Abstract
Upper and lower respiratory infections are common in early childhood and may be exacerbated by air pollution. We investigated short-term changes in ambient air pollutant concentrations, including speciated particulate matter less than 2.5 μm in diameter (PM2.5), in relation to emergency department (ED) visits for respiratory infections in young children. Daily counts of ED visits for bronchitis and bronchiolitis (n = 80,399), pneumonia (n = 63,359), and upper respiratory infection (URI) (n = 359,246) among children 0–4 years of age were collected from hospitals in the Atlanta, Georgia, area for the period 1993–2010. Daily pollutant measurements were combined across monitoring stations using population weighting. In Poisson generalized linear models, 3-day moving average concentrations of ozone, nitrogen dioxide, and the organic carbon fraction of particulate matter less than 2.5 μm in diameter (PM2.5) were associated with ED visits for pneumonia and URI. Ozone associations were strongest and were observed at low (cold-season) concentrations; a 1–interquartile range increase predicted a 4% increase (95% confidence interval: 2%, 6%) in visits for URI and an 8% increase (95% confidence interval: 4%, 13%) in visits for pneumonia. Rate ratios tended to be higher in the 1- to 4-year age group compared with infants. Results suggest that primary traffic pollutants, ozone, and the organic carbon fraction of PM2.5 exacerbate upper and lower respiratory infections in early life, and that the carbon fraction of PM2.5 is a particularly harmful component of the ambient particulate matter mixture.
Keywords: air pollution, bronchiolitis, children, lower respiratory infection, pneumonia, upper respiratory infection
Children may be more vulnerable to the health effects of ambient air pollution because of their higher rates of breathing, narrower airways, developing lungs and immune systems, and frequent exposure to outdoor air. The disease burden of respiratory infections such as bronchiolitis, bronchitis, pneumonia, and upper respiratory infection in early life is high, and globally, pneumonia is the largest cause of death among young children (1). Despite this, relatively few large-scale analyses of air pollution and respiratory infection have focused on infancy and the first few years of life. In particular, investigations assessing specific chemical components of ambient particulate matter in relation to these outcomes in young children are lacking.
Experimental studies have demonstrated that the severity of viral respiratory infection in animal and in vitro models can be enhanced by exposure to air pollutants (2–4). The results of several epidemiologic studies are consistent with these findings, including a natural experiment in the 1980s, when a steel mill closing in the Utah Valley, Utah, temporarily reduced pollution levels; during the period of reduced air pollution, hospital admissions for bronchitis and pneumonia decreased, most dramatically among preschool children (5). Subsequent epidemiologic studies have provided additional evidence of associations between short-term changes in air pollution and upper and lower respiratory infection symptoms and emergency health care among young children (6–10). However, the pollutants implicated are not consistent; associations for ozone have been particularly inconsistent, with reports of positive, null, and inverse associations with these outcomes (6–10).
We further examined these questions using a large database of emergency department (ED) visits in the Atlanta, Georgia, metropolitan area over an 18-year period between 1993 and 2010, including 80,399 visits for bronchiolitis and bronchitis, 63,359 visits for pneumonia, and 359,246 visits for upper respiratory infection (URI) among children under the age of 5 years. The study period includes 12 years (1998–2010) when chemical components of particulate matter less than 2.5 μm in diameter (PM2.5) were monitored daily at several monitoring sites in the study area, allowing assessment of the constituents of particulate matter that may exacerbate respiratory disease in young children. We conducted analyses to describe the shape of the concentration-response, assess season-specific effects, and explore confounding between pollutants, including among chemical components of PM2.5.
METHODS
We obtained ED visit data directly from 41 metropolitan Atlanta hospitals for the period January 1, 1993, to December 31, 2004 (not all hospitals contributed the full period), and from the Georgia Hospital Association for the period January 1, 2005, to June 30, 2010. We identified ED visits among children who were 0–4 years of age with billing zip codes within the 20-county Atlanta metropolitan area and the following primary diagnoses of respiratory infection based on International Classification of Diseases, Ninth Revision (ICD-9), diagnosis codes: acute bronchitis or bronchiolitis (code 466); pneumonia (codes 480–486), including viral, pneumococcal, other bacterial, unspecified organism, and bronchopneumonia; and upper respiratory infection (codes 460–465), including nasopharyngitis, sinusitis, pharyngitis, tonsillitis, laryngitis, tracheitis, and multiple or unspecified sites.
We obtained data on daily concentrations of ambient 1-hour maximum carbon monoxide and nitrogen dioxide; 8-hour maximum ozone; and 24-hour average particulate matter less than 10 µm in diameter (PM10), PM2.5, and the major chemical constituents of PM2.5 (sulfate, nitrate, ammonium, elemental carbon, and organic carbon) from ambient monitoring networks in the study area (11–14). Daily measurement of PM10 began in January 1996, and daily measurement of PM2.5 and its chemical components began in August 1998. Pollutant measurements were averaged across monitors for each day by population weighting based on the 2000 US Census, in an approach described elsewhere (15) and summarized in Web Appendix 1, available at http://aje.oxfordjournals.org/. The study population was concentrated in the center of the 20-county area (covering 6,208 miles2; 1 mile = 1.61 km). Data on daily ambient airborne pollen concentrations were obtained from the Atlanta Allergy and Asthma Clinic (Atlanta, Georgia).
In a time-series analysis using Poisson generalized linear models allowing for overdispersion, we estimated associations between the 3-day moving average pollutant concentration (the average of concentrations today (lag 0), yesterday (lag 1), and 2 days ago (lag 2)) and the daily counts of ED visits for 1) bronchiolitis and bronchitis, 2) pneumonia, and 3) URI. We chose the 3-day moving average as our a priori lag exposure on the basis of our previous work (16), but we assessed alternative lags in sensitivity analyses. For each outcome group, we first estimated associations separately for infants less than 1 year of age and children 1–4 years of age. Primary models included pollutant concentrations as linear terms, but we also flexibly modeled the shape of the concentration-response using loess smoothers in generalized additive models. All models included cubic polynomials for the 3-day moving average of average dew point (average of lags 0, 1, and 2) and the 3-day moving average of maximum temperature; cubic splines with 1 knot per month (12 df per year) to control for seasonality and longer-term trends (e.g., changes in ED usage over time); indicator terms for day of week, season, holiday, and lag holiday (i.e., holidays occurring in the previous 2 days); indicator terms for whether a hospital, or group of hospitals, was contributing visits on that day (not all hospitals contributed during the entire period); and interactions between season and day of week. We also assessed confounding by pollen concentrations (for ragweed, pine, oak, juniper, grass, and birch) and by influenza epidemics by including the log of the count of ED visits for influenza (using ICD-9 code 487).
To assess model misspecification and detect possible residual confounding, we conducted analyses in which we added a term for the pollutant concentration on the day after the ED visit (lag negative 1 pollution). Because tomorrow's pollution level cannot cause today's ED visit, observed associations with future pollution concentrations after controlling for the average of lag 0, 1, and 2 pollution (i.e., the hypothesized causal window) must be due to bias (or type 1 error) (17). In subanalyses, we estimated pollutant effects separately for November–February and for the warmer months in Atlanta of March–October using interaction terms, also allowing control of meteorological variables to vary seasonally. In multipollutant models, we estimated associations for PM2.5 components adjusted for other PM2.5 components. We also estimated the joint effect of a combined increase in multiple pollutants by calculating the exponentiated sum (of the pollutants in the combination) of the product of each pollutant's model coefficient and that pollutant's interquartile range (IQR); standard errors were calculated using the variance-covariance matrices for the pollutant coefficients. We conducted a variety of sensitivity analyses, described in more detail with the modeling approach in Web Appendix 1, including consideration of alternative approaches to controlling for meteorological factors (including precipitation), seasonality, and longer-term trends. Analyses were performed using SAS, version 9.3, software (SAS Institute, Inc., Cary, North Carolina) and R, version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Descriptive statistics of the daily pollutant concentrations are presented in Table 1. Correlations were strong between many of the pollutants even after accounting for season and meteorological factors (Table 2). ED visit counts for each outcome, age group, and monitoring period for each pollutant are presented in Table 3; in the earliest years of the study, few hospitals were participating, so ED counts were low. The strong seasonal patterns for these outcomes are shown in Web Figure 1. For the bronchiolitis/bronchitis outcome group, 90% of ED visits in infants less than 1 year of age were coded as bronchiolitis, whereas in children 1–4 years of age, only 52% were coded as bronchiolitis (ICD-9 code 466.1).
Table 1.
Daily Population-Weighted Ambient Air Pollutant Concentrations and Maximum Temperature, Atlanta, Georgia, 1993–2010
| Pollutant | Mean (SD) | Seasonal Mean |
Range | Percentile |
IQR | Missing Days, % | No. of Monitorsa | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| March–October | November–February | 5th | 25th | 50th | 75th | 95th | ||||||
| 8-hour ozone, ppbb | 45.9 (19.8) | 53.4 | 29.0 | 3.0–127.1 | 17.6 | 30.9 | 43.8 | 58.7 | 80.6 | 27.8 | 3 | 5 |
| 1-hour nitrogen dioxide, ppbb | 21.4 (8.7) | 20.9 | 22.4 | 2.1–95.2 | 8.8 | 15.3 | 20.5 | 26.4 | 36.7 | 11.1 | <1 | 6 |
| 1-hour carbon monoxide, ppmb | 0.70 (0.46) | 0.66 | 0.79 | 0.09–5.50 | 0.21 | 0.37 | 0.58 | 0.90 | 1.63 | 0.54 | <1 | 5 |
| 24-hour PM10, µg/m3c | 22.2 (10.4) | 24.6 | 17.6 | 3.6–93.8 | 8.5 | 14.6 | 20.7 | 28.1 | 40.9 | 13.5 | 4 | 9 |
| 24-hour PM2.5, µg/m3d | ||||||||||||
| All PM2.5 | 14.1 (6.9) | 15.1 | 12.1 | 2.1–75.2 | 5.6 | 9.1 | 12.8 | 17.8 | 27.4 | 8.8 | 0 | 11 |
| Sulfate | 4.2 (2.9) | 4.9 | 2.7 | 0.1–52.0 | 1.3 | 2.2 | 3.4 | 5.2 | 9.7 | 3.0 | <1 | 6 |
| Nitrate | 0.8 (0.6) | 0.6 | 1.2 | 0.0–5.7 | 0.2 | 0.4 | 0.6 | 1.0 | 2.0 | 0.6 | <1 | 6 |
| Ammonium | 1.5 (1.0) | 1.7 | 1.2 | 0.1–11.8 | 0.5 | 0.9 | 1.3 | 1.8 | 3.3 | 1.0 | <1 | 6 |
| Elemental carbon | 0.9 (0.5) | 0.9 | 0.9 | 0.1–7.9 | 0.3 | 0.5 | 0.8 | 1.1 | 1.9 | 0.6 | <1 | 6 |
| Organic carbon | 3.2 (1.4) | 3.2 | 3.3 | 0.6–14.6 | 1.5 | 2.2 | 3.0 | 4.0 | 5.8 | 1.7 | 1 | 6 |
| Maximum temperature, °C | 22.3 (8.4) | 26 | 14 | −8–40 | 7 | 16 | 23 | 29 | 34 | 13 | 0 | 1 |
Abbreviations: IQR, interquartile range; PM2.5, particulate matter less than 2.5 μm in diameter; PM10, particulate matter less than 10 μm in diameter; SD, standard deviation.
a All gaseous monitors operated daily, 9 PM2.5 monitors operated daily, 2 PM10 monitors operated daily, and 4 PM2.5 component monitors operated daily; where not monitored daily, particulate matter was monitored every 3 or 6 days.
b Measurements available from January 1, 1993, to June 30, 2010 (n = 6,390). Ozone was not measured from December 1994 to February 1995 or from December 1995 to February 1996.
c Measurements available from January 1, 1996, to June 30, 2010 (n = 5,295).
d Measurements available from August 1, 1998, to June 30, 2010 (n = 4,352).
Table 2.
Partial Spearman Correlation Coefficientsa for Daily Air Pollutant Concentrations, Atlanta, Georgia, 1993–2010
| Pollutant | Ozone | Nitrogen Dioxide | Carbon Monoxide | PM10 | PM2.5 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| All PM2.5 | Sulfate | Nitrate | Ammonium | Elemental Carbon | Organic Carbon | |||||
| Ozone | 1 | |||||||||
| Nitrogen dioxide | 0.37 | 1 | ||||||||
| Carbon monoxide | 0.21 | 0.57 | 1 | |||||||
| PM10 | 0.32 | 0.46 | 0.48 | 1 | ||||||
| PM2.5 | ||||||||||
| All PM2.5 | 0.30 | 0.41 | 0.45 | 0.82 | 1 | |||||
| Sulfate | 0.30 | 0.20 | 0.18 | 0.61 | 0.74 | 1 | ||||
| Nitrate | −0.02 | 0.16 | 0.19 | 0.37 | 0.45 | 0.38 | 1 | |||
| Ammonium | 0.28 | 0.20 | 0.16 | 0.62 | 0.76 | 0.89 | 0.46 | 1 | ||
| Elemental carbon | 0.20 | 0.60 | 0.68 | 0.59 | 0.57 | 0.32 | 0.30 | 0.31 | 1 | |
| Organic carbon | 0.29 | 0.50 | 0.57 | 0.69 | 0.72 | 0.44 | 0.36 | 0.45 | 0.77 | 1 |
Abbreviations: PM10, particulate matter less than 10 μm in diameter; PM2.5, particulate matter less than 2.5 μm in diameter.
a Adjusted for seasonality and long-term trends (cubic splines with 1 knot/month), maximum temperature (cubic terms), average dew point (cubic terms), weekday, weekday × season (winter, spring, summer, fall), and holidays.
Table 3.
Number of Emergency Department Visits by Outcome, Patient Age, and Monitoring Period, Atlanta, Georgia 1993–2010
| Outcome by Patient Age | Emergency Department Visits |
|||||
|---|---|---|---|---|---|---|
| January 1993–June 2010a |
January 1996–June 2010b |
August 1998–June 2010c |
||||
| No. | Daily Mean | No. | Daily Mean | No. | Daily Mean | |
| Bronchiolitis or bronchitis | ||||||
| <1 year | 50,222 | 47,227 | 42,705 | |||
| 1–4 years | 30,177 | 27,513 | 24,355 | |||
| Total | 80,399 | 12.6 | 74,740 | 14.1 | 67,060 | 15.4 |
| Pneumonia | ||||||
| <1 year | 14,686 | 13,696 | 12,056 | |||
| 1–4 years | 48,673 | 44,687 | 39,773 | |||
| Total | 63,359 | 9.9 | 58,383 | 11.0 | 51,829 | 11.9 |
| Upper respiratory infection | ||||||
| <1 year | 124,746 | 116,867 | 107,454 | |||
| 1–4 years | 234,500 | 214,342 | 195,886 | |||
| Total | 359,246 | 56.2 | 331,209 | 62.6 | 303,340 | 69.7 |
a Monitoring period for ozone, nitrogen dioxide, and carbon monoxide.
b Monitoring period for particulate matter less than 10 μm in diameter.
c Monitoring period for particulate matter less than 2.5 μm in diameter and components.
Table 4 presents results of the primary analysis of 3-day moving average pollutant concentrations as linear terms in single-pollutant models scaled to a 1–IQR increase (Table 1) to facilitate comparisons between pollutants for the same relative degree of variability. Rate ratios for all outcomes were generally higher among children aged 1–4 years compared with infants less than 1 year of age. All rate ratios for ozone and the carbon fractions of PM2.5 were above the null value (P < 0.05 for pneumonia and URI). ED visits for URI among children aged 0–4 years were positively associated with all pollutants except the nitrate fraction of PM2.5. The number of ED visits for pneumonia was positively associated with ozone, nitrogen dioxide, PM10 (for children aged 1–4 years only), and the organic carbon fraction of PM2.5. The nitrate fraction of PM2.5 was negatively associated with bronchiolitis and bronchitis; no pollutants were positively associated with this outcome, although some of the point estimates in the children aged 1–4 years were similar to those observed for URI and pneumonia (e.g., PM10 and the organic carbon fraction of PM2.5). Results of analyses adding future pollution (lag negative 1) to the primary models to assess model misspecification are shown in Web Figure 2. For bronchiolitis/bronchitis in infants less than 1 year of age, the point estimates for future pollution are systematically below the null and often statistically significant, suggesting model misspecification. For the other outcomes, the estimates from the lag negative 1 analysis did not indicate problems with the analysis.
Table 4.
Adjusted Rate Ratios for Interquartile Range Increases in 3-Day Moving Average Ambient Air Pollutant Concentrationsa and Emergency Department Visits Among Children, Atlanta, Georgia, 1993–2010
| Outcome by Pollutant | 0–4 Years of Age |
<1 Year of Age |
1–4 Years of Age |
|||
|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Bronchiolitis/bronchitisb | ||||||
| Ozone | 1.029 | 0.985, 1.075 | 1.040 | 0.986, 1.098 | 1.014 | 0.949, 1.083 |
| Nitrogen dioxide | 1.000 | 0.980, 1.021 | 0.990 | 0.966, 1.015 | 1.014 | 0.982, 1.047 |
| Carbon monoxide | 1.012 | 0.992, 1.031 | 1.017 | 0.993, 1.041 | 1.000 | 0.970, 1.032 |
| PM10 | 1.013 | 0.991, 1.037 | 1.007 | 0.981, 1.035 | 1.020 | 0.985, 1.058 |
| PM2.5 | ||||||
| All PM2.5 | 1.005 | 0.983, 1.027 | 0.998 | 0.972, 1.024 | 1.016 | 0.982, 1.051 |
| Sulfate | 1.007 | 0.984, 1.030 | 0.996 | 0.969, 1.025 | 1.022 | 0.988, 1.057 |
| Nitrate | 0.980c | 0.965, 0.996 | 0.977d | 0.960, 0.994 | 0.987 | 0.962, 1.013 |
| Ammonium | 0.997 | 0.977, 1.017 | 0.991 | 0.966, 1.015 | 1.007 | 0.977, 1.039 |
| Elemental carbon | 1.011 | 0.993, 1.029 | 1.010 | 0.989, 1.031 | 1.013 | 0.984, 1.043 |
| Organic carbon | 1.013 | 0.995, 1.031 | 1.008 | 0.987, 1.029 | 1.020 | 0.990, 1.051 |
| Pneumoniae | ||||||
| Ozone | 1.083f | 1.038, 1.131 | 1.033 | 0.949, 1.124 | 1.100f | 1.047, 1.155 |
| Nitrogen dioxide | 1.025c | 1.003, 1.047 | 1.021 | 0.979, 1.066 | 1.026c | 1.001, 1.051 |
| Carbon monoxide | 1.015 | 0.994, 1.037 | 1.011 | 0.969, 1.054 | 1.016 | 0.992, 1.041 |
| PM10 | 1.020 | 0.997, 1.045 | 0.997 | 0.951, 1.044 | 1.027c | 1.001, 1.056 |
| PM2.5 | ||||||
| All PM2.5 | 1.010 | 0.988, 1.033 | 0.994 | 0.952, 1.039 | 1.015 | 0.990, 1.041 |
| Sulfate | 1.005 | 0.983, 1.027 | 1.000 | 0.959, 1.043 | 1.006 | 0.982, 1.031 |
| Nitrate | 1.000 | 0.983, 1.017 | 0.991 | 0.959, 1.025 | 1.002 | 0.983, 1.022 |
| Ammonium | 1.000 | 0.980, 1.020 | 1.003 | 0.965, 1.042 | 0.999 | 0.977, 1.022 |
| Elemental carbon | 1.014 | 0.994, 1.034 | 1.005 | 0.967, 1.045 | 1.016 | 0.994, 1.039 |
| Organic carbon | 1.020c | 1.000, 1.040 | 1.015 | 0.977, 1.056 | 1.021 | 0.998, 1.044 |
| Upper respiratory infectiong | ||||||
| Ozone | 1.041f | 1.019, 1.064 | 1.059f | 1.026, 1.092 | 1.032c | 1.006, 1.058 |
| Nitrogen dioxide | 1.027f | 1.016, 1.039 | 1.017c | 1.001, 1.034 | 1.033f | 1.019, 1.047 |
| Carbon monoxide | 1.023f | 1.011, 1.035 | 1.006 | 0.989, 1.023 | 1.031f | 1.017, 1.046 |
| PM10 | 1.023f | 1.012, 1.036 | 1.008 | 0.992, 1.026 | 1.031f | 1.018, 1.047 |
| PM2.5 | ||||||
| All PM2.5 | 1.015d | 1.004, 1.027 | 1.002 | 0.986, 1.018 | 1.023f | 1.010, 1.037 |
| Sulfate | 1.013c | 1.003, 1.024 | 1.010 | 0.995, 1.024 | 1.015c | 1.003, 1.028 |
| Nitrate | 1.000 | 0.990, 1.009 | 0.986 | 0.973, 0.999 | 1.008 | 0.997, 1.019 |
| Ammonium | 1.011c | 1.001, 1.021 | 1.009 | 0.995, 1.022 | 1.013c | 1.001, 1.025 |
| Elemental carbon | 1.015d | 1.005, 1.026 | 1.002 | 0.988, 1.017 | 1.023f | 1.010, 1.036 |
| Organic carbon | 1.019f | 1.008, 1.030 | 1.010 | 0.996, 1.025 | 1.024f | 1.011, 1.037 |
Abbreviations: CI, confidence interval; IQR, interquartile range; PM10, particulate matter less than 10 μm in diameter; PM2.5, particulate matter less than 2.5 μm in diameter; RR, rate ratio.
a IQR units presented in Table 1.
b For age 0–1 years, n = 50,222; for age 1–4 years, n = 30,177.
c P < 0.05 from Wald χ2 test.
d P < 0.01 Wald χ2 test.
e For age 0–1 years, n = 14,686; for age 1–4 years, n = 48,673.
f P < 0.001 Wald χ2 test.
g For age 0–1 years, n = 124,746; for age 1–4 years, n = 234,500.
Results showed some sensitivity to the choice of degrees of freedom included in the cubic time splines. For the positive associations observed, rate ratios tended to be slightly higher when fewer knots were included and slightly lower when more knots were included (Web Figure 3). Results were not sensitive to the choice of meteorological control, including control for mean temperature instead of maximum temperature, additional degrees of freedom, controlling separately for lag 0 and lag 1–2 moving average temperature, or controlling for precipitation. Control for pollen concentrations or influenza visit counts had virtually no impact on the pollutant estimates and was not performed in the final models. Assessment of individual lag days 0–7 (Web Figure 4) generally showed stronger associations for lag days 0–3, but there was some indication of elevated risk at longer lags for some pollutant-outcome combinations (e.g., pneumonia and PM10). Because the indicated model misspecification for bronchiolitis was not resolved after extensive analyses considering other model specifications, subsequent analyses focused on pneumonia and URI. Figure 1 shows season-specific results for the November–February and March–October seasons scaled to the same IQR increase (see Table 1). For both pneumonia and URI, the rate ratio for a 1–IQR increase in ozone was highest in the November–February period, when ozone levels are lowest.
Figure 1.
Season-specific (circles = March–October; triangles = November–February) rate ratios per 1–interquartile range increase in 3-day moving average population-weighted ambient air pollutant concentrations for A) pneumonia and B) upper respiratory infections, Atlanta, Georgia, 1993–2010. Bars, 95% confidence intervals. CO, carbon monoxide; EC, elemental carbon fraction of PM2.5; NH4, ammonium fraction of PM2.5; NO2, nitrogen dioxide; NO3, nitrate fraction of PM2.5; O3, ozone; PM10, particulate matter less than 10 μm in diameter; OC, organic carbon fraction of PM2.5; PM2.5, particulate matter less than 2.5 μm in diameter; SO4, sulfate fraction of PM2.5.
Estimation of nonlinear associations between 3-day average pollutant concentrations and ED visits using loess smoothers indicated that linearity of the pollutant associations was a reasonable approximation for most pollutant-outcome combinations (Web Figure 5). Relative to the fifth percentile of ozone concentrations (20 ppb), ED visits for pneumonia and URI were significantly elevated at concentrations as low as 30 ppb. In contrast to ozone, some of the plots for nitrogen dioxide, PM10, and the organic carbon fraction of PM2.5 showed higher rates of ED visits only at the middle or top of the concentration distribution.
Joint-effect estimates from multipollutant models are presented in Figure 2 for the following pollutant combinations: 1) nitrogen dioxide, carbon monoxide, and the elemental carbon fraction of PM2.5, which are 3 markers of primary traffic exhaust; 2) ozone and carbon monoxide; 3) ozone and the elemental carbon fraction of PM2.5; 4) ozone and nitrogen dioxide; and 5) ozone and PM2.5. For both URI and pneumonia, the rate ratio for a combined 1–IQR increase in nitrogen dioxide, carbon monoxide, and the elemental carbon fraction of PM2.5 is similar to the highest rate ratio observed among these primary traffic pollutants in their respective single-pollutant models (confidence intervals for pneumonia are wider because of fewer visits). In contrast, when ozone and any of the primary traffic pollutants were included in a model together, the joint effect was higher than either single-pollutant rate ratio. When ozone and PM2.5 were included together in a multipollutant model, the joint effect was similar to the estimate for ozone from a single-pollutant model.
Figure 2.
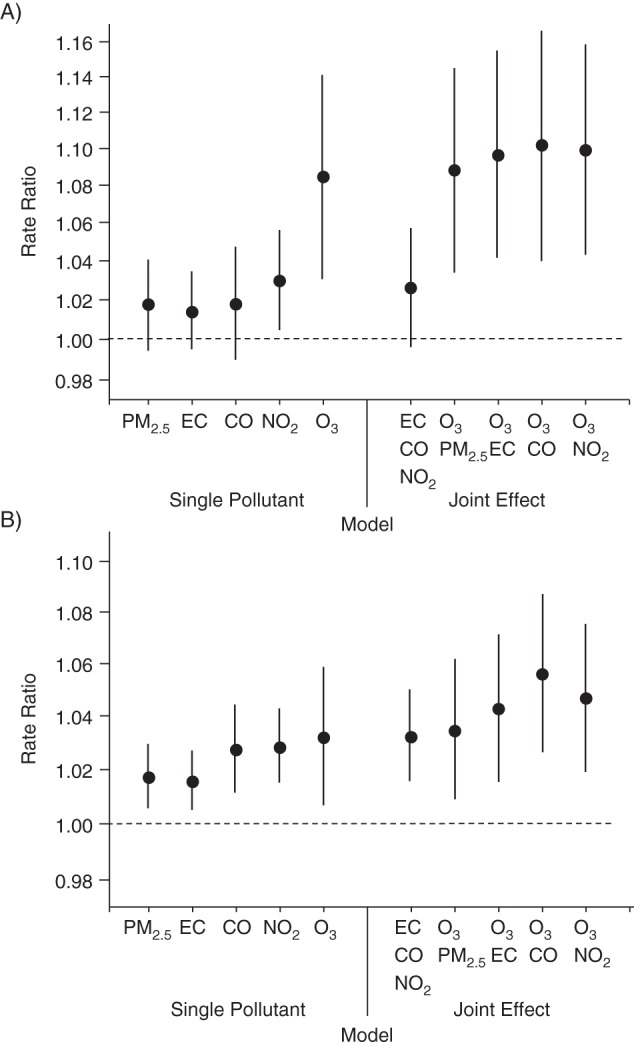
Rate ratios per 1–interquartile range increase in ozone (O3), nitrogen dioxide (NO2), carbon monoxide (CO), particulate matter less than 2.5 µm in diameter (PM2.5), and the elemental carbon fraction of PM2.5 (EC) from single-pollutant models and joint-effects models (for a combined increase in the interquartile range of all pollutants listed) for A) pneumonia and B) upper respiratory infection, Atlanta, Georgia, 1998–2010. All estimates are based on 4,186 observation days with measurements for all 5 pollutants. Bars, 95% confidence intervals.
Figure 3 shows results from the models including multiple PM2.5 components. The ammonium fraction of PM2.5 was excluded from this model because of model instability due to its high correlation with the sulfate fraction of PM2.5 (r = 0.9). To a lesser extent, this instability also affected organic carbon and elemental carbon, which were included despite a high correlation (r = 0.8). Among the PM2.5 components included, the organic carbon fraction of PM2.5 was most strongly associated with URI and pneumonia.
Figure 3.
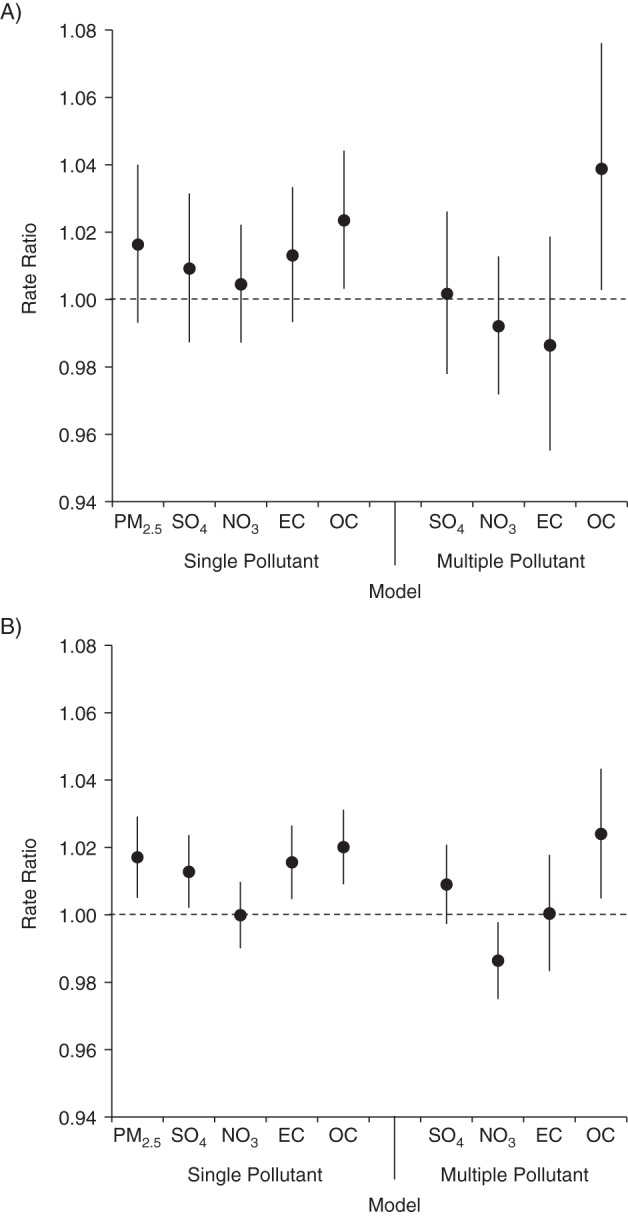
Rate ratios per 1–interquartile range increase in components of particulate matter less than 2.5 μm in diameter (PM2.5) from single-pollutant models and from a multipollutant model including the sulfate fraction of PM2.5 (SO4), nitrate (NO3), elemental carbon (EC), and organic carbon (OC) for A) pneumonia and B) upper respiratory infection, Atlanta, Georgia, 1998–2010. Bars, 95% confidence intervals.
DISCUSSION
In our large population-based study we observed evidence that ED visits for respiratory infection among children less than 5 years of age were associated with short-term increases in pollutant concentrations. ED visits for pneumonia and URI were associated with both ozone and markers of primary traffic pollution (e.g., nitrogen dioxide, carbon monoxide, and the elemental carbon fraction of PM2.5) over the previous 3 days. Of the chemical constituents of PM2.5 we assessed, the strongest associations were observed with the carbon fraction of PM2.5, particularly organic carbon. For all outcomes, rate ratios tended to be higher in children aged 1–4 years compared with infants less than 1 year of age. Although ED visits for bronchiolitis/bronchitis were not positively associated with any pollutants, rate ratio estimates in children aged 1–4 years were similar to those observed for URI and pneumonia for several of the particulate matter measures. For infant bronchiolitis, there was evidence of model misspecification indicated by the lag negative 1 analyses (future pollution).
The major limitation of our study, as in similar studies of this kind, is misclassification of exposures and outcomes. We defined outcome groups on the basis of the primary ICD-9 codes listed for ED visits, and misclassification of the type of respiratory infection is likely, particularly in this age group. Nonetheless, temporal patterns of the outcomes did show some anticipated differences, with visits for bronchiolitis showing steep peaks during the respiratory syncytial virus (RSV) season and more variable, sustained increases in visits for URI starting in the fall. Despite differences in the temporal patterns of the outcomes, associations with air pollutants were generally similar among the outcomes, with the possible exception of infant bronchiolitis, discussed below. We did not present results for a combined respiratory outcome group because these results would be driven by visits for URI, which constituted the vast majority of respiratory visits. Finally, we note that our outcome measure of ED visits likely includes children with more severe disease or less access to primary health care; associations with air pollution may be weaker or stronger for other population subgroups.
To calculate daily pollutant concentrations for the study area, we used a spatially averaged approach with population weights that used all available monitoring data on a given day (15). However, the spatial variability of the pollutants examined varies considerably, with secondary pollutants (e.g., ozone and the sulfate and nitrate fractions of PM2.5) being more spatially homogeneous and spatially correlated than primary pollutants (e.g., carbon monoxide, nitrogen dioxide, and the elemental carbon fraction of PM2.5) and pollutants with both primary and secondary sources in between (i.e., the organic carbon fraction of PM2.5). We decided a priori not to assess sulfur dioxide in this study because of the limited number of available monitors and extreme spatial heterogeneity in our study area. Our previous work exploring the effects of measurement error in Atlanta suggests that the net bias caused by measurement error in our study is likely toward the null, especially for the more spatially heterogeneous pollutants (e.g., nitrogen dioxide, carbon monoxide, and the elemental carbon fraction of PM2.5) (16, 18).
The strong positive correlations between the pollutants and differences in spatial heterogeneity among the pollutants further complicate interpretation, because any pollutant may be serving as a surrogate of a less well-measured or unmeasured copollutant (19). Although it is difficult to attribute effects to specific pollutants in this study setting, our joint-effects models suggested that individual associations with primary traffic pollutants (i.e., nitrogen dioxide, carbon monoxide, and the elemental carbon fraction of PM2.5) could all be reflecting the same underlying association, possibly with other unmeasured traffic-related pollutants such as ultrafine particles. In contrast, multipollutant models combining ozone with nitrogen dioxide, carbon monoxide, or the elemental carbon fraction of PM2.5 suggested independent effects of primary traffic pollution and ozone.
Examination of the concentration-response for ozone showed elevated rates of pneumonia and ED visits for URI as low as 30 ppb (relative to 20 ppb), well below the National Ambient Air Quality Standard of an 8-hour maximum of 75 ppb and lower than some estimates of policy-relevant background concentrations (i.e., concentrations that would exist in the absence of anthropogenic emissions) (20). Controlled human exposure studies on healthy volunteers show reduced lung function at ozone levels as low as 60 ppb, although few experimental studies have assessed lower concentrations (20). Young children with underlying respiratory infections may be more sensitive to low concentrations of ozone than healthy volunteers. We also observed higher rate ratios for ozone (for the same 27.8-ppb increase) in November to February, when concentrations are the lowest. With few indoor sources of ozone, higher associations may be observed for ozone in the coldest months because of greater differences between personal exposure and ambient concentrations in the warmer season. Children may be likely to be outside on unusually warm, sunny days in the winter and, thus, high ambient ozone is correlated with high personal ozone exposure in the winter, whereas in the warmer months, high-ozone days are also the most unpleasantly hot days in Atlanta, when children stay inside and actually experience low personal ozone exposure. Alternatively, our results could reflect unmeasured confounding involving behavior changes on high-ozone days, such as increased vigorous activity on sunny winter days leading to the exacerbation of respiratory disease.
The toxicity of airborne particles is likely to vary by chemical composition, and a major strength of our study was the availability of daily measurements of the major PM2.5 components from multiple monitors in the study area over a 12-year period. We observed significant associations between the organic carbon fraction of PM2.5 and pneumonia and URI and a suggestive association with bronchiolitis/bronchitis among children aged 1–4 years, both of which were robust to control for other PM2.5 components. Model results for the particulate matter components should be interpreted in light of differences in measurement error across pollutants in our study area, with increasing spatial heterogeneity of pollutant concentrations observed from sulfate to nitrate to organic carbon to elemental carbon (15). Elemental carbon is most spatially heterogeneous because it is a primary pollutant from traffic sources; organic carbon in Atlanta is from both primary combustion sources and secondary formation, derived from both biogenic and anthropogenic precursors (21). Given the high correlation between organic carbon and elemental carbon (r = 0.8) and the greater expected bias toward the null for elemental carbon due to measurement error (17, 18), it is possible that associations with organic carbon capture some of the true effect of elemental carbon. We also observed an association between the sulfate fraction of PM2.5, a secondary pollutant formed from coal-fired power plant emissions, and URI and a suggestive association with bronchitis/bronchiolitis among children aged 1–4 years, but little evidence of an association with pneumonia. We note that the power to detect associations was far greater for URI than the other outcomes because of the larger number of visits.
Our extensive sensitivity analyses showed that results were robust to alternative approaches for meteorological control but were somewhat sensitive to the degrees of freedom included in the cubic splines. Relative to the literature, our primary models included a large number of degrees of freedom to control for time (12 knots/year) (22, 23). As shown in Web Figure 3, fewer degrees of freedom (less control for time) generally led to slightly higher rate ratios, and more degrees of freedom led to slightly lower rate ratios. Visual inspection of the time splines (Web Figure 1) showed that our primary models captured the systematic seasonal trends in the outcomes of greatest concern for confounding but also accounted for some of the finer-scale temporal variability in the outcome. If our approach erred on the side of overcontrol for time, our sensitivity analyses indicate that the true rate ratios for pollution would be slightly higher than those reported for our primary analysis.
Our analysis of future pollution as an indicator of model misspecification suggested that estimated associations for the bronchiolitis outcome, particularly among infants, were biased. Associations with the future pollution indicator were not reduced by the inclusion of additional degrees of freedom in the smooth functions of time, suggesting that residual confounding by seasonality or longer-term trends was not the problem. Notably, at least 2 previous investigations reported inverse associations between daily pollutant concentrations and bronchiolitis hospitalizations, findings that also suggest the presence of an unmeasured confounder, because it is unlikely that air pollution has a protective effect on bronchiolitis. In southern California, inverse associations between traffic-related pollutants (nitrogen dioxide, carbon monoxide, and PM2.5) over the previous 1–8 days and bronchiolitis hospitalization among infants in winter were observed (24). Similarly, in Vietnam, inverse associations between PM10, nitrogen dioxide, and ozone averaged over the previous 6 days and hospitalization for acute lower respiratory infection in children less than 5 years of age were observed only in the rainy (i.e., the RSV) season (8). There is evidence that RSV epidemics are influenced by meteorological factors, either directly or through indirect effects on behavior (25), although these relationships are poorly understood. Similar to the Vietnam study, our control for rainfall over the previous 3 days did not change the pollutant estimates. Although we did not observe a protective association, our future pollution analyses similarly suggest a bias in our bronchiolitis results. It is possible that there is a complex interaction between meteorological factors and circulating RSV that is not captured adequately by our models or those used in previous studies. A better understanding of the relationship between meteorological factors and RSV may help improve estimation of potential acute associations between air pollution and bronchiolitis.
In summary, we observed independent effects of ozone and primary traffic pollutants on ED visits for pneumonia and URI and evidence that the carbon fraction of PM2.5 may be a particularly harmful component of the PM2.5 mixture for respiratory infections in children. The modest increases in risk that we observed translate into large public health impacts when considering the prevalence of the exposure and pediatric diseases assessed.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia (Lyndsey A. Darrow, W. Dana Flanders); Department of Environmental Health, Rollins School of Public Health, Emory University, Atlanta, Georgia (Mitchel Klein, Paige E. Tolbert, Matthew J. Strickland); and School of Civil and Environmental Engineering, Georgia Institute of Technology, Atlanta, Georgia (James A. Mulholland).
This study was supported by the National Institutes of Health (NIH) (grants R03ES018963 and K01ES019877) and the United States Environmental Protection Agency (EPA) (grant R834799).
This work is solely the responsibility of the grantee and does not necessarily represent the official views of the EPA or NIH. Further, the EPA and NIH do not endorse the purchase of any commercial products or services mentioned in the publication.
Conflict of interest: none declared.
REFERENCES
- 1.World Health Organization. World Health Statistics 2013. Geneva, Switzerland: World Health Organization Press; 2013. [Google Scholar]
- 2.Castranova V, Ma JY, Yang HM, et al. Effect of exposure to diesel exhaust particles on the susceptibility of the lung to infection. Environ Health Perspect. 2001;109(Suppl 4):609–612. doi: 10.1289/ehp.01109s4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrod KS, Jaramillo RJ, Rosenberger CL, et al. Increased susceptibility to RSV infection by exposure to inhaled diesel engine emissions. Am J Respir Cell Mol Biol. 2003;28(4):451–463. doi: 10.1165/rcmb.2002-0100OC. [DOI] [PubMed] [Google Scholar]
- 4.Mikerov AN, Haque R, Gan X, et al. Ablation of SP-A has a negative impact on the susceptibility of mice to Klebsiella pneumoniae infection after ozone exposure: sex differences. Respir Res. 2008;9:77. doi: 10.1186/1465-9921-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pope CA., 3rd Respiratory hospital admissions associated with PM10 pollution in Utah, Salt Lake, and Cache Valleys. Arch Environ Health. 1991;46(2):90–97. doi: 10.1080/00039896.1991.9937434. [DOI] [PubMed] [Google Scholar]
- 6.Barnett AG, Williams GM, Schwartz J, et al. Air pollution and child respiratory health: a case-crossover study in Australia and New Zealand. Am J Respir Crit Care Med. 2005;171(11):1272–1278. doi: 10.1164/rccm.200411-1586OC. [DOI] [PubMed] [Google Scholar]
- 7.Gouveia N, Fletcher T. Respiratory diseases in children and outdoor air pollution in São Paulo, Brazil: a time series analysis. Occup Environ Med. 2000;57(7):477–483. doi: 10.1136/oem.57.7.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le TG, Ngo L, et al. HEI Collaborative Working Group on Air Pollution, Poverty, and Health in Ho Chi Minh City. Effects of short-term exposure to air pollution on hospital admissions of young children for acute lower respiratory infections in Ho Chi Minh City, Vietnam. Res Rep Health Eff Inst. 2012;(169):5–72. [PubMed] [Google Scholar]
- 9.Hertz-Picciotto I, Baker RJ, Yap PS, et al. Early childhood lower respiratory illness and air pollution. Environ Health Perspect. 2007;115(10):1510–1518. doi: 10.1289/ehp.9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ségala C, Poizeau D, Mesbah M, et al. Winter air pollution and infant bronchiolitis in Paris. Environ Res. 2008;106(1):96–100. doi: 10.1016/j.envres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 11.United States Environmental Protection Agency. Air Quality System 2013. http://www.epa.gov/airquality/airdata/ Updated July 11, 2014. Accessed July 22, 2014.
- 12.Butler AJ, Andrew MS, Russell AG. Daily sampling of PM2.5 in Atlanta: results of the first year of the Assessment of Spatial Aerosol Composition in Atlanta Study. J Geophys Res-Atmos. 2003;108(D7):SOS3-1–SOS3-11. [Google Scholar]
- 13.Hansen DA, Edgerton E, Hartsell B, et al. Air quality measurements for the Aerosol Research and Inhalation Epidemiology Study. J Air Waste Manag Assoc. 2006;56(10):1445–1458. doi: 10.1080/10473289.2006.10464549. [DOI] [PubMed] [Google Scholar]
- 14.Van Loy M, Bahadori T, Wyzga R, et al. The Aerosol Research and Inhalation Epidemiology Study (ARIES): PM2.5 mass and aerosol component concentrations and sampler intercomparisons. J Air Waste Manag Assoc. 2000;50(8):1446–1458. doi: 10.1080/10473289.2000.10464187. [DOI] [PubMed] [Google Scholar]
- 15.Ivy D, Mulholland JA, Russell AG. Development of ambient air quality population-weighted metrics for use in time-series health studies. J Air Waste Manag Assoc. 2008;58(5):711–720. doi: 10.3155/1047-3289.58.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strickland MJ, Gass KM, Goldman GT, et al. Effects of ambient air pollution measurement error on health effect estimates in time-series studies: a simulation-based analysis. J Expo Sci Environ Epidemiol. doi: 10.1038/jes.2013.16. [published online ahead of print April 1, 2013] ( doi:10.1038/jes.2013.16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flanders WD, Klein M, Darrow LA, et al. A method for detection of residual confounding in time-series and other observational studies. Epidemiology. 2011;22(1):59–67. doi: 10.1097/EDE.0b013e3181fdcabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldman GT, Mulholland JA, Russell AG, et al. Ambient air pollutant measurement error: characterization and impacts in a time-series epidemiologic study in Atlanta. Environ Sci Technol. 2010;44(19):7692–7698. doi: 10.1021/es101386r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolbert PE, Klein M, Peel JL, et al. Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. J Expo Sci Environ Epidemiol. 2007;17(Suppl 2):S29–S35. doi: 10.1038/sj.jes.7500625. [DOI] [PubMed] [Google Scholar]
- 20.United States Environmental Protection Agency. Integrated Science Assessment for Ozone and Related Photochemical Oxidants (EPA 600/R-10/076F) Washington, DC: Office of Research and Development; National Center for Environmental Assessment; 2013. [Google Scholar]
- 21.Ding X, Zheng M, Yu L, et al. Spatial and seasonal trends in biogenic secondary organic aerosol tracers and water-soluble organic carbon in the southeastern United States. Environ Sci Technol. 2008;42(14):5171–5176. doi: 10.1021/es7032636. [DOI] [PubMed] [Google Scholar]
- 22.Dominici F, McDermott A, Daniels M, et al. Revised analyses of the National Morbidity, Mortality, and Air Pollution Study: mortality among residents of 90 cities. J Toxicol Environ Health A. 2005;68(13-14):1071–1092. doi: 10.1080/15287390590935932. [DOI] [PubMed] [Google Scholar]
- 23.Katsouyanni K, Samet JM, Anderson HR, et al. Air pollution and health: a European and North American Approach (APHENA) Res Rep Health Eff Inst. 2009;(142):5–90. [PubMed] [Google Scholar]
- 24.Karr C, Lumley T, Shepherd K, et al. A case-crossover study of wintertime ambient air pollution and infant bronchiolitis. Environ Health Perspect. 2006;114(2):277–281. doi: 10.1289/ehp.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welliver R. The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Paediatr Respir Rev. 2009;10(Suppl 1):6–8. doi: 10.1016/S1526-0542(09)70004-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



