Abstract
Though expressed in relatively few neurons in insect nervous systems, pigment-dispersing factor (PDF) plays many roles in the control of behavior and physiology. PDF’s role in circadian timekeeping is its best-understood function and the focus of this review. Here we recount the isolation and characterization of insect PDFs, review the evidence that PDF acts as a circadian clock output factor, and discuss emerging models of how PDF functions within circadian clock neuron network of Drosophila, the species in which this peptide’s circadian roles are best understood.
Keywords: Neuropeptide, Circadian, Neuromodulation, Pigment Dispersing Factor, Pigment Dispersing Hormone
1.1: Introduction
Pigment-dispersing factors (PDFs) are highly conserved 18-amino acid, α- amidated neuropeptides [1–3]. Though expressed in relatively few neurons in insect nervous systems [4–12], PDF plays many roles in the control of behavior and physiology, including circadian rhythmicity [13], geotaxis [14], sleep and arousal [15–18], copulation [19], flight [20], the modulation of visceral muscle contraction [21] and tracheal growth [22]. PDF’s role in circadian timekeeping is its bestunderstood function and the focus of this review.
1.2: Isolation and Identification of PDFs As Clock Output Components
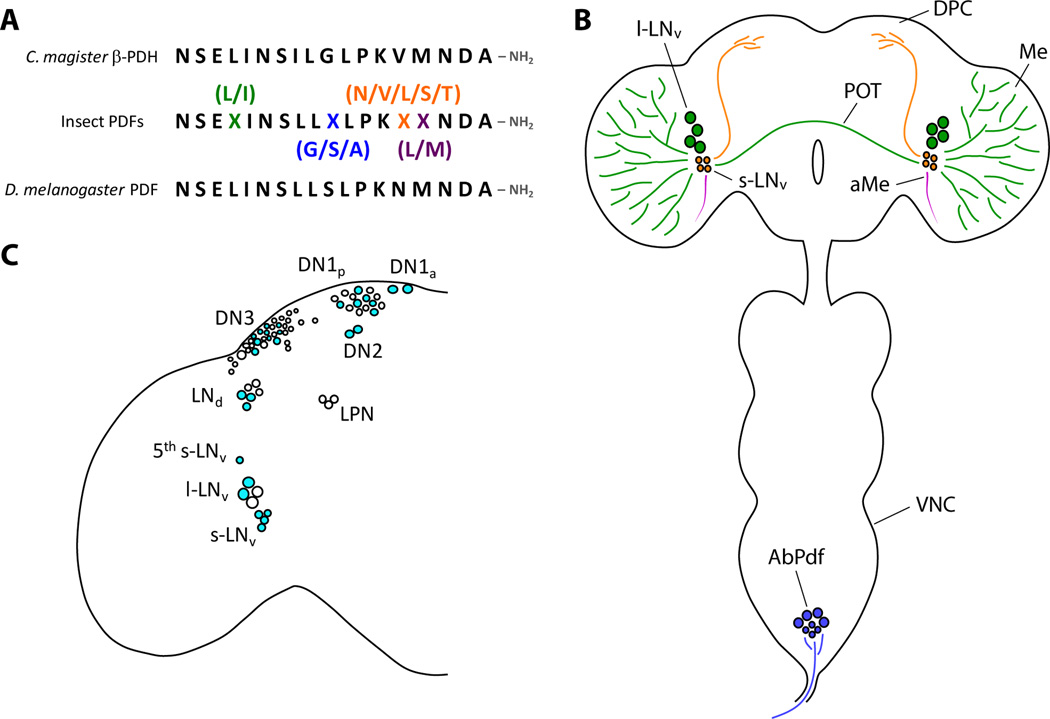
PDFs were first identified based on their similarity to crustacean pigment dispersing hormones (PDHs), which regulate the dispersion and migration of pigment in chromophores and photoreceptors, including daily rhythms of the latter [1,23]. Insect PDFs were first isolated based on their ability to induce pigment dispersion in crustaceans when applied exogenously and have very high sequence similarity to crustacean β-PDHs (Figure 1A) [23]. Insect brains contain small numbers of neurons, approximately six to 20, that are immunoreactive to antisera raised against crustacean PDH (PDHir neurons) [4–12]. Though the numbers and positions of PDHir neurons vary among the insect orders, most species studied contain anterior-ventrally located somata near the accessory medulla, an accessory visual neuropil [4–12]. These neurons typically extend neural processes within the accessory medulla, the optic lobes and the dorsal protocerebrum, as schematized for Drosophila melanogaster in Figure 1B [4–12]. Some species display additional PDHir somata and projections in other regions of the central nervous system, most notably in the tritocerebrum, suboesophageal ganglia, and the corpora cardiaca (e.g., [9,24]).
Figure 1.
(A) The highly conserved sequence of insect PDFs. Insect PDFs have highly similar sequences to crustacean β-PDH hormones. Crustacean nervous systems also contain α-PDF, to which insect PDFs display no homology. Top: the sequence of β-PDH from Cancer magister. Middle: a generalized amino acid sequence for insect PDFs. Variable amino acids are indicated by a colored X with common variants shown above or below the variable amino acid. Bottom: the sequence of PDF from Drosophila melanogaster. (B) A schematic of PDF neuron anatomy in the Drosophila central nervous system. The three classes of PDF neurons, the large ventral lateral neurons (l-LNvs), the small ventral lateral neurons (s-LNvs) and the abdominal PDF neurons (AbPdf) are indicated. The l-LNvs innervate the medullae (Me) of the optic lobes and project across the posterior optic tract (POT). The s-LNvs project to the dorsal protocerebrum (DPC). Both the l-LNvs and s-LNvs project to the accessory medullae (aMe), shown in magenta. The AbPdf neurons reside in the abdominal ganglia of the ventral nerve cord (VNC), project to the viscera and are a likely source of circulating PDF. Note, PDF is also expressed within neurons of the tritocerebrum of adult Drosophila (not shown), though they undergo programed cell death in the days following adult eclosion [13]. (C) A cell body map of clock neurons in the Drosophila brain. The various neuron classes are indicated. PdfR expression is indicated in cyan. PdfR expression in the lateral posterior neurons (LPNs) was not determined.
PDHir neurons with somata situated near the accessory medulla were implicated as circadian pacemakers in orthopteroid insects based on anatomical criteria, physiological observations, and ablation and transplant experiments [25]. The discovery that the PDHir neurons of the Drosophila melanogaster brain express the circadian clock gene period (per) [26] and that these neurons are missing in visual system mutants with weak or absent circadian rhythms further supported the hypothesis that PDHir neurons function as circadian pacemakers in insects [4,27].
The cloning of Pdf from Drosophila melanogaster [2] and subsequently from other insects (e.g., [3,28,29]), made it possible to address the role that this peptide plays in the control of circadian locomotor rhythms using molecular/genetic approaches. Flies bearing the loss-of-function Pdf01 mutation display a syndrome of circadian phenotypes. These mutants are characterized by the loss of the anticipatory morning peak of activity and an advanced evening peak of activity under light/dark (LD) conditions [13]. Pdf01 mutants also display significantly higher levels of arrhythmicity under constant darkness and temperature (DD), indicating that the ability to produce endogenous circadian rhythms is compromised in the absence of PDF [13]. Pdf01 mutants that do display rhythmic locomotion under DD have relatively weak rhythms with significantly shorter periods [13]. The loss of PDF in the fly is also accompanied by an inability to delay the evening peak of activity during long days [30] and the absence of increased nighttime activity in response to nocturnal light [31].
RNA-interference mediated knockdown of Pdf in the cockroach Blattella germanica resulted in significant increases in arrhythmicity in both LD and DD conditions, but had no obvious effects on the period of locomotor rhythms [29]. In contrast, Pdf knockdown in the cricket Gryllus bimaculatus resulted in a shortening of the free-running period but did not result in increases in arrhythmicity under DD conditions [32]. Pdf knockdown also reduced levels of nighttime activity in the cricket, and caused a more rapid resynchronization to shifted LD cycles [32]. Injection of PDH or PDF into the brains of free-running cockroaches and crickets produces dose-dependent phase changes in both insects [33,34]. Thus, PDF is required for normal circadian locomotor rhythms in several insects and likely acts to adjust the period or phase of circadian rhythms. However its specific roles may differ among species.
1.3: Mechanisms of PDF function in the clock neuron network of Drosophila
The circadian functions of PDF are best understood for D. melanogaster, whose clock neuron network can be manipulated with a precision unavailable in other species. This network consists of approximately 150 neurons, which support daily rhythms in clock gene expression and can be divided into nine distinct anatomical classes (Figure 1C) [35–37]. Two classes of clock neurons express PDF, which are readily divisible by anatomical criteria. The four to five large ventral lateral neurons (l-LNvs) residing in each hemisphere project across the medulla of the ipsilateral optic lobe and across the posterior optic tract to the contralateral medulla and accessory medulla (Figure 1B) [4,13,24,38]. The four small ventral lateral neurons (s-LNvs) in each hemisphere ipsilaterally innervate both the accessory medulla and dorsal protocerebrum (Figure 1B) where they reside in proximity to the projections of the other classes of clock neuron [4,13,24,38]. These dorsal projections display diurnal and circadian rhythms in PDF immunoreactivity, consistent with rhythmic PDF release in the dorsal brain [39].
Visualization of clock gene rhythms in various classes of neurons of Pdf01 mutants indicates that some clock neuron classes rely on PDF for the maintenance of coherent and synchronized molecular oscillations, including the PDF-expressing s-LNvs themselves [30,40]. The fact that molecular rhythms within some clock neurons become progressively desynchronized under DD conditions fits well with the observation that locomotor rhythms of Pdf01 mutants are relatively strong during the first few days of DD but weaken progressively over the subsequent circadian cycles [13,40]. Flies lacking functional molecular clocks within the PDF neurons display high levels of arrhythmicity under DD conditions [41,42], indicating that circadian timekeeping within these neurons is critical for the maintenance of endogenous circadian rhythms. Furthermore, speeding up the molecular clock specifically within the PDF neurons shortens the free-running period of locomotor rhythms and speeds-up the molecular clocks within most PDF-negative clock neuron classes [43], an effect that requires PDF signaling [44]. Based on these results, PDF is hypothesized to function as a synchronizing factor within the network of clock neurons, a function remarkably similar to that of vasoactive intestinal polypeptide (VIP) in the clock center of the mammalian brain [45,46].
The identification of PDF’s receptor PdfR (also known as Han) revealed that, like the receptor for VIP, it is a secretin-like G protein-coupled receptor that signals through increases in intracellular cyclic AMP (cAMP) [47–49]. The genetic loss of PdfR results in the same circadian behavioral phenotypes as those described for Pdf01 mutants [47–49]. The observation that the majority of clock neuron classes, including the PDF-expressing s-LNvs but not the l-LNvs, respond to bath-applied PDF peptide with cAMP increases provided further support of the hypothesis that PDF released from the LNvs acts to coordinate the clock neuron network of Drosophila [50]. The rescue of PdfR expression within the clock neuron network of a loss-of-function PdfR mutant was sufficient for the rescue of the mutant’s circadian behavioral phenotypes, further supporting the hypothesis that PDF acts to coordinate and synchronize circadian timekeeping throughout the clock neuron network [47,51]. Furthermore the expression of membrane-tethered PDF peptide in the clock neurons of Pdf01 mutants rescued some aspects of the mutant’s circadian behavior [52], further supporting the hypothesis that PDF’s circadian function is based on its modulation of clock neurons.
Despite the evidence supporting a synchronizing function for PDF within the clock neuron network, other observations suggest a more complex role for PDF in circadian timekeeping. The loss of PDF has differential effects on the molecular clocks within the various clock neuron classes. The posterior dorsal neuron one (DN1p) class appears to require PDF for the maintenance of molecular rhythms [30], whereas the dorsal lateral neurons (LNds) do not [30,40]). Strikingly, PDF has opposing effects on the synchrony of molecular rhythms within different clock neuron classes. For example, under free-running conditions the s-LNvs become phase-dispersed with respect to their molecular rhythms in the absence of PDF [40], whereas the molecular clocks of the LNds display increased synchrony in the absence of PDF [30].
The short period phenotypes of the rhythmic subsets of Pdf and PdfR mutants suggest that PDF normally acts to lengthen the period of the circadian clock [13,47–49]. However, when PDF levels are increased in the dorsal protocerebrum through the ectopic expression of Pdf, the free-running period of locomotor rhythms is not simply lengthened [53,54]. Though Pdf overexpression is associated with increases in the period of locomotor rhythms in some individuals, it also causes an increase in the incidence of arrhythmicity and internal desynchronization, a condition marked by the presence of multiple periodicities in individual flies [53,54]. Such PDF-induced behavioral desynchrony was accompanied by a loss of synchrony among the clock neuron classes, with the s-LNvs along with a subset of dorsal neurons (DNs) displaying short-period molecular rhythms, with other DNs and a subset of the LNds displaying longer-period rhythms [30]. Similar effects are observed when PDF neurons are constitutively hyperexcited through the directed expression of a modified Na+ channel [55]. Furthermore, when PdfR is constitutively activated specifically in the s-LNvs using a membrane-tethered PDF peptide, the behavioral output of these neurons (the anticipatory morning peak of activity) was phase-advanced, consistent with an accelerated molecular clock in these neurons [56]. These results indicate that PDF has differential effects on neuronal oscillators, increasing the period of some while decreasing the period of others, at least when PDF is overexpressed or PdfR is constitutively activated.
1.4: Network Properties of PDF function in Drosophila
The results outlined in the preceding two paragraphs indicate that PDF has several and in some cases opposing effects within the clock neuron network. The nature of PdfR expression within the network provides a partial explanation for these findings. Because specific antisera for the immunocytochemical visualization of PdfR expression do not exist and the PdfR gene is too large for standard transgenic methods of expression mapping, Im and Taghert (2010) created flies in which the entire PdfR gene was introduced with a C-terminal MYC tag into loss-offunction PdfR mutants [57]. This large (~70kB) construct (PdfR-MYC) fully rescues the behavioral phenotypes of PdfR mutants and allows PdfR-MYC expression to be assayed using anti-MYC immunocytochemistry [57]. This expression mapping revealed that the clock neuron network is mosaic for PdfR expression, with only approximately half of the neurons expressing the receptor [57] (Figure 1C). PdfR expression is not uniform within most clock neurons classes, for example, only half the LNds and less than half the DN1p and DN3 neurons expressed detectable levels of PdfR-MYC [57]. Live imaging experiments using cAMP sensor specifically expressed within the PdfR-positive and -negative LNds revealed that PdfR-expressing LNds but not PdfR-negative LNds, respond to bath-applied PDF [44]. Thus, only subsets of clock neurons are receptive to PDF. PdfR expression is closely associated with the expression of cryptochrome (cry), a blue light circadian photoreceptor [58,59], and flies lacking both PdfR and cry function display severe circadian phenotypes [59–61].
The mosaic nature of PdfR expression in the clock neuron network may partially explain some of the findings outlined above. For example, the internal desynchronization observed when PDF levels are increased in the dorsal protocerebum [30,53,54] likely reflects desynchronization between the PdfR-positive and PdfR-negative clock neurons. Furthermore, the fact that only half of the LNds express PdfR [57] may explain why their molecular oscillations are more synchronous in the absence of PDF [30], as PDF could normally act to desynchronize these two types of LNds. Thus, PDF could act as a synchronizing factor among PdfR-expressing neurons while acting to desynchronize classes composed of PdfR-positive and -negative clock neurons. In support of this hypothesis, synchronization of PDF-negative neurons to experimentally slowed PDF neurons requires PdfR and neurons lacking PdfR expression do not synchronize to slowed PDF neurons [44]. Furthermore, the strength of molecular clock coupling to PDF neurons varies among PdfR-expressing neurons, with some clock neurons being strongly coupled and others showing relatively weak coupling to PDF neuron clocks [44]. In fact, when the speed of the molecular clock within the PDF neurons is specifically altered, the presence of a physiological connection between PDF neurons and their PdfRexpressing targets does not always insure the coupling of their molecular clocks [44]. Thus, the efficacy of PDF mediated synchronization likely varies even among clock neurons expressing PdfR.
1.5: PDF as a circulating peptide hormone
In addition to the clock-associated PDF neurons of the central brain, four to eight PDF neurons are present in the posterior abdominal segments of Drosophila’s ventral nerve chord (AbPdf neurons) (Figure 1C) [4,5,24], a pattern of expression that is also found in the locust Locusta migratoria [7], the blowfly Phormia terraenovae [5], and the cabbage fly Delia radicum [62]. In adult Drosophila, these neurons project out of ventral nerve cord and terminate superficially along the surface of the rectum and at the junction of the posterior midgut, anterior hindgut and the ureters [5,21]. PDF likely serves as a circulating peptide hormone in insects. Some insects appear to release PDH from the corpora cardiaca, the brain’s neurohemal organ [9] and the AbPdf neurons are strong candidates as a source of circulating PDF [7], The AbPdf neurons do not express the molecular clock and PDF from these neurons is not required for normal locomotor rhythms in Drosophila [63]. However circulating PDF adjusts the phase of the molecular clock in oenocytes, the peripheral sites of pheromone production in the fly [64]. Interestingly, the normal phasing of the oenocyte molecular clock requires PDF in both the LNvs and the AbPDF neurons [64]. Thus, circulating PDF appears to influence at least some non-neuronal peripheral clocks in Drosophila.
1.6: The Cellular Basis of PDF Signaling
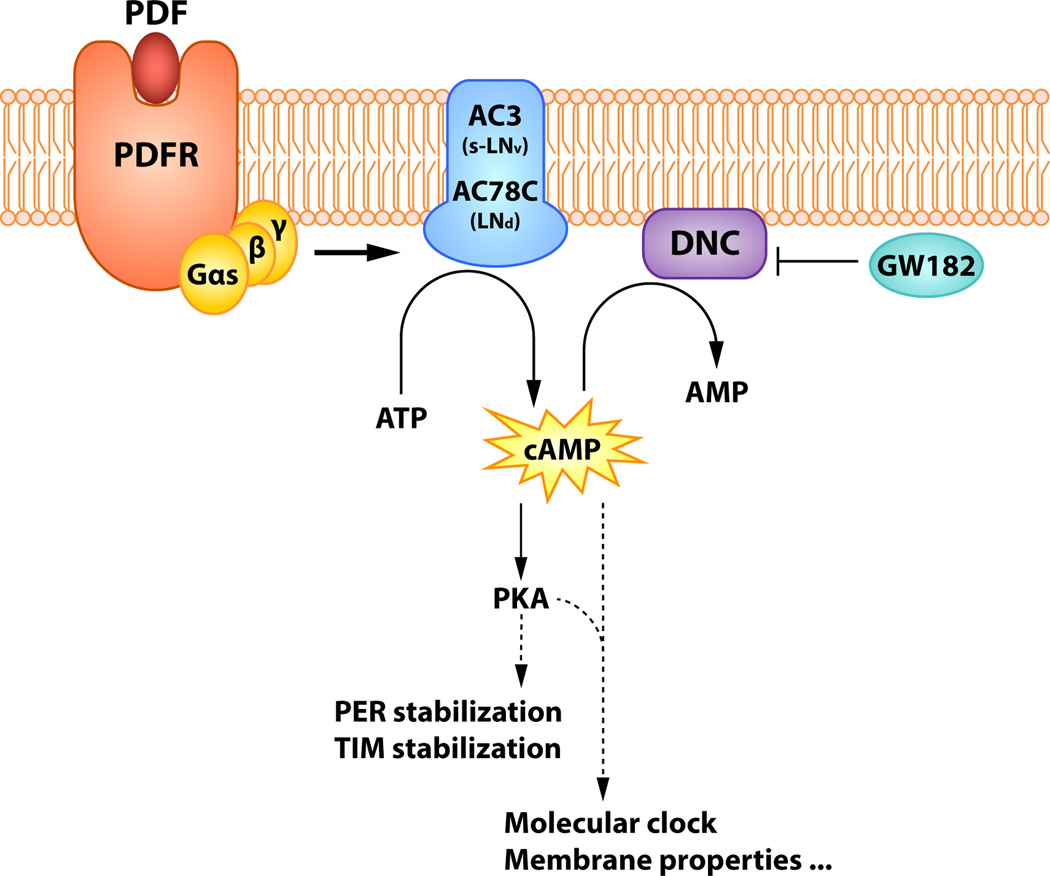
Moasic expression of PdfR among clock neurons may not fully explain the varied effects of PDF within the circadian system. How can the same peptide functioning through the same G protein-coupled receptor have opposing effects on different clock neuron targets? One possible explanation comes from the discovery that different clock neuron classes utilize different adenylyl cyclases (ACs) for PdfR signaling. In the s-LNvs PdfR signaling produces cAMP increases through AC3, while AC78C (an ortholog of mammalian AC8) and at least one additional unidentified adenylyl cyclase are used in the PdfR-expressing LNds [65,66]. These findings indicate that clock neurons differ in PdfR signalosome composition, leading to the hypothesis that distinct signalosomes transduce cAMP increases into distinct or perhaps even opposing effects on the molecular clocks of PDF-receptive neurons [66].
The PdfR signaling pathway in clock neurons has been partially revealed and is summarized in Figure 2. Upon binding to PDF peptide, PdfR activates Gαs (also known as Gsα60A) [56,67], resulting in activation of ACs and the production of cAMP [49,50]. This action is opposed by the phosphodiesterase Dunce, whose abundance is regulated by GW182, a protein essential for microRNA-mediated gene silencing [67].
Figure 2.
A schematic representation of the PdfR signaling pathway. PdfR signals through Gαs and activates distinct adenylyl cyclases (ACs) in different classes of PdfR-expressing clock neurons. The action of PdfR is opposed by the phosphodiesterase Dunce (DNC), the abundance of which is regulated by GW182, a protein essential for microRNA-mediated gene silencing. Increases of cAMP levels stimulate protein kinase A (PKA), which leads to the stabilization of PERIOD (PER) protein, thereby adjusting the molecular clock. Please see section 1.6 for a full description.
How do PdfR-induced cAMP increases eventually lead to changes in the phase or amplitudes of molecular clocks and/or changes in the membrane properties of target neurons? Recent work has established that PdfR-induced cAMP increases activate protein kinase A (PKA) in clock neurons [68,69]. PKA signaling enhances the stability of the Period (PER) and Timeless (TIM) protein, resulting in changes in the phase and/or period of the molecular clocks within target clock neurons [68,69]. It is interesting to note that the Drosophila cyclic-AMP response element binding protein B (dCREB2), a well-established mediator of cAMP/PKA pathway, interacts with the core molecular clock feedback loop [70]. Furthermore, a mutation of dCREB2 produces circadian locomotor phenotypes remarkably similar to those of Pdf or PdfR mutants [70]. Thus, dCREB2 is a clear candidate component of PdfR signalosomes.
Though PdfR is thought to signal predominantly through increases of cAMP, it can produce changes in Ca2+ [49,69]. Though the acute excitation of PDF neurons does not result in measureable Ca2+ changes in PdfR-expressing LNds [71], focal application of PDF does cause small Ca2+ increases in a subset of the DN1p class of clock neurons [69]. Furthermore, PdfR has recently been identified as an IP3/Ca2+ coupled receptor required for the maintenance of flight [20]. Thus, it appears that PdfR signaling causes Ca2+ increases at least in some PdfR-expressing neurons, including some clock neurons [69,72]. Finally, PDF signaling causes depolarization in some neurons, including the DN1ps, which display increased firing rates in response to the focal application of the peptide [69].
1.7: A summary of PDF functions in the Drosophila nervous system
The limited expression of PDF within the Drosophila nervous system, along with highly specific molecular genetic tools for the manipulation of PDF- and PdfR-expressing neurons in the fly, have allowed researchers to identify diverse functions for this peptide. The many circadian and non-circadian roles played by PDF likely reflect the existence of diverse sets of PDF targets (Figure 3) and multiple modes of signaling downstream of PdfR. The targets of PDF are likely to reside in both the central nervous system and peripheral tissues and to include both clock and nonclock cells [21,57,64,73]. It seems clear that the study of PDF, its cellular targets, its modes of signaling, and its behavioral and physiological functions will continue to enrich our understanding of neuromodulatory control for many years to come.
Figure 3.
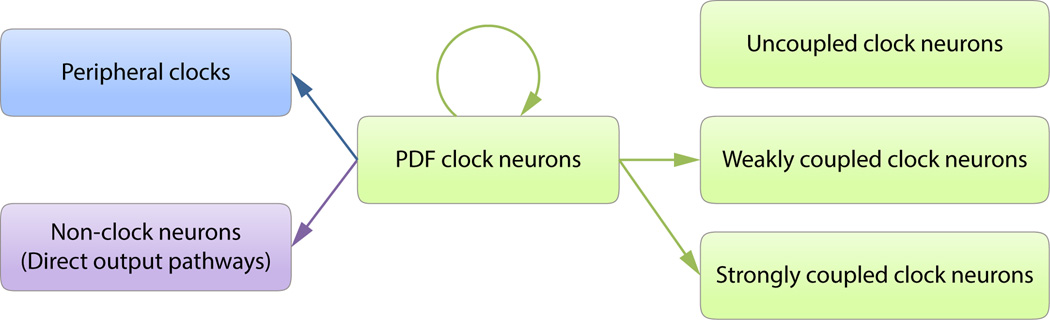
A summary of PDF function in the circadian system of Drosophila. PDF-negative clock neurons are variably coupled to PDF-expressing clock neurons, with some PdfR-expressing neurons strongly coupled and others more weakly coupled to the molecular clocks of PDF neurons. Other clock neurons do not express PdfR and do not appear to be physiologically connected to PDF neurons. PDF also modulates neurons that do not express the molecular clock and these neurons may represent output targets of the circadian system. Finally, circulating PDF, coordinates molecular clocks in peripheral tissues and mediates non-circadian functions of PDF. Please see sections 1.5 and 1.7 for details.
Highlights.
We recount the isolation and characterization of insect pigment-dispersing factors.
We discuss the expression of pigment-dispersing factor within insect central nervous systems.
We review the evidence that pigment-dispersing factor acts as a circadian clock output factor in insects.
We present a model of pigment-dispersing factor function within the circadian clock neuron network of Drosophila.
Acknowledgments
We thank Michael Rosbash for communicating results before publication and Paul Taghert, Charlotte Helfrich-Förster, Justin Blau, and Michael Rosbash for insightful discussions about PDF function. We are also grateful to an anonymous reviewer whose critique of a draft of this resulted in a significantly improved manuscript. We acknowledge a great volume of excellent work that we were not able to include due to the space constraints of this review. The authors were supported by NIH (NINDS) grants R00NS062953 and R01NS077933 to O. T. S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicting interests.
References
- 1.Rao KR, Reihm JP. The Pigment-Dispersing Hormone Family: Chemistry, Structure-Activity Relations, and Distribution. The Biological Bulletin. 1989;177:225–229. [Google Scholar]
- 2.Park JH, Hall JC. Isolation and Chronobiological Analysis of a Neuropeptide Pigment-Dispersing Factor Gene in Drosophila melanogaster. Journal of Biological Rhythms. 1998;13:219–228. doi: 10.1177/074873098129000066. [DOI] [PubMed] [Google Scholar]
- 3.Matsushima A, Sato S, Chuman Y, Takeda Y, Yokotani S, Nose T, Tominaga Y, Shimohigashi M, Shimohigashi Y. cDNA cloning of the housefly pigment-dispersing factor (PDF) precursor protein and its peptide comparison among the insect circadian neuropeptides. Journal of Peptide Science. 2004;10:82–91. doi: 10.1002/psc.511. [DOI] [PubMed] [Google Scholar]
- 4.Helfrich-Förster C, Homberg U. Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. The Journal of Comparative Neurology. 1993;337:177–190. doi: 10.1002/cne.903370202. [DOI] [PubMed] [Google Scholar]
- 5.Nässel DR, Shiga S, Mohrherr CJ, Rao KR. Pigment-dispersing hormone-like peptide in the nervous system of the flies Phormia and Drosophila: Immunocytochemistry and partial characterization. The Journal of Comparative Neurology. 1993;331:183–198. doi: 10.1002/cne.903310204. [DOI] [PubMed] [Google Scholar]
- 6.Stengl M, Homberg U. Pigment-dispersing hormone-immunoreactive neurons in the cockroach Leucophaea maderae share properties with circadian pacemaker neurons. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1994;175:203–213. doi: 10.1007/BF00215116. [DOI] [PubMed] [Google Scholar]
- 7.Persson MGS, Eklund MB, Dircksen H, Muren JE, Nässel DR. Pigment-dispersing factor in the locust abdominal ganglia may have roles as circulating neurohormone and central neuromodulator. Journal of Neurobiology. 2001;48:19–41. doi: 10.1002/neu.1040. [DOI] [PubMed] [Google Scholar]
- 8.Sato S, Chuman Y, Matsushima A, Tominaga Y, Shimohigashi Y, Shimohigashi M. A Circadian Neuropeptide, Pigment-Dispersing Factor–PDF, in the Last-Summer Cicada Meimuna opalifera: cDNA Cloning and Immunocytochemistry. Zoological Science. 2002;19:821–828. doi: 10.2108/zsj.19.821. [DOI] [PubMed] [Google Scholar]
- 9.Sehadová H, Sauman I, Sehnal F. Immunocytochemical distribution of pigment-dispersing hormone in the cephalic ganglia of polyneopteran insects. Cell and Tissue Research. 2003;312:113–125. doi: 10.1007/s00441-003-0705-5. [DOI] [PubMed] [Google Scholar]
- 10.Závodská R, Šauman I, Sehnal F. Distribution of PER Protein, Pigment-Dispersing Hormone, Prothoracicotropic Hormone, and Eclosion Hormone in the Cephalic Nervous System of Insects. Journal of Biological Rhythms. 2003;18:106–122. doi: 10.1177/0748730403251711. [DOI] [PubMed] [Google Scholar]
- 11.Wei H, el Jundi B, Homberg U, Stengl M. Implementation of pigment-dispersing factor-immunoreactive neurons in a standardized atlas of the brain of the cockroach Leucophaea maderae. The Journal of Comparative Neurology. 2010;518:4113–4133. doi: 10.1002/cne.22471. [DOI] [PubMed] [Google Scholar]
- 12.Vafopoulou X, Terry KL, Steel CGH. The circadian timing system in the brain of the fifth larval instar of Rhodnius prolixus (hemiptera) The Journal of Comparative Neurology. 2010;518:1264–1282. doi: 10.1002/cne.22274. [DOI] [PubMed] [Google Scholar]
- 13. Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf Neuropeptide Gene Mutation and Ablation of PDF Neurons Each Cause Severe Abnormalities of Behavioral Circadian Rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. A technical turning-point in the field and a centrally important study establishing PDF as a critical neuroptide for circadian timekeeping in Drosophila.
- 14.Toma DP, White KP, Hirsch J, Greenspan RJ. Identification of genes involved in Drosophila melanogaster geotaxis, a complex behavioral trait. Nat Genet. 2002;31:349–353. doi: 10.1038/ng893. [DOI] [PubMed] [Google Scholar]
- 15.Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou Y-T, Sharma VK, Holmes TC. Large Ventral Lateral Neurons Modulate Arousal and Sleep in Drosophila. Current Biology. 2008;18:1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang Y, Griffith L, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proceedings of the National Academy of Sciences U.S.A. 2008;105:19587–19594. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJL, Kang K, Liu X, Garrity PA, Rosbash M, et al. PDF Cells Are a GABA-Responsive Wake-Promoting Component of the Drosophila Sleep Circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung BY, Kilman VL, Keath JR, Pitman JL, Allada R. The GABAA Receptor RDL Acts in Peptidergic PDF Neurons to Promote Sleep in Drosophila. Current biology : CB. 2009;19:386–390. doi: 10.1016/j.cub.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim WJ, Jan LY, Jan YN. A PDF/NPF Neuropeptide Signaling Circuitry of Male Drosophila melanogaster Controls Rival-Induced Prolonged Mating. Neuron. 2013;80:1190–1205. doi: 10.1016/j.neuron.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agrawal T, Sadaf S, Hasan G. A Genetic RNAi Screen for IP3/Ca2+ Coupled GPCRs in Drosophila Identifies the PdfR as a Regulator of Insect Flight. PLoS Genet. 2013;9:e1003849. doi: 10.1371/journal.pgen.1003849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talsma AD, Christov CP, Terriente-Felix A, Linneweber GA, Perea D, Wayland M, Shafer OT, Miguel-Aliaga I. Remote control of renal physiology by the intestinal neuropeptide pigment-dispersing factor in Drosophila. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1200247109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linneweber G, Jacobson J, Busch KE, Hudry B, Christov CP, Dormann D, Yuan M, Otani T, Knust E, de Bono M, et al. Neuronal Control of Metabolism through Nutrient-Dependent Modulation of Tracheal Branching. Cell. 2014;156:69–83. doi: 10.1016/j.cell.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao KR, Riehm JP. Pigment-Dispersing Hormonesa. Annals of the New York Academy of Sciences. 1993;680:78–88. doi: 10.1111/j.1749-6632.1993.tb19676.x. [DOI] [PubMed] [Google Scholar]
- 24.Helfrich-Förster C. Development of pigment-dispersing hormoneimmunoreactive neurons in the nervous system of<I>Drosophila melanogaster</I>. The Journal of Comparative Neurology. 1997;380:335–354. doi: 10.1002/(sici)1096-9861(19970414)380:3<335::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Stengl M, Homberg U. Pigment-dispersing hormone-immunoreactive neurons in the cockroach Leucophaea maderae share properties with circadian pacemaker neurons. Journal of Comparative Physiology A. 1994;175:203–213. doi: 10.1007/BF00215116. [DOI] [PubMed] [Google Scholar]
- 26.Helfrich-Förster C. The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proceedings of the National Academy of Sciences U.S.A. 1995;92:612–616. doi: 10.1073/pnas.92.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helfrich-Förster C. Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brainbehavioral study of disconnected mutants. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1998;182:435–453. doi: 10.1007/s003590050192. [DOI] [PubMed] [Google Scholar]
- 28.Chuman Y, Matsushima A, Sato S, Tomioka K, Tominaga Y, Meinertzhagen IA, Shimohigashi Y, Shimohigashi M. cDNA Cloning and Nuclear Localization of the Circadian Neuropeptide Designated as Pigment-Dispersing Factor PDF in the Cricket Gryllus bimaculates. Journal of Biochemistry. 2002;131:895–903. doi: 10.1093/oxfordjournals.jbchem.a003180. [DOI] [PubMed] [Google Scholar]
- 29.Lee C-M, Su M-T, Lee HJ. Pigment Dispersing Factor: An Output Regulator of the Circadian Clock in the German Cockroach. Journal of Biological Rhythms. 2009;24:35–43. doi: 10.1177/0748730408327909. [DOI] [PubMed] [Google Scholar]
- 30.Yoshii T, Wülbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, Helfrich-Förster C. The Neuropeptide Pigment-Dispersing Factor Adjusts Period and Phase of Drosophila's Clock. The Journal of Neuroscience. 2009;29:2597–2610. doi: 10.1523/JNEUROSCI.5439-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helfrich-Förster C. Does the Morning and Evening Oscillator Model Fit Better for Flies or Mice? J Biol Rhythms. 2009;24:259–270. doi: 10.1177/0748730409339614. [DOI] [PubMed] [Google Scholar]
- 32.Hassaneen E, El-Din Sallam A, Abo-Ghalia A, Moriyama Y, Karpova SG, Abdelsalam S, Matsushima A, Shimohigashi Y, Tomioka K. Pigment-Dispersing Factor Affects Nocturnal Activity Rhythms, Photic Entrainment, and the Free-Running Period of the Circadian Clock in the Cricket Gryllus bimaculatus. Journal of Biological Rhythms. 2011;26:3–13. doi: 10.1177/0748730410388746. [DOI] [PubMed] [Google Scholar]
- 33.Petri B, Stengl M. Pigment-Dispersing Hormone Shifts the Phase of the Circadian Pacemaker of the Cockroach Leucophaea maderae. The Journal of Neuroscience. 1997;17:4087–4093. doi: 10.1523/JNEUROSCI.17-11-04087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singaravel M, Fujisawa Y, Hisada M, Saifullah ASM, Tomioka K. Phase Shifts of the Circadian Locomotor Rhythm Induced by Pigment-Dispersing Factor in the Cricket Gryllus bimaculatus. Zoological Science. 2003;20:1347–1354. doi: 10.2108/zsj.20.1347. [DOI] [PubMed] [Google Scholar]
- 35.Ewer J, Frisch B, Hamblen-Coyle M, Rosbash M, Hall J. Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cells' influence on circadian behavioral rhythms. J. Neurosci. 1992;12:3321–3349. doi: 10.1523/JNEUROSCI.12-09-03321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaneko M, Hall JC. Neuroanatomy of cells expressing clock genes in Drosophila: Transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. The Journal of Comparative Neurology. 2000;422:66–94. doi: 10.1002/(sici)1096-9861(20000619)422:1<66::aid-cne5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Shafer OT, Helfrich-Förster C, Renn SCP, Taghert PH. Reevaluation of Drosophila melanogaster's neuronal circadian pacemakers reveals new neuronal classes. The Journal of Comparative Neurology. 2006;498:180–193. doi: 10.1002/cne.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helfrich-Förster C, Shafer OT, W¸lbeck C, Grieshaber E, Rieger D, Taghert P. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. The Journal of Comparative Neurology. 2007;500:47–70. doi: 10.1002/cne.21146. [DOI] [PubMed] [Google Scholar]
- 39.Park JH, Helfrich-Förster C, Lee G, Liu L, Rosbash M, Hall JC. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proceedings of the National Academy of Sciences. 2000;97:3608–3613. doi: 10.1073/pnas.070036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin Y, Stormo GD, Taghert PH. The Neuropeptide Pigment-Dispersing Factor Coordinates Pacemaker Interactions in the Drosophila Circadian System. J. Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 42.Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 43.Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- 44. Yao Z, Shafer OT. The Drosophila Circadian Clock Is a Variably Coupled Network of Multiple Peptidergic Units. Science. 2014;343:1516–1520. doi: 10.1126/science.1251285. This study establishes that the various clock neuron classes likely display a variety of PdfR mediated clock coupling strengths and that PDF mediated physiological connection do not always ensure the coupling of molecular clocks.
- 45.Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- 46.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hyun S, Lee Y, Hong S-T, Bang S, Paik D, Kang J, Shin J, Lee J, Jeon K, Hwang S, et al. Drosophila GPCR Han Is a Receptor for the Circadian Clock Neuropeptide PDF. 2005;48:267–278. doi: 10.1016/j.neuron.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 48.Lear BC, Merrill CE, Lin J-M, Schroeder A, Zhang L, Allada R. A G Protein-Coupled Receptor, groom-of-PDF, Is Required for PDF Neuron Action in Circadian Behavior. 2005;48:221–227. doi: 10.1016/j.neuron.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. PDF Receptor Signaling in Drosophila Contributes to Both Circadian and Geotactic Behaviors. Neuron. 2005;48:213–219. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread Receptivity to Neuropeptide PDF throughout the Neuronal Circadian Clock Network of Drosophila Revealed by Real-Time Cyclic AMP Imaging. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lear BC, Zhang L, Allada R. The Neuropeptide PDF Acts Directly on Evening Pacemaker Neurons to Regulate Multiple Features of Circadian Behavior. PLoS Biol. 2009;7:e1000154. doi: 10.1371/journal.pbio.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi C, Fortin J-P, McCarthy Ev, Oksman L, Kopin AS, Nitabach MN. Cellular Dissection of Circadian Peptide Signals with Genetically Encoded Membrane-Tethered Ligands. Current biology : CB. 2009;19:1167–1175. doi: 10.1016/j.cub.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helfrich-Förster C, Täuber M, Park JH, Mühlig-Versen M, Schneuwly S, Hofbauer A. Ectopic Expression of the Neuropeptide Pigment-Dispersing Factor Alters Behavioral Rhythms in Drosophila melanogaster. The Journal of Neuroscience. 2000;20:3339–3353. doi: 10.1523/JNEUROSCI.20-09-03339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wülbeck C, Grieshaber E, Helfrich-Förster C. Pigment-Dispersing Factor (PDF) Has Different Effects on Drosophila's Circadian Clocks in the Accessory Medulla and in the Dorsal Brain. Journal of Biological Rhythms. 2008;23:409–424. doi: 10.1177/0748730408322699. [DOI] [PubMed] [Google Scholar]
- 55.Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH, Holmes TC. Electrical Hyperexcitation of Lateral Ventral Pacemaker Neurons Desynchronizes Downstream Circadian Oscillators in the Fly Circadian Circuit and Induces Multiple Behavioral Periods. The Journal of Neuroscience. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Choi C, Cao G, Tanenhaus AK, McCarthy Ev, Jung M, Schleyer W, Shang Y, Rosbash M, Yin JCP, Nitabach MN. Autoreceptor Control of Peptide/Neurotransmitter Corelease from PDF Neurons Determines Allocation of Circadian Activity in Drosophila. Cell Reports. 2012;2:332–344. doi: 10.1016/j.celrep.2012.06.021. This study established the specific G-Protein signalling pathways down stream of PdfR in the PDF expressing LNv neurons and provided evidence that the PDF neurons are accelerated by PdfR signalling.
- 57. Im SH, Taghert PH. PDF receptor expression reveals direct interactions between circadian oscillators in Drosophila. The Journal of Comparative Neurology. 2010;518:1925–1945. doi: 10.1002/cne.22311. This study established that the clock neuron network of Drosophila is mosaic for PdfR expression.
- 58.Yoshii T, Todo T, Wülbeck C, Stanewsky R, Helfrich-Förster C. Cryptochrome is present in the compound eyes and a subset of Drosophila's clock neurons. The Journal of Comparative Neurology. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
- 59.Im SH, Li W, Taghert PH. PDFR and CRY Signaling Converge in a Subset of Clock Neurons to Modulate the Amplitude and Phase of Circadian Behavior in Drosophila. PLoS ONE. 2011;6:e18974. doi: 10.1371/journal.pone.0018974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cusumano P, Klarsfeld A, Chelot E, Picot M, Richier B, Rouyer F. PDF-modulated visual inputs and cryptochrome define diurnal behavior in Drosophila. Nat Neurosci. 2009;12:1431–1437. doi: 10.1038/nn.2429. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L, Lear BC, Seluzicki A, Allada R. The CRYPTOCHROME Photoreceptor Gates PDF Neuropeptide Signaling to Set Circadian Network Hierarchy in Drosophila. Current biology : CB. 2009;19:2050–2055. doi: 10.1016/j.cub.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zoephel J, Reiher W, Rexer K-H, Kahnt J, Wegener C. Peptidomics of the Agriculturally Damaging Larval Stage of the Cabbage Root Fly Delia radicum (Diptera: Anthomyiidae) PLoS ONE. 2012;7:e41543. doi: 10.1371/journal.pone.0041543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shafer OT, Taghert PH. RNA-Interference Knockdown of Drosophila Pigment Dispersing Factor in Neuronal Subsets: The Anatomical Basis of a Neuropeptide's Circadian Functions. PLoS ONE. 2009;4:e8298. doi: 10.1371/journal.pone.0008298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Krupp JJ, Billeter J-C, Wong A, Choi C, Nitabach MN, Levine JD. Pigment-Dispersing Factor Modulates Pheromone Production in Clock Cells that Influence Mating in Drosophila. Neuron. 2013;79:54–68. doi: 10.1016/j.neuron.2013.05.019. This study indicates that the clock targets of PDF are not limitted to central clock neurons by establishing that PDF modulates the clocks of some peripheral, non-neuronal cell types.
- 65.Duvall LB, Taghert PH. The Circadian Neuropeptide PDF Signals Preferentially through a Specific Adenylate Cyclase Isoform AC3 in M Pacemakers of Drosophila. PLoS Biol. 2012;10:e1001337. doi: 10.1371/journal.pbio.1001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Duvall LB, Taghert PH. E and M Circadian Pacemaker Neurons Use Different PDF Receptor Signalosome Components in Drosophila. Journal of Biological Rhythms. 2013;28:239–248. doi: 10.1177/0748730413497179. This study established that PdR targets distinct adenylyl cyclases in different classes of clock neuron suggesting that the receptor signals through distinct signalsomes throughout the network.
- 67.Zhang Y, Emery P. GW182 Controls Drosophila Circadian Behavior and PDF-Receptor Signaling. Neuron. 2013;78:152–165. doi: 10.1016/j.neuron.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li Y, Guo F, Shen J, Rosbash M. PDF and cAMP enhance PER stability in Drosophila clock neurons. Proceedings of the National Academy of Sciences. 2014;111:E1284–E1290. doi: 10.1073/pnas.1402562111. This study, along with the following study, establishes a link between PdfR signalling and the molecular clock, identifying PKA as a stabilizer if clock proteins.
- 69. Seluzicki A, Flourakis M, Kula-Eversole E, Zhang L, Kilman V, Allada R. Dual PDF Signaling Pathways Reset Clocks Via TIMELESS and Acutely Excite Target Neurons to Control Circadian Behavior. PLoS Biol. 2014;12:e1001810. doi: 10.1371/journal.pbio.1001810. This study, along with the previous study, establishes a link between PdfR signalling and the molecular clock, identifying PKA as a stabilizer if clock proteins.
- 70.Belvin MP, Zhou H, Yin JCP. The Drosophila dCREB2 Gene Affects the Circadian Clock. Neuron. 1999;22:777–787. doi: 10.1016/s0896-6273(00)80736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao Z, Macara AM, Lelito KR, Minosyan T, Shafer OT. Analysis of Functional Neuronal Connectivity in the Drosophila Brain. Journal of Neurophysiology. 2012 doi: 10.1152/jn.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vecsey CG, Pírez N, Griffith LC. The Drosophila neuropeptides PDF and sNPF have opposing electrophysiological and molecular effects on central neurons. Journal of Neurophysiology. 2014;111:1033–1045. doi: 10.1152/jn.00712.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pírez N, Christmann BL, Griffith LC. Daily rhythms in locomotor circuits in Drosophila involve PDF. Journal of Neurophysiology. 2013;110:700–708. doi: 10.1152/jn.00126.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]