Abstract
Circular dichroism (CD) spectropolarimeters typically employ one photoelastic modulator. However, spectropolarimeters employing two or even four modulators are more versatile and can be used to subvert common measurement errors arising from imperfectly isotropic samples or sample holders. Small linear anisotropies that can cause large errors in CD measurement can be associated with multi-well sample holders. Thus, high-throughput CD analyses in multi-well plates have not yet been demonstrated. One such application is the determination of enantiomeric excess of a library of reaction products. Herein, a spectropolarimeter employing four photoelastic modulators and a translation stage was used to determine the enantiomeric excess of a family of chiral amine complexes much more rapidly than could be achieved with a robotic fluid injection system. These experiments are proof of concept for high-throughput CD analysis. In practice, commercially available glass bottomed well plates are sufficiently strain free such that a simple instrument with just one photoelastic modulator and a vertical optical train should be able to deliver the CD without special considerations given herein. On the other hand, polystyrene well plates cannot be used in this way.
Introduction
Chiral HPLC and GC are the most common methods for determining enantiomeric excess (ee), but they are serial and slow.1-5 Demand for simple, fast assays for the determination of ee has grown over the last several years driven by the development of high-throughput screening (HTS) protocols for reaction development using parallel synthesis6-10 in which reagent or catalyst ligands and/or reaction conditions are varied systematically.11-21 Parallel synthesis has become popular with the advent of micro-well plates, micro-reactors, mini-block reactors, and automated liquid handlers.22,23 Given the synthetic efficiency of parallel syntheses, a limiting step in asymmetric catalyst discovery is the determination of ee. Unfortunately, a fast CD plate reader that can deliver ee in a high-throughput platform has not been demonstrated. By contrast, Leung and Anslyn utilized colorimetric indicator displacement assays28 that require only 1 to 10 minutes to read a 96-well plate with a simple UV-vis spectrophotometer, bona fide high-throughput screens that can analyze thousands of samples per day.24,25
The design and construction of an apparatus able to perform the sampling operations and read a 96-well plate may bring CD into the realm of HTS, a boon to the synthesis community because a growing number of ee assays involve chiroptical measurement.29 For example, the groups of Anslyn and Wolf have developed and tested CD methods for the analysis of chiral analytes including amino acids30, alcohols,31 amino alcohols32,33 carboxylates34 and amines.35-38 Biological/biochemical sciences may benefit likewise. CD spectroscopy is sensitive to the conformational analysis of peptides, proteins, and nucleic acids.39,40 CD can also be used to study dynamic processes such as binding interactions between a macromolecule and small molecule drugs, dyes, guests, and ligands.39-41
Manufacturers of CD spectropolarimeters have attempted to streamline the acquisition of data by designing auto-samplers or robotic injectors,42 but they only marginally speed measurement; CD “robots” are not true plate readers but automated flow injectors, drawing each sample and injecting it sequentially. Moreover, they can be sensitive to non-aqueous media, malfunctioning when handling common organic solvents.43
Why is a HTS-CD spectrometer not currently available? Presumably, this is because multi-well plates are not necessarily optically isotropic. They can be burdened with strain linear birefringence (LB), i.e. refractive index variance. Parasitic LB has plagued measurements of the CD of anisotropic media for decades.44-50 Moreover, menisci -- non-normal interfaces -- in well plates can introduce Fresnel linear dichroism (LD) into the optical system. LB and LD can be much larger than circular birefringence (CB or optical rotation) and CD. The minor chiroptical perturbation to the polarization state of light in anisotropic media can be of the same order of magnitude as parasitic ellipticities from imperfect samples, containers, and optical components.
Experimental Strategy
Instrument Design
CD spectropolarimeters typically modulate between left and right circular polarization states by using a photoelastic modulator (PEM) placed before the sample. The differential transmission is recorded. But the measured CD can be grossly affected by the admixture of linear polarization in the input light and its coupling to linear anisotropies of the medium (in solid samples) or the container. Multiple photoelastic modulators (PEMs) provide a strategy for separating and eliminating contributions to the change in the polarization of light due to parasitic linear anisotropies that have traditionally given rise to artifactual chiral signatures.58,59
Most polarization techniques rely on measuring particular elements of the sample Mueller matrix, the linear operator that contains all of the information about the polarization transforming properties of an analyte.60 Increasingly complex samples spread the desired optical effects over all 16 elements of the Mueller matrix.45 In 2011, we introduced a polarimeter using four PEMs (4-PEM spectropolarimeter or simply “4-PEM” in brief) that measures simultaneously all Mueller matrix elements without reconfiguration, thus avoiding errors associated with moving optical components.58,59 A schematic is shown in Figure 1. This instrument is fast, sensitive, and accurate. It will be used here as a high-speed automated plate reader.
Figure 1.
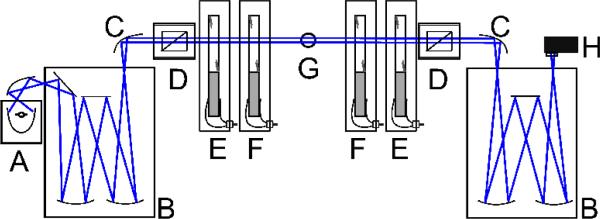
Four photoelastic modulator (4-PEM) spectropolarimeter. A: light source, B: monochromators, C: mirrors, D: linear polarizers, E, F: photoelastic modulators set at 0° (E) and 45° (F). G: Sample holder, H: photomultiplier
In our instrument, light from a Xe arc lamp coupled to a monochromator (290 nm to 850 nm) was normally incident on the sample. The polarization state generator and the polarizer state analyzer are each composed of a linear polarizer and two PEMs (Hinds Instruments) operating at a different frequencies. The PEMs were set at relative orientations of ±45°. The advantage of this setup over other Mueller matrix polarimeters is that measurements are obtained without any moving parts. The transmitted light was detected with a photomultiplier. The 4-PEM measures the 16 parameters of a normalized Mueller matrix at each wavelength.
A 96-well glass bottom plate (In Vitro Scientific, P96-1.5H-N, with a MatTek Corporation top cover glass, PCS10872-1.0) was mounted on two linear actuators (Newport) with a range of only three wells in the vertical and horizontal directions, thus only a 3×3 block of wells could be measured without repositioning the plate. The cover glass was secured with elastic bands because our polarimeter has a horizontal light path. There are no obstacles to building a comparable instrument with a vertical optical train and a high-speed translation stage outfitted for well plates. But, for proof of concept here, we used the instrument currently in operation (in fact, two PEMs will suffice). With average reading times of about two seconds for a single wavelength and around 30 seconds for a 120 nm-wide scan per sample, the rate of CD analysis can truly be called high-throughput. Single ellipticity values (as used herein) can be read for the entire plate in less than three minutes.
Chemical System
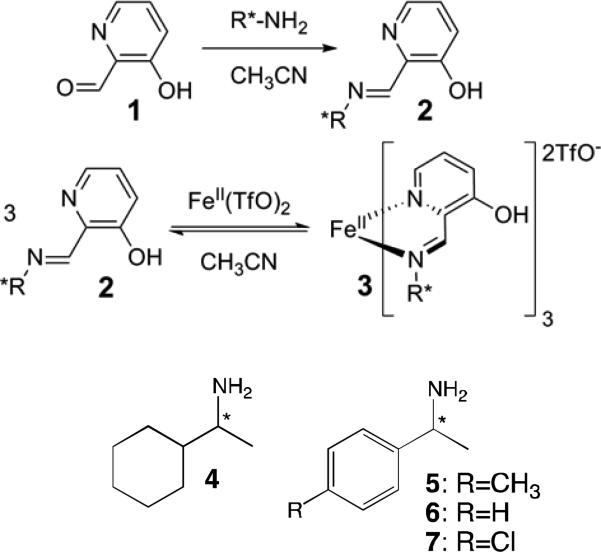
With the goal of testing the performance of a multiple PEM polarimeter in HTS measurements, we chose a validated CD assay for ee of α-chiral amines.35 Aldehyde 1 reacts with the a chiral amine (4-7) to give a bidentate imine 2. Fe(II), in turn, binds three equivalents of that imine to give several helical octahedral complexes represented as 3, whose handedness is dictated by the chirality of the amine (Scheme 1). We determined the enantioenrichment of amine 4-7 by measuring CD in the strong metal-to-ligand charge transfer bands that arise upon formation of complex 3.35 This reaction is fast, simple, relies on commercially available reagents and can be analyzed in situ.
Scheme 1.
Two-step assembly that leads to the CD-active Fe(II) complex used to report the ee of chiral amines 4-7.
Results and Discussion
Mueller Matrix Polarimetry
A Mueller matrix is a real valued 4×4 matrix that represents an optical element or sample. Stokes-Mueller calculus tracks the transformation of the state of polarization of light passing through the 4-PEM and the sample.
The Mueller matrix of an optically active solution, (Msol ) in (1), has only two parameters where α = CD and, β = CD, and α2 + β2 + γ2 = 1. It has been shown previously that Mueller matrix which introduces small, parasitic linear anisotropies (LB and LD less than ~0.5 rad) and negligible depolarization can be expressed as the explicit matrix (Manis) in (1).61 Here, LB' and LD' refer to a set of orthogonal axes at ±45° with respect to the instrument coordinate system.
In the absence of linear anisotropies, CD can be taken directly from either the M03 or M30 elements. Commercial CD spectrometers typically measure M03 using a single PEM positioned before the sample. However, even small ellipticities introduced by the sample holder will perturb the measured Mueller matrix. To evaluate the Mueller matrix with anisotropy introduced into sample holder before or after the sample, i.e. closer to or further from the light source, we analyzed the
| (1) |
respective matrix products or MsolManis or ManisMsol.
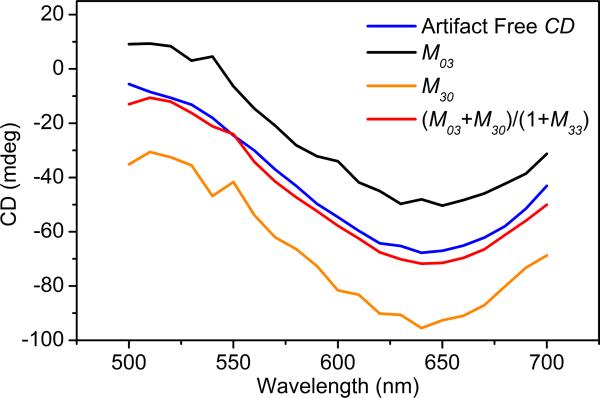
As explained in the supporting information, regardless of the sample position, analysis of the matrix products shows that CD can be approximated from the elements of measured Mueller matrix as CD = (M03+M30)/(1+M33). For very small linear anisotropies, M33 approaches 1, and the correction for CD approaches the simple average of M03and M30. For large linear anisotropies, M03 and M30 will be very different from one another, and the correction fails. Figure 2 illustrates the deviations from the artifact-free CD that result from measurement of only M03 or M30 in the presence of modest anisotropy.
Figure 2.
CD errors when using only one PEM. A 0.02 M solution of Cu(II) tartrate measured without (artifact-free CD) and with (all other spectra) a birefringent piece of adhesive tape placed after the solution cuvette using the 4-PEM.
We emulated a well-plate measurement of a 0.02 M Cu(II)-D-tartrate solution in a quartz cuvette placed just before a transparent birefringent material (a piece of adhesive tape). The complete M was measured with the 4-PEM. M03 and M30 by themselves did not match the CD measured directly in the glass cuvette without the interfering linearly birefringent tape. But, (M03+M30)/(1+M33) matched quite well.
Using the In Vitro Scientific glass bottomed well plates, in practice M03 and M30 were equal and either could be taken as the CD. This implies that glass bottomed well plates, at least from this manufacturer, can be used along with a single photoelastic modulator. That is a HTS CD instrument could well be fashioned from a commercial device with a vertical optical train and a high speed scanning stage of the type already adapted to simple UV-vis HTS. However, there is no direct way to verify the CD measurements with a single PEM, while for two (or in this case four) PEMs, M03 , M30 , and M33 can be monitored simultaneously to increase data reliability. Additionally, two PEMs deliver other elements of the Mueller matrix that can be used to estimate the magnitude of the linear anisotropies.62,63 We caution that polystyrene well plates are unsuitable for the measurement of CD as the perturbing LB is much too large. Such a plate was analyzed with the 4-PEM and the heterogeneities in the optical properties were captured with an imaging Mueller matrix microscope described previously.64
Enantiomeric Excess Determination in Well Plates
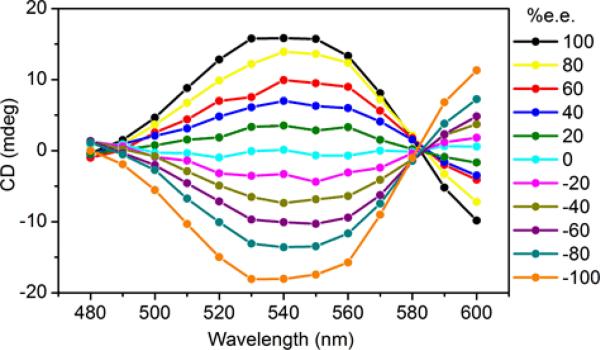
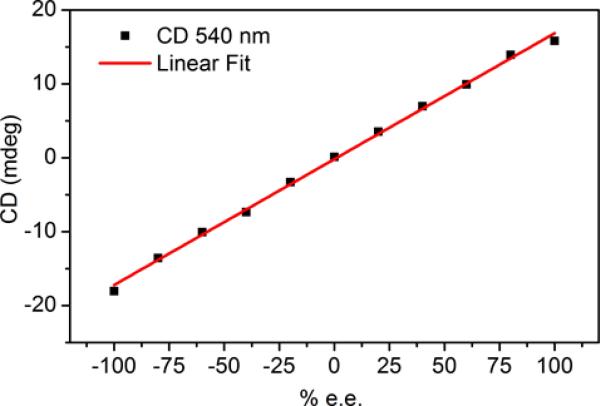
Amines 4-7 in Fe complexes in Scheme 1 were analyzed by the 4-PEM. CD spectra were measured (Figure 3) for solutions spanning the enantiomeric composition between 100% R configuration and 100% S configuration. A calibration curve was built from M03 values (Figure 4) and fit to a line in the case of 4. The R2 value was 0.998.
Figure 3.
CD spectra for the assembly with 4.
Figure 4.
Calibration curve for the assembly with 4 at 540 nm.
While the calibration curve in Figure 4 is a straight line for 4, they are not generally linear because the CD represents mixtures of stereoisomers at intermediate concentrations. The curves in the supporting material are generally sigmoidal and fit to 3rd order polynomials. See supporting information.
Once the calibration curves that relate CD signal intensity to ee for each amine were obtained, a number of “unknown” samples were analyzed from new stock solutions of products. The CD values were converted to values of ee using the calibration curves previously established. The calculated values of ee were compared to the actual values of the solutions measured. The ee errors ranged between |5-9|%. These are errors in chemistry (weighing reagents and volumes) and are consistent with those found when measuring such samples individually and fall within the range of accuracy that is expected for a HTS method.28
Conclusions
With a spectropolarimeter carrying more than one photoelastic modulator, CD was measured rapidly in a multi-well plate format. The technology has been tested with an existing sensing ensemble and the results are consistent with previous data. There should be few obstacles to HTS-CD in high quality well plates with glass interfaces.
Supplementary Material
Acknowledgements
AE gratefully acknowledges the financial support from the National Institutes of Health (GM077437) and the Welch Foundation (F-1151). BK thanks the National Science Foundation (DMR-1105000) and SMN is grateful for a National Science Foundation Predoctoral Fellowship (DGE-12342536).
Footnotes
Electronic Supplementary Information (ESI) available: details of experimental conditions, CD and ee calculations, Mueller matrix data. See DOI: 10.1039/b000000x/
References
- 1.Alves G, Fortuna A, Falcao A. Trends Chromatogr. 2008;4:1–10. [Google Scholar]
- 2.Stambuli JP, Hartwig JF. Curr. Opin. Chem. Biol. 2003;7:420. doi: 10.1016/s1367-5931(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu KD, Snapper ML, Hoveyda AH. Chem-Eur J. 1998;4:1885. [Google Scholar]
- 4.Shabbir SH, Regan CJ, Anslyn EV. Natl. Acad. Sci. USA. 2009;106:10487. doi: 10.1073/pnas.0809530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reetz MT. Angew. Chem. Int. Edit. 2002;41:1335. doi: 10.1002/1521-3773(20020415)41:8<1335::aid-anie1335>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Traverse JF, Snapper ML. Drug Discov. Today. 2002;7(19):1002–12. doi: 10.1016/s1359-6446(02)02436-4. [DOI] [PubMed] [Google Scholar]
- 7.Jaekel C, Paciello R. Chem. Rev. 2006;106(7):2912–42. doi: 10.1021/cr040675a. [DOI] [PubMed] [Google Scholar]
- 8.Gennari C, Piarulli U. Chem. Rev. 2003;103(8):3071–100. doi: 10.1021/cr020058r. [DOI] [PubMed] [Google Scholar]
- 9.Wahler D, Reymond J-L. Curr. Opin. Biotechnol. 2001;12(6):535–44. doi: 10.1016/s0958-1669(01)00260-9. [DOI] [PubMed] [Google Scholar]
- 10.Stambuli JP, Hartwig JF. Curr. Opin. Chem. Biol. 2003;7(3):420–6. doi: 10.1016/s1367-5931(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 11.de Vries JG, Lefort L. Chem. Eur. J. 2006;12(18):4722–34. doi: 10.1002/chem.200500819. [DOI] [PubMed] [Google Scholar]
- 12.Ding K. Chem. Commun. 2008;8:909–21. doi: 10.1039/b710668h. [DOI] [PubMed] [Google Scholar]
- 13.Lefort L, Boogers JAF, de Vries AHM, de Vries JG. Top. Catal. 2006;40(1-4):185–91. [Google Scholar]
- 14.Minnaard AJ, Feringa BL, Lefort L, de Vries JG. Acc. Chem. Res. 2007;40(12):1267–77. doi: 10.1021/ar7001107. [DOI] [PubMed] [Google Scholar]
- 15.Seiche W, Breit B. In: Catalysts with Chiral Self-assembling Ligands in Phosphorus Ligands in Asymmetric Catalysis. Börner A, editor. Wiley-VCH; Weinheim: 2008. [Google Scholar]
- 16.Sakthivel K, Notz W, Bui T, Barbas CF. J. Am. Chem. Soc. 2001;123(22):5260–7. doi: 10.1021/ja010037z. [DOI] [PubMed] [Google Scholar]
- 17.Lefort L, Boogers JAF, de Vries AHM, de Vries JG. Org. Lett. 2004;6(11):1733–5. doi: 10.1021/ol049510e. [DOI] [PubMed] [Google Scholar]
- 18.Feringa BL. Acc. Chem. Res. 2000;33(6):346–53. doi: 10.1021/ar990084k. [DOI] [PubMed] [Google Scholar]
- 19.Pamies O, Dieguez M. Chem. Eur. J. 2008;14(3):944–60. doi: 10.1002/chem.200700852. [DOI] [PubMed] [Google Scholar]
- 20.Pescarmona PP, Van der Waal JC, Maxwell IE, Maschmeyer T. Catal. Lett. 1999;63(1,2):1–11. [Google Scholar]
- 21.Crabtree RH. Chem. Commun. 1999;17:1611–6. [Google Scholar]
- 22.Powers DG, Coffen DL. Drug Discov. Today. 1999;4(8):377–83. doi: 10.1016/s1359-6446(99)01364-1. [DOI] [PubMed] [Google Scholar]
- 23.Jagt RBC, Toullec PY, Schudde EP, De Vries JG, Feringa BL, Minnaard AJ. J. Comb. Chem. 2007;9(3):407–14. doi: 10.1021/cc060161k. [DOI] [PubMed] [Google Scholar]
- 24.Tarasow TM, Tarasow SL, Eaton BE. Nature. 1997;389(6646):54–7. doi: 10.1038/37950. [DOI] [PubMed] [Google Scholar]
- 25.Reetz MT, Kuhling KM, Wilensek S, Husmann H, Hausig UW, Hermes M. Catal. Today. 2001;67(4):389–96. [Google Scholar]
- 26.Takacs JM, Han J. Org. Lett. 2004;6(18):3099–102. doi: 10.1021/ol0488791. [DOI] [PubMed] [Google Scholar]
- 27.Bromidge S, Wilson PC, Whiting A. Tetrahedron Lett. 1998;39(48):8905–8. [Google Scholar]
- 28.Leung D, Anslyn EV. J. Am. Chem. Soc. 2008;130(37):12328–12333. doi: 10.1021/ja8038079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf C, Bentley KW. Chem. Soc. Rev. 2013 [Google Scholar]
- 30.Ghosn MW, Wolf C. Tetrahedron. 2010;66(23):3989–3994. [Google Scholar]
- 31.You L, Berman JS, Anslyn EV. Nat. Chem. 2011;3(12):943–948. doi: 10.1038/nchem.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosn MW, Wolf C. J. Am. Chem. Soc. 2009;131(45):16360–16361. doi: 10.1021/ja907741v. [DOI] [PubMed] [Google Scholar]
- 33.Iwaniuk DP, Bentley KW, Wolf C. Chirality. 2012;24(7):584. doi: 10.1002/chir.22066. [DOI] [PubMed] [Google Scholar]
- 34.Joyce LA, Maynor MS, Dragna JM, da Cruz GM, Lynch VM, Canary JW, Anslyn EV. J. Am. Chem. Soc. 2011;133(34):13746–13752. doi: 10.1021/ja205775g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dragna JM, Pescitelli G, Tran L, Lynch VM, Anslyn EV, Di Bari L. J. Am. Chem. Soc. 2012;134(9):4398–4407. doi: 10.1021/ja211768v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metola P, Anslyn EV, James TD, Bull SD. Chem. Sci. 2011;3(1):156–161. [Google Scholar]
- 37.Ghosn MW, Wolf C. Tetrahedron. 2011;67(36):6799–6803. [Google Scholar]
- 38.Iwaniuk DP, Wolf C. Org. Lett. 2011;13(10):2602–2605. doi: 10.1021/ol200574x. [DOI] [PubMed] [Google Scholar]
- 39.Berova N, Nakanishi K, Woody RW. Circular dichroism: principles and applications. Wiley-VCH; New York: 2000. [Google Scholar]
- 40.Purdie N, Brittain HG. Analytical applications of circular dichroism. Elsevier; 1993. [DOI] [PubMed] [Google Scholar]
- 41.Eriksson M, Nordén B. Meth. Enzymol. 2001;340:68–98. doi: 10.1016/s0076-6879(01)40418-6. [DOI] [PubMed] [Google Scholar]
- 42.Jasco ASU-800 Autosampler [Google Scholar]
- 43.In our experience, the tubing used is not compatible with acetonitrile or halogenated solvents, and thus the robotic handler rapidly plugs with the use of these solvents. Additionally, the threshold for concentration tolerated by the plastic tubes before clogging occurs is low enough that it is another cause of concern.
- 44.Hipps KW, Crosby GA. J. Phys. Chem. 1979;83:555–562. [Google Scholar]
- 45.Schellman J, Jensen HP. Chem. Rev. 1987;87:1359–1399. [Google Scholar]
- 46.Michl J, Thulstrup EW. Spectroscopy with Polarized Light. VCH; New York: 1986. [Google Scholar]
- 47.Kuroda R, Harada T, Shindo Y. Rev. Sci. Instrum. 2001;72:3802–3810. [Google Scholar]
- 48.Maestre MF, Salzman GC, Tobey RA, Bustamante C. Biochemistry. 1985;24:5152–5157. doi: 10.1021/bi00340a600. [DOI] [PubMed] [Google Scholar]
- 49.Moxon JRL, Renshaw AR, Phys J. Condens. Matter. 1990;2:6807–6836. [Google Scholar]
- 50.Kremers M, Meekes H. J. Phys. D: Appl. Phys. 1995;28:1212–1224. [Google Scholar]
- 51.Arago F. Mem. Inst. Fr. 1811;1:93–134. [Google Scholar]
- 52.O'Loane JK. Chem. Rev. 1980;80:41–61. [Google Scholar]
- 53.Applequist J. Am. Sci. 1987;75:59–68. [Google Scholar]
- 54.Kaminsky W. Rep. Prog. Phys. 2000;63:1575–1640. [Google Scholar]
- 55.Kahr B, Arteaga O. Chemphyschem. 2012;16:79–88. doi: 10.1002/cphc.201100660. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi J, Asahi T, Takahashi S, Glazer AM. J. Appl. Crystallogr. 1988;21:479–484. [Google Scholar]
- 57.Arteaga O, Canillas A, Jellison GE., Jr. Appl. Opt. 2009;48:5307–5317. doi: 10.1364/AO.48.005307. [DOI] [PubMed] [Google Scholar]
- 58.Arteaga O, Freudenghtal J, Wang B, Nichols S, Kahr B. Chim. Oggi. 2012;30:31–36. [Google Scholar]
- 59.Arteaga O, Freudenthal J, Wang B, Kahr B. Appl. Opt. 2012;51:6805–6817. doi: 10.1364/AO.51.006805. [DOI] [PubMed] [Google Scholar]
- 60.Goldstein D, Goldstein DH. Polarized Light, revised and expanded. Vol. 83. CRC Press; 2003. [Google Scholar]
- 61.Arteaga O., Ph.D. Thesis. University of Barcelona; 2011. [Google Scholar]
- 62.Jellison GE, Jr., Modine FA. Appl. Opt. 1997;36:8184–8189. doi: 10.1364/ao.36.008184. [DOI] [PubMed] [Google Scholar]
- 63.Jellison GE, Jr., Modine FA. Appl. Opt. 1997;36:8190–8198. doi: 10.1364/ao.36.008190. [DOI] [PubMed] [Google Scholar]
- 64.Shtukenberg AG, Freudenthal J, Kahr B. J. Am. Chem. Soc. 2010;132:9341–9349. doi: 10.1021/ja101491n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.