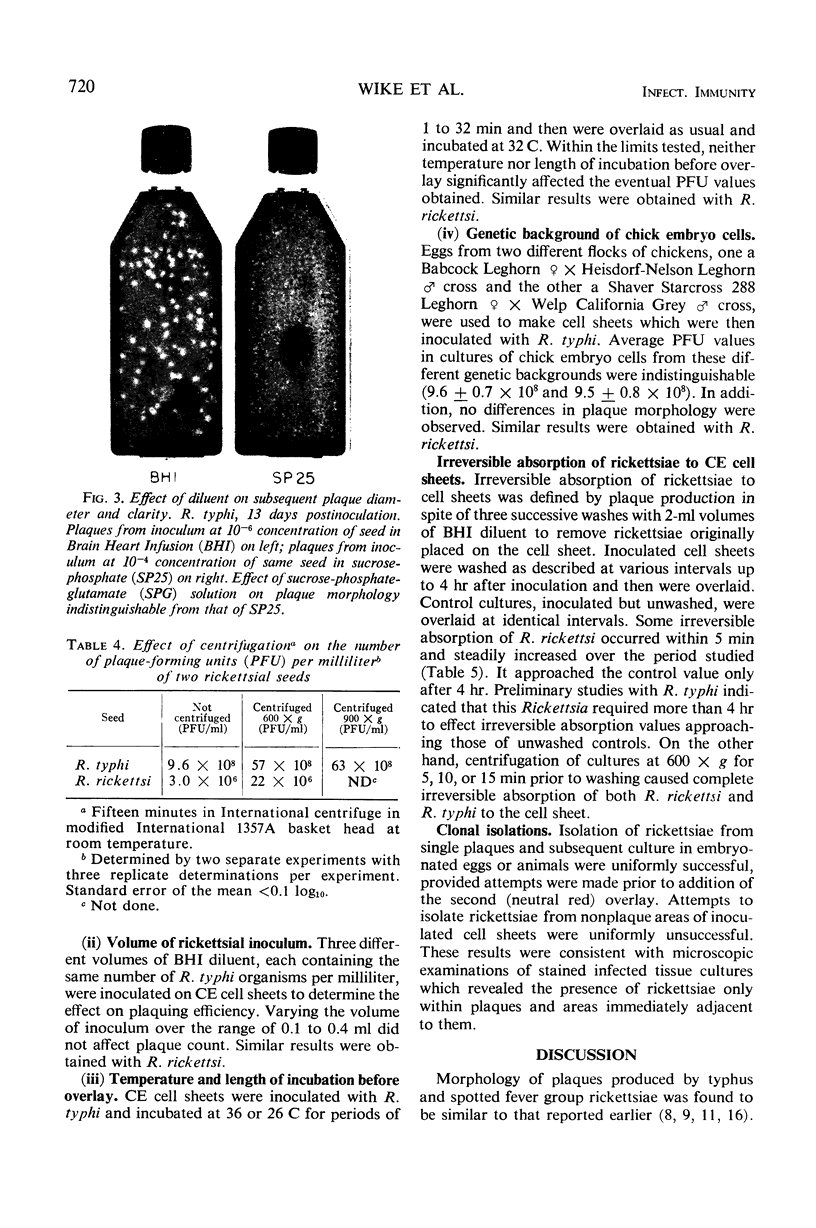


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A., Silberman R., Peacock M., Spielman S. H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969 Jan;13(1):60–66. [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Hahon N., Cooke K. O. Assay of Coxiella burnetii by enumeration of immunofluorescent infected cells. J Immunol. 1966 Oct;97(4):492–497. doi: 10.21236/ad0482256. [DOI] [PubMed] [Google Scholar]
- Kordová N. Plaque assay of rickettsiae. Acta Virol. 1966 May;10(3):278–278. [PubMed] [Google Scholar]
- McDade J. E. Determination of antibiotic susceptibility of Rickettsia by the plaque assay technique. Appl Microbiol. 1969 Jul;18(1):133–135. doi: 10.21236/ad0857810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade J. E., Gerone P. J. Plaque assay for Q fever and scrub typhus rickettsiae. Appl Microbiol. 1970 Jun;19(6):963–965. doi: 10.1128/am.19.6.963-965.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade J. E., Stakebake J. R., Gerone P. J. Plaque assay system for several species of Rickettsia. J Bacteriol. 1969 Sep;99(3):910–912. doi: 10.1128/jb.99.3.910-912.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICKENS E. G., BELL E. J., LACKMAN D. B., BURGDORFER W. USE OF MOUSE SERUM IN IDENTIFICATION AND SEROLOGIC CLASSIFICATION OF RICKETTSIA AKARI AND RICKETTSIA AUSTRALIS. J Immunol. 1965 Jun;94:883–889. [PubMed] [Google Scholar]
- Rees H. B., Jr, Weiss E. Glutamate catabolism of Rickettsia rickettsi and factors affecting retention of metabolic activity. J Bacteriol. 1968 Feb;95(2):389–396. doi: 10.1128/jb.95.2.389-396.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman R., Fiset P. Method for counting Rickettsiae and Chlamydiae in purified suspensions. J Bacteriol. 1968 Jan;95(1):259–261. doi: 10.1128/jb.95.1.259-261.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS E., DRESSLER H. R. Centrifugation and Rickettsiae and viruses onto cells and its effect on infection. Proc Soc Exp Biol Med. 1960 Apr;103:691–695. doi: 10.3181/00379727-103-25637. [DOI] [PubMed] [Google Scholar]
- Weinberg E. H., Stakebake J. R., Gerone P. J. Plaque assay for Rickettsia rickettsii. J Bacteriol. 1969 May;98(2):398–402. doi: 10.1128/jb.98.2.398-402.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]