Abstract
Extracellular adenosine-5'-triphosphate (ATP) triggers biological responses in a wide variety of cells and tissues and activates signaling cascades that affect cell membrane potential and excitability. It has been demonstrated that compressive loading promotes ATP production and release by intervertebral disc (IVD) cells, while a high level of extracellular ATP accumulates in the nucleus pulposus (NP) of the IVD. In this study, a noninvasive system was developed to measure ATP-induced changes in the membrane potential of porcine IVD cells using the potential sensitive dye di-8-butyl-amino-naphthyl-ethylene-pyridinium-propyl-sulfonate (di-8-ANEPPS).The responses of NP and annulus fibrosus (AF) cells to ATP were examined in monolayer and 3-dimensional cultures. It was found that the pattern and magnitude of membrane potential change in IVD cells induced by extracellular ATP depended on cell type, culture condition, and ATP dose. In addition, gene expression of P2X4 purinergic receptor was found in both cell types. Inhibition of the ATP-induced response by pyridoxalphosphate-6-azophenyl-2', 4'-disulfonate (PPADS), a non-competitive inhibitor of P2 receptors, suggests that ATP may modulate the biological activities of IVD cells via P2 purinergic receptors.
Keywords: ATP, membrane potential, intervertebral disc, purinergic receptors, di-8-ANEPPS
INTRODUCTION
In addition to its role as an intracellular energy source, adenosine-5'-triphosphate (ATP) is an extracellular signaling molecule actively involved in the regulation of short-term and long-term processes through the modification of proliferation, differentiation, and apoptosis of cells via purinergic pathways1. The receptors involved in the transduction of purinergic signals are distributed in a wide variety of tissues throughout the body and therefore, biological responses to extracellular ATP have been reported in tissues as diverse as skin, skeletal muscle, bone and the immune system2. In previous studies of cells and tissues that exhibited a biological response to extracellular ATP, signaling cascades that affected the cell membrane potential and excitability were activated by extracellular ATP3.
The intervertebral disc (IVD) cells maintain the proper homeostatic balance of biosynthesis, breakdown, and accumulation of extracellular matrix (ECM) molecules4. These cellular processes determine the quality and integrity of the ECM and thus, the mechanical response of the IVD5. Our recent studies have demonstrated that compressive loading affects ATP production and release by IVD cells in the 3-dimensional agarose gel model 6, 7 and in-situ energy metabolism in the IVD 8. A high accumulated level of extracellular ATP (~165 μM) compared to physiological concentrations (1–50 nM)9 has been found in the nucleus pulposus (NP) of the IVD 8. However, whether extracellular ATP can induce a signaling response in IVD cells has not been investigated.
Membrane potentials are the difference in electric potential across the bilayer membrane of a cell 10. The combined actions of ion channels and transporters establish membrane potentials by regulating intracellular and extracellular ionic concentrations10, 11. Increasing evidence suggests that membrane potentials are not only involved in the cellular processes of proliferation, migration and differentiation 12–14, but are also related to complex processes such as control of wound healing 15, 16, regeneration17, 18, left-right patterning 19 and development15. Thus, it is suggested that membrane potentials are a fundamental control mechanism of cell functions 10. While patch clamp techniques are successfully and widely used to control and measure membrane potentials, system set-up is laborious and requires extensive expertise 10. Another approach to monitor membrane potentials is the use of potential sensitive dyes, which provide ease of use and a noninvasive set up. The aminonaphthylethenylpyridinium (ANEP) group of dyes offers rapid shifts in their spectra in response to changes in the surrounding electric field and modify their electronic structure, thus allowing fast optical responses (in the millisecond-range) to membrane potential changes 20. Among the ANEP dyes, di-8-butyl-amino-naphthyl-ethylene-pyridinium-propyl-sulfonate (di-8-ANEPPS) is more appropriate for long term experiments because the dye is maintained in the outer leaflet of the plasma membrane due to its lipophilic property 21. Upon binding to the cell membrane, di-8-ANEPPS becomes strongly fluorescent. A membrane potential change is followed by a redistribution of the intramolecular charge. Consequently, the spectral profile and fluorescence intensity of di-8-ANEPPS change, and the variations of fluorescence intensity are proportional to variations in membrane potential 22. Di-8-ANEPPS has been used to measure membrane potentials induced by electrical stimulation22. It has also been used to complement electrophysiological techniques23, 24. However, to our knowledge, noninvasive measurements of transient membrane potential changes induced by exogenous ATP using di-8-ANEPPS have not been reported.
In this paper, we developed a system to perform noninvasive measurements of ATP-induced membrane potential changes using the fast voltage sensitive dye di-8-ANEPPS and digital imaging processing techniques. By using this system, transient membrane potential changes of IVD cells to exogenous ATP stimulation were studied in monolayer and 3-dimensional cultures.
MATERIALS AND METHODS
Sample preparation
IVD tissues from the NP and the annulus fibrosus (AF) regions were harvested from the spines of three mature pigs (~ 250 lbs., ~ 1.5 years old) within 2 hours of sacrifice (Cabrera Farms, Hialeah, FL). The IVD tissues were enzymatically digested in Dulbecco's Modified Eagle Medium supplemented with 4.5 g/L of D-Glucose and 584 mg/L of L-Glutamine (DMEM; Invitrogen Corp., Carlsbad, CA) containing 1 mg/mL collagenase type II (Worthington Biochemical Corp., Lakewood, NJ) and 0.6 mg/mL protease (Sigma Aldrich, St. Louis, MO) for about 24 hours at 37 °C, 5% CO2. AF tissue was broken down mechanically by using a syringe to facilitate tissue digestion while in its respective digestion solution. The mixtures containing IVD cells were filtered using a 70μm strainer (BD Biosciences, San Jose, CA) to remove undigested tissue. The cells were re-suspended in DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen Corp.) and 1% antibiotic-antimycotic and were mixed at a 1:1 ratio with 4% agarose gel. Cell-agarose constructs were made by casting 100 μL of the cell-agarose mixture in a custom-made mold to obtain a construct of 1 × 106 cells in 2% agarose. The constructs were incubated overnight at 37°C, 5% CO2 in DMEM supplemented with 10% fetal bovine serum and 1% antibiotics. Additionally, some NP and AF cells were expanded in monolayer culture. The cells of primary culture were plated on cover slides. The samples, either cells seeded in agarose or cells plated on cover slides, were placed in a RC-21BR large volume closed bath imaging chamber (Warner Instruments, Hamden, CT). The samples were washed with phosphate buffered saline (PBS; Lonza, Walkersville, MD) for 5 minutes at 0.3 mL/min using a syringe pump (Harvard Apparatus, Holliston, MA) and then were incubated for 10 minutes (cells on cover slides) or 15 minutes (cells seeded in agarose) at 4°C in a staining solution, which is PBS containing 30 μM of di-8-ANEPPS and 0.05% of Pluronic F-127 (both Biotium, Hayward, CA) 25. Uneven membrane staining and progressive dye internalization prevent di-8-ANEPPS from being used for absolute measurements such as resting membrane potential unless measured in conjunction with microelectrodes 22, 26, 27. In this study, uneven membrane staining was improved by using Pluronic F-127 (0.05%) to aid solubilization of the dye; dye internalization was inhibited by incubation of the samples at 4°C22. The staining solution was gently washed away with PBS for 5 minutes at 0.3 mL/min. A single cell was selected and observed using a fluorescence microscope (Olympus IX70, Melville, NY) equipped with a 20X objective (LCPlan FL, 0.60 Ph2, Olympus), an advanced fluorescence illuminator (Lumen 200, Prior Scientific Inc., Rockland, MA), a filter for di-8-ANEPPS (QD625-A-OMF, Semrock, Rochester, NY [excitation: 435 nm, emission: 625 nm]) and a digital CCD camera (Retiga 2000R, Q-Imaging, Canada). In the 3-dimensional culture, a cell located on the periphery of the gel was selected in order to prevent any time delay of the response due to ATP diffusion in agarose gel.
ATP injection
ATP at either 100, 200, 300 or 500 μM concentration was injected into the chamber at 1.6 mL/min for 10 seconds. The time and rate of injection were selected to ensure that the contents of the chamber (260 μl volume) were substituted by the injected ATP. A sequence of fluorescence images was recorded starting at 10 seconds before ATP injection and continued during and after the injection for a total of 50 seconds. The sequence of fluorescence images was acquired in a movie structure at a rate of over 120 frames per second using QCapture Pro v6 (Media Cybernetics Inc. and Q-Imaging Inc., Canada) for further image processing.
Data Processing
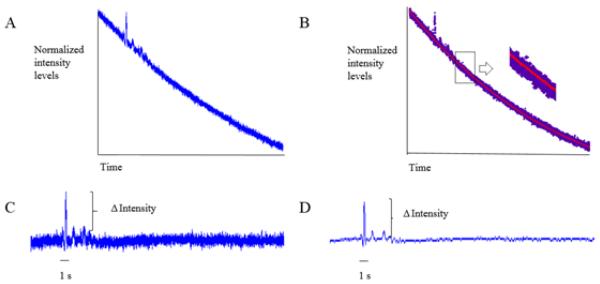
The cell membrane was labeled by a program implemented in MATLAB v7.1 (The MathWorks Inc., Natick, MA). Briefly, noise was reduced using blind deconvolution on every image in the recording sequence22. Then, the first image in the sequence was used as a reference to obtain an intensity threshold level used to accentuate the ring stain pattern defining the cell membrane. A binary image of the cell membrane was then created from the first image based on the defined intensity threshold level 24. Finally, all the images in the recording sequence were multiplied by the binary image in order to label the same region of interest on the cell membrane (Figure 1).
Figure 1.
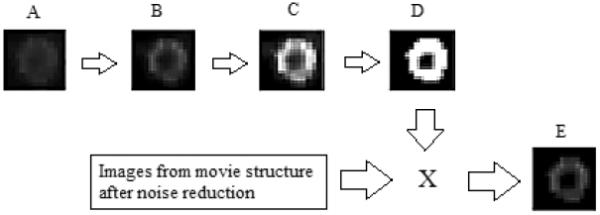
Image processing procedure. A. first image of the recording sequence. B. blind deconvolution. C. gray level adjustment. D. binary image creation. E. example of a resultant image.
After image processing, the average intensity of the cell membrane was computed for images at all time-points and was then normalized by the average intensity of the cell membrane before ATP response. A normalized intensity levels vs. time curve, which exhibited the time decay of intensity due to photobleaching, was obtained (Figure 2A). An exponential model I = aebt + cedt, where I is the normalized intensity, t is the time, and a, b, c and d are the coefficients, was used to describe the time decay of fluorescence intensity (Figure 2B). The coefficients were determined by curve fitting the theoretical model with the experimental intensity curve (Figure 2B). Then, the experimental intensity curve was compensated using the theoretical model (Figure 2C). Finally, the compensated experimental intensity curve was filtered with a 4th order low pass Butterworth filter with a cutoff frequency of 15 Hz to yield a smooth curve (Figure 2D). The increase in membrane potential in response to ATP stimulation was expressed as a percentage compared to the normalized average intensity of the cell membrane before ATP response.
Figure 2.
Compensation procedure for the time decay of fluorescence intensity due to photobleaching. A. a typical original experimental intensity curve showing the photobleaching effect. B. theoretical curve fitting (the red curve is an exponential model). C. the experimental intensity curve in (A) compensated by the exponential model obtained in (B). D. the compensated intensity curve after filtering.
Gene expression of purinergic receptors
Total RNA from IVD cells in monolayer and 3-dimensional agarose gel cultures was extracted using the trizol (Tri-Reagent, Molecular Research Center, Cincinnati, OH) protocol. Total RNA was quantified using the Qubit RNA BR assay kit (Life Technologies, Carlsbad, CA) and reverse transcribed to cDNA using the High Capacity cDNA reverse transcription kit (Applied Biosystems, Foster, CA), according to the manufacturers' specifications. Gene expression of P2X4 purinergic receptor and 18S (endogenous control) was examined using RT-PCR (One step Plus, Applied Biosystems). The PCR products were electrophoresed on a 2% ethidium bromide-stained agarose gel in order to examine the expression of the purinergic receptor. The primer sequences were as follow: P2X4 forward primer: CCGGGACGTGGACCACAACG; P2X4 reverse primer: TGCGGCATGGGCTTCACTGG; 18S forward primer: CGGCTACCACATCCAAGGA; 18S reverse primer: AGCTGGAATTACCGCGGCT. The sizes of PCR products for P2X4 and 18S were 245 and 188 bp, respectively.
Inhibition of P2 receptors
The involvement of the purinergic pathway in the cell membrane depolarization response was studied using pyridoxalphosphate-6-azophenyl-2', 4'-disulfonate (PPADS), a non-competitive inhibitor of P2 receptors, on IVD cells cultured on cover slides. Before an inhibitor treatment, the samples were washed with PBS for 5 minutes at 0.3 mL/min and then incubated at room temperature in PBS for an additional 5 minutes. Then, the samples were incubated with PBS containing 3, 10 or 30 μM of PPADS at room temperature for 20 minutes. After the inhibitor treatment, 500 μM ATP was injected to the chamber at 1.6 mL/min for 10 seconds while fluorescence images were acquired for further processing.
Statistical analysis
A two- way ANOVA was conducted to compare the magnitude of the response between cell types and ATP concentrations in the monolayer culture. Since different ATP concentrations were used for AF and NP cells in the 3-dimensional agarose culture, student's t-test analyses were performed to compare the magnitude of the response between different concentrations of ATP for each cell type and between NP and AF cells under 500 μM ATP only. A two-way ANOVA followed by post hoc Tukey test was performed to compare differences in the response of IVD cells to PPADS at different concentrations. The statistical analyses were performed using SPSS software (IBM, Chicago, IL). Differences in all cases were considered significant at p < 0.05.
RESULTS
AF cells
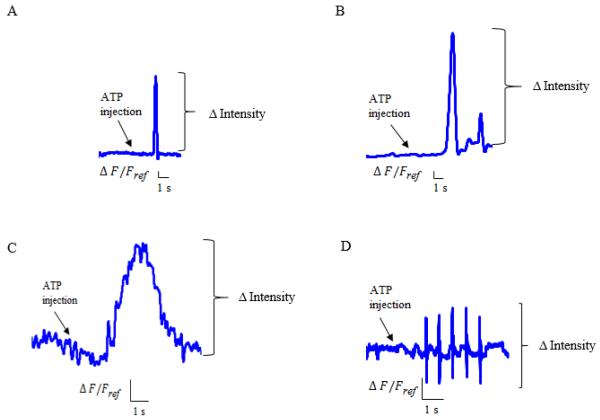
The membrane potential change response of AF cells in monolayer culture was a single spike (Figure 3A). The change in fluorescence intensity was 4.87% ± 0.83% for 200 μM ATP and 8.52% ± 1.93% for 500 μM ATP. In the 3-dimensional agarose gel culture, the membrane potential change response was a wide upward deflection (Figure 3C) resulting in a change in fluorescence intensity of 3.7% ± 0.73% for 200 μM ATP and 7.98% ± 1.53% for 500 μM ATP (mean ± SEM; n = 5 for each measurement). The fluorescence intensity induced by 500 μM ATP was significantly higher than the 200 μM ATP in the monolayer and 3-dimensional cultures (Figure 4A, p = 0.031 and p = 0.036, respectively). In both culture environments, no responses were observed from the 100 μM ATP injection. No responses were observed by injection of PBS only.
Figure 3.
Typical responses in monolayer and 3-dimensional agarose gel cultures. A. fluorescence intensity change of the cell membrane in AF cells in monolayer culture induced by 500 μM ATP. B. fluorescence intensity change of the cell membrane in NP cells in monolayer culture induced by 500 μM ATP.C. fluorescence intensity change of the cell membrane in AF cells in 3-dimensional agarose gel culture induced by 500 μM ATP. D. fluorescence intensity change of the cell membrane in NP cells in 3-dimensional agarose gel culture induced by 500 μM ATP. ΔF/Fref is the normalized change in fluorescence intensity. ΔF: Fluorescence variation, Fref : Fluorescence of the first image.
Figure 4.
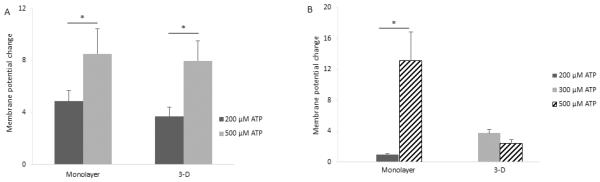
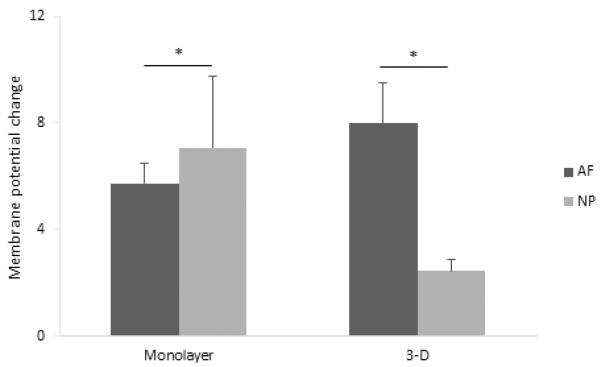
Comparison of the membrane potential change of IVD cells in monolayer and 3-dimensional cultures (n = 5 for each measurement). A. AF cells (Monolayer: p = 0.031, 3-D: p = 0.036). B. NP cells (Monolayer: p = 0.031, 3-D: p = 0.08).
NP cells
The membrane potential change response of NP cells in monolayer culture was a main spike followed by attenuated spikes (Figure 3B). The change in fluorescence intensity was 1% ±0.08% for 200 μM ATP and 13.1% ± 3.74% for 500 μM ATP. The fluorescence intensity induced by 500 μM ATP was significantly higher than 200 μM ATP (Figure 4B, p = 0.031). No response was observed for the injection of 100 μM ATP. In the 3-dimensional culture, NP cells responded with several interspersed spikes (Figure 3D). The fluorescence intensity was altered by only the injection of 300 μM or 500 μM ATP, with the changes being 3.75% ± 0.46% for 300 μM ATP and 2.45% ± 0.42% for 500 μM ATP (mean ± SEM; n = 5 for each measurement). No responses were observed by injection of PBS only.
Comparison between NP and AF cells
A significant difference between AF and NP cells was found in the monolayer culture as determined by two- way ANOVA (Figure 5, p = 0.043). In the 3-dimensional agarose gel culture, the fluorescence intensity induced by 500 μM ATP was significantly higher in AF cells compared to NP cells (Figure 5, p = 0.017). It was also observed that ATP-induced responses had a longer duration in agarose culture than in monolayer culture. In addition, in both cell culture environments, ATP elicited a transient depolarization in which the membrane potential returned to its previous state while ATP was still present.
Figure 5.
Comparison of the membrane potential change between AF and NP cells (n = 5 for each measurement) in monolayer culture (200 μM + 500 μM: p = 0.043) and 3-dimensional agarose gel culture (500 μM ATP, p = 0.017).
Gene expression of purinergic receptors
The gene of the P2X4 purinergic receptor was expressed by AF and NP cells in monolayer and 3-dimensional agarose gel cultures (Figure 6).
Figure 6.
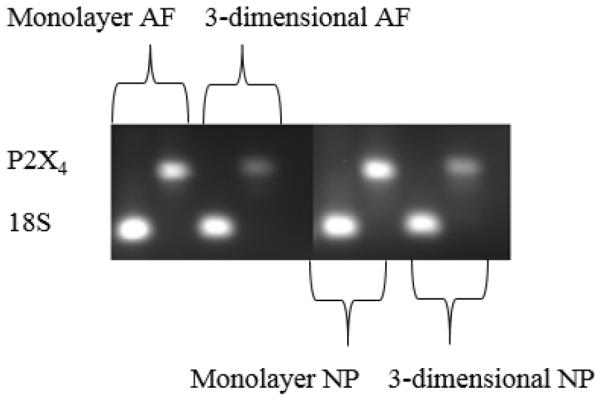
Gene expression of purinergic receptor P2X4 in AF and NP cells cultured in monolayer and 3-dimensional agarose gel. 18S is the endogenous control.
Inhibition of P2 receptors
PPADS was used in the ATP injection experiments to examine whether the purinergic pathway has a role in the membrane potential change of IVD cells. No significant difference in the response of IVD cells treated with PPADS at 3, 10 or 30 μM of PPADS (n = 3 for each concentration of PPADS) was found (p = 0.364). Furthermore, a significant difference in response to ATP stimulation between IVD cells with and without PPADS was found, confirming that PPADS abolished the ATP-induced membrane potential response of IVD cells (p = 0.001).
DISCUSSION
This study established a noninvasive system to measure ATP-induced membrane potential changes using the potentiometric dye di-8-ANEPPS and digital imaging processing techniques. Using this system, ATP-induced transient membrane potential changes of IVD cells were detected in both monolayer and 3-dimensional cultures. To our knowledge, this is the first study to show transient membrane potential changes that exogenous ATP triggers in IVD cells using a potentiometric dye.
Unlike electrodes, potentiometric dyes are easy to use and are free from electrical stimulus artifacts that may easily arise when currents are applied to the samples. However, environmental factors such as variations in the system set-up and cell membrane composition, which is likely to vary from cell to cell, can affect the spectral properties of the dye 28. Differences in membrane staining and illumination that derive from the staining process and the microscope and camera system set up accounted for day-to-day variations in the system. Therefore, normalization of the average intensity of the cell membrane at all time-points was used to reduce cell-to-cell variation of fluorescence. In addition, no membrane potential changes induced by PBS injections before and after the ATP injection experiment ensured that the cell response was attributed to ATP stimulation, not a result of injection.
Depolarizing responses due to exogenous ATP have been extensively measured electrophysiologically29–31. In neurons, ATP analogues such as 2-methylthio-ATP, ADP and α,β methylene ATP reported depolarizing responses while neither adenosine nor AMP evoked any response 30. Normal chondrocytes stimulated by exogenous ATP, UTP 2-methylthioadenosine and α,β-methylene ATP have shown membrane hyperpolarization while no significant response was observed from chondrocytes isolated from osteoarthritis cartilage 32. Purinergic receptors in the plasma membrane of cells are excited when bound to extracellular ATP and initiate signaling cascades33, 34. In chondrocytes, Ca2+signaling through P2 receptors activation was demonstrated by using antagonists of P2 receptors 35, 36. In IVD cells, the expression of mRNA for P2Xpurinergic receptors (P2X4,) is consistent with previous studies documenting the involvement of P2X4 receptors in cell membrane depolarization37, 38. It was suggested that P2X4 activation permits the influx of Ca2+ and Na+, which in turn depolarizes the cell membrane. Depolarization of the cell membrane opens voltage-dependent calcium channels that enhance Ca2+ influx and, as a result, induces ATP release. This mechanism has been proposed to initiate Ca2+ oscillations that drive ATP oscillations during chondrogenesis37. In monocyte-derived human macrophages, transient depolarization of the cell membrane was reported to be induced by extracellular ATP possibly via P2X4 receptor activation38. In our study, the inhibition of the membrane potential change response to ATP by PPADS (an inhibitor of P2 receptors) also confirmed the involvement of P2 receptor activation, especially P2X4, suggesting that ATP may modulate the biological activities of IVD cells via the P2 purinergic receptors. Although PPADS has been reported to have modest to poor inhibition effects at blocking P2X439, it has also been documented that mouse and human P2X4 receptors displayed more sensitivity to PPADS than the rat P2X4 receptor under the same experimental conditions 40. Moreover, differences in antagonist binding and desensitization were reported between rat and human P2X4 receptors that display 87% sequence homology 41. The human P2X4 receptor showed higher sensitivity to PPADS and more rapid desensitization than the rat P2X4 receptor. Therefore, sensitivity to PPADS may be different among different species.
Since a 3-dimensional agarose gel maintains the original cellular phenotype by resembling the in vivo environment42, different responses to ATP observed between the two culture conditions may be due to differences in cell phenotype. In addition, the distinct responses of membrane potential change between AF and NP cells may be due to different embryonic origins. AF cells are derived from the mesenchyme, while NP cells are derived from the notochord. Moreover, AF cells are elongated and resemble fibroblasts, whereas NP cells are spheroidal and chondrocyte-like in vivo 43.
In our study, the ATP level required to induce membrane potential changes in IVD cells was higher than the reported in electrophysiological recordings in which the EC50 of P2 receptors is in the low micromolar range 39. There are two possible reasons for this difference. Firstly, our system detects the potential change of the whole cell membrane while the patch clamp technique can precisely measure transmembrane current induced by activation of a single P2 receptor. Therefore, based on small changes in the florescence intensity of the whole cell membrane triggered by ATP in our study, the major limitation of using a voltage sensitive dye to measure membrane potential changes is its sensitivity compared to precise measurements of the traditional patch clamp technique. Secondly, a high extracellular ATP level (~165 μM8) found in the IVD may desensitize the P2 receptors of IVD cells. For instance, previous studies found that normal and osteoarthritic human chondrocytes exhibited different responses to ATP, although there were no differences in expression of P2 receptors between them44. That observation suggests an ATP signaling desensitization effect due to increased levels of ATP found in osteoarthritic synovial fluid32, 44.
In summary, we developed a noninvasive system for detecting membrane potential variations induced by ATP stimulation using a potentiometric dye and image processing techniques. Both NP and AF cells exhibited different responses to exogenous ATP stimulation in monolayer and 3-dimensional cultures, which may maintain cellular phenotypes. Distinct patterns and magnitudes of membrane potential changes of AF and NP cells were also observed, which may be due to different embryonic origins of cells. In addition, the results showed that extracellular ATP induces changes in membrane potential via activation of P2 purinergic receptors in IVD cells, suggesting that extracellular ATP signaling is involved in the regulation of cellular activities in the IVD.
ACKNOWLEDGEMENT
This study was supported by the grant AR056101 from the NIH.
ETHICAL STANDARDS All animal studies were carried out in accordance with NIH and University of Miami guidelines, and approved by the Animal Care and Use Committee of the University of Miami. No human studies were carried out by the authors for this article.
Footnotes
CONFLICTS OF INTEREST Silvia Gonzales, Brittany Rodriguez, Carlos Barrera and Chun-Yuh Charles Huang declare that they have no conflicts of interest.
REFERENCES
- 1.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36(9):1127–39. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G, Knight GE. Cellular Distribution and Functions of P2 Receptor Subtypes in Different Systems. International Review of Cytology: Academic Press; 2004. pp. 31–304. [DOI] [PubMed] [Google Scholar]
- 3.Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265(3 Pt 1):C577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 4.Ohshima H, Urban JPG, Bergel DH. Effect of static load on matrix synthesis rates in the intervertebral disc measured in vitro by a new perfusion technique. Journal of Orthopaedic Research. 1995;13(1):22–9. doi: 10.1002/jor.1100130106. [DOI] [PubMed] [Google Scholar]
- 5.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. Journal of Cell Science. 1995;108(4):1497–508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 6.Fernando HN, Czamanski J, Yuan T-Y, Gu W, Salahadin A, Huang C-YC. Mechanical loading affects the energy metabolism of intervertebral disc cells. Journal of Orthopaedic Research. 2011;29(11):1634–41. doi: 10.1002/jor.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salvatierra JC, Yuan TY, Fernando H, et al. Difference in Energy Metabolism of Annulus Fibrosus and Nucleus Pulposus Cells of the Intervertebral Disc. Cell Mol Bioeng. 2011;4(2):302–10. doi: 10.1007/s12195-011-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Gonzales S, Levene H, Gu W, Huang CY. Energy metabolism of intervertebral disc under mechanical loading. J Orthop Res. 2013;10(10):22436. doi: 10.1002/jor.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trabanelli S, Ocadlikova D, Gulinelli S, et al. Extracellular ATP exerts opposite effects on activated and regulatory CD4+ T cells via purinergic P2 receptor activation. Journal of immunology (Baltimore, Md : 1950) 2012;189(3):1303–10. doi: 10.4049/jimmunol.1103800. [DOI] [PubMed] [Google Scholar]
- 10.Sundelacruz S, Levin M, Kaplan DL. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev. 2009;5(3):231–46. doi: 10.1007/s12015-009-9080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang M, Brackenbury WJ. Membrane potential and cancer progression. Frontiers in Physiology. 2013;4 doi: 10.3389/fphys.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binggeli R, Weinstein RC. Membrane potentials and sodium channels: Hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. Journal of Theoretical Biology. 1986;123(4):377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- 13.Blackiston DJ, McLaughlin KA, Levin M. Bioelectric controls of cell proliferation: Ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009;8(21):3527–36. doi: 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundelacruz S, Levin M, Kaplan DLP. Depolarization alters phenotype, maintains plasticity of pre-differentiated mesenchymal stem cells. Tissue Eng Part A. 2013;27:27. doi: 10.1089/ten.tea.2012.0425.rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuccitelli R. Endogenous electric fields in embryos during development, regeneration and wound healing. Radiation Protection Dosimetry. 2003;106(4):375–83. doi: 10.1093/oxfordjournals.rpd.a006375. [DOI] [PubMed] [Google Scholar]
- 16.Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol. 2003;58:1–26. doi: 10.1016/s0070-2153(03)58001-2. [DOI] [PubMed] [Google Scholar]
- 17.Levin M. Large-scale biophysics: ion flows and regeneration. Trends in Cell Biology. 2007;17(6):261–70. doi: 10.1016/j.tcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Levin M. Bioelectric mechanisms in regeneration: Unique aspects and future perspectives. Seminars in Cell & Developmental Biology. 2009;20(5):543–56. doi: 10.1016/j.semcdb.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111(1):77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- 20.Molecular Probes. 2006. Invitrogen. Potential-sensitive ANEP dyes. [Google Scholar]
- 21.Product and safety data sheet: di-8-ANEPPS. Biotium. [Google Scholar]
- 22.Pucihar G, Kotnik T, Miklavcic D. Measuring the induced membrane voltage with Di-8-ANEPPS. J Vis Exp. 2009;19(33) doi: 10.3791/1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiFranco M, Capote J, Vergara JL. Optical imaging and functional characterization of the transverse tubular system of mammalian muscle fibers using the potentiometric indicator di-8-ANEPPS. J Membr Biol. 2005;208(2):141–53. doi: 10.1007/s00232-005-0825-9. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Davidson RM, Wei MD, Loew LM. Membrane electric properties by combined patch clamp and fluorescence ratio imaging in single neurons. Biophys J. 1998;74(1):48–53. doi: 10.1016/S0006-3495(98)77765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fromherz P, Hubener G, Kuhn B, Hinner MJ. ANNINE-6plus, a voltage-sensitive dye with good solubility, strong membrane binding and high sensitivity. Eur Biophys J. 2008;37(4):509–14. doi: 10.1007/s00249-007-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beach JM, McGahren ED, Xia J, Duling BR. Ratiometric measurement of endothelial depolarization in arterioles with a potential-sensitive dye. Am J Physiol. 1996;270(6 Pt 2):H2216–27. doi: 10.1152/ajpheart.1996.270.6.H2216. [DOI] [PubMed] [Google Scholar]
- 27.Knisley SB, Justice RK, Kong W, Johnson PL. Ratiometry of transmembrane voltage-sensitive fluorescent dye emission in hearts. American journal of physiology Heart and circulatory physiology. 2000;279(3):H1421–33. doi: 10.1152/ajpheart.2000.279.3.H1421. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi Y, Zviman MM, Brand JG, Teeter JH, Restrepo D. Measurement of membrane potential and [Ca2+]i in cell ensembles: application to the study of glutamate taste in mice. Biophysical Journal. 1996;71(2):1057–70. doi: 10.1016/S0006-3495(96)79306-2. 8// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grubb BD, Evans RJ. Characterization of cultured dorsal root ganglion neuron P2X receptors. European Journal of Neuroscience. 1999;11(1):149–54. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- 30.Ueno S, Harata N, Inoue K, Akaike N. ATP-gated current in dissociated rat nucleus solitarii neurons. J Neurophysiol. 1992;68(3):778–85. doi: 10.1152/jn.1992.68.3.778. [DOI] [PubMed] [Google Scholar]
- 31.Huber-Lang M, Fischer KG, Gloy J, et al. UTP and ATP induce different membrane voltage responses in rat mesangial cells. Am J Physiol. 1997;272(6 Pt 2):F704–11. doi: 10.1152/ajprenal.1997.272.6.F704. [DOI] [PubMed] [Google Scholar]
- 32.Millward-Sadler SJ, Wright MO, Flatman PW, Salter DM. ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology. 2004;41(3–4):567–75. [PubMed] [Google Scholar]
- 33.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–92. [PubMed] [Google Scholar]
- 34.Burnstock G. Purinergic Nerves. Pharmacological Reviews. 1972;24(3):509–81. [PubMed] [Google Scholar]
- 35.Elfervig MK, Graff RD, Lee GM, Kelley SS, Sood A, Banes AJ. ATP induces Ca2+signaling in human chondrons cultured in three-dimensional agarose films. Osteoarthritis and Cartilage. 2001;9(6):518–26. doi: 10.1053/joca.2000.0435. 8// [DOI] [PubMed] [Google Scholar]
- 36.Pingguan-Murphy B, El-Azzeh M, Bader DL, Knight MM. Cyclic compression of chondrocytes modulates a purinergic calcium signalling pathway in a strain rate- and frequency-dependent manner. Journal of cellular physiology. 2006;209(2):389–97. doi: 10.1002/jcp.20747. [DOI] [PubMed] [Google Scholar]
- 37.Kwon HJ. Extracellular ATP signaling via P2X(4) receptor and cAMP/PKA signaling mediate ATP oscillations essential for prechondrogenic condensation. The Journal of endocrinology. 2012;214(3):337–48. doi: 10.1530/JOE-12-0131. [DOI] [PubMed] [Google Scholar]
- 38.Hanley PJ, Musset B, Renigunta V, et al. Extracellular ATP induces oscillations of intracellular Ca2+ and membrane potential and promotes transcription of IL-6 in macrophages. Proc Natl Acad Sci U S A. 2004;101(25):9479–84. doi: 10.1073/pnas.0400733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stühmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proceedings of the National Academy of Sciences. 1996;93(8):3684–8. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones CA, Chessell IP, Simon J, et al. Functional characterization of the P2X4 receptor orthologues. British Journal of Pharmacology. 2000;129(2):388–94. doi: 10.1038/sj.bjp.0703059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Guzman M, Soto F, Gomez-Hernandez JM, Lund P-E, Stühmer W. Characterization of Recombinant Human P2X4 Receptor Reveals Pharmacological Differences to the Rat Homologue. Molecular Pharmacology. 1997;51(1):109–18. doi: 10.1124/mol.51.1.109. [DOI] [PubMed] [Google Scholar]
- 42.Gruber HE, Fisher EC, Jr., Desai B, Stasky AA, Hoelscher G, Hanley EN., Jr. Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235(1):13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 43.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20(11):1307–14. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 44.Knight MM, McGlashan SR, Garcia M, Jensen CG, Poole CA. Articular chondrocytes express connexin 43 hemichannels and P2 receptors - a putative mechanoreceptor complex involving the primary cilium? J Anat. 2009;214(2):275–83. doi: 10.1111/j.1469-7580.2008.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]