Abstract
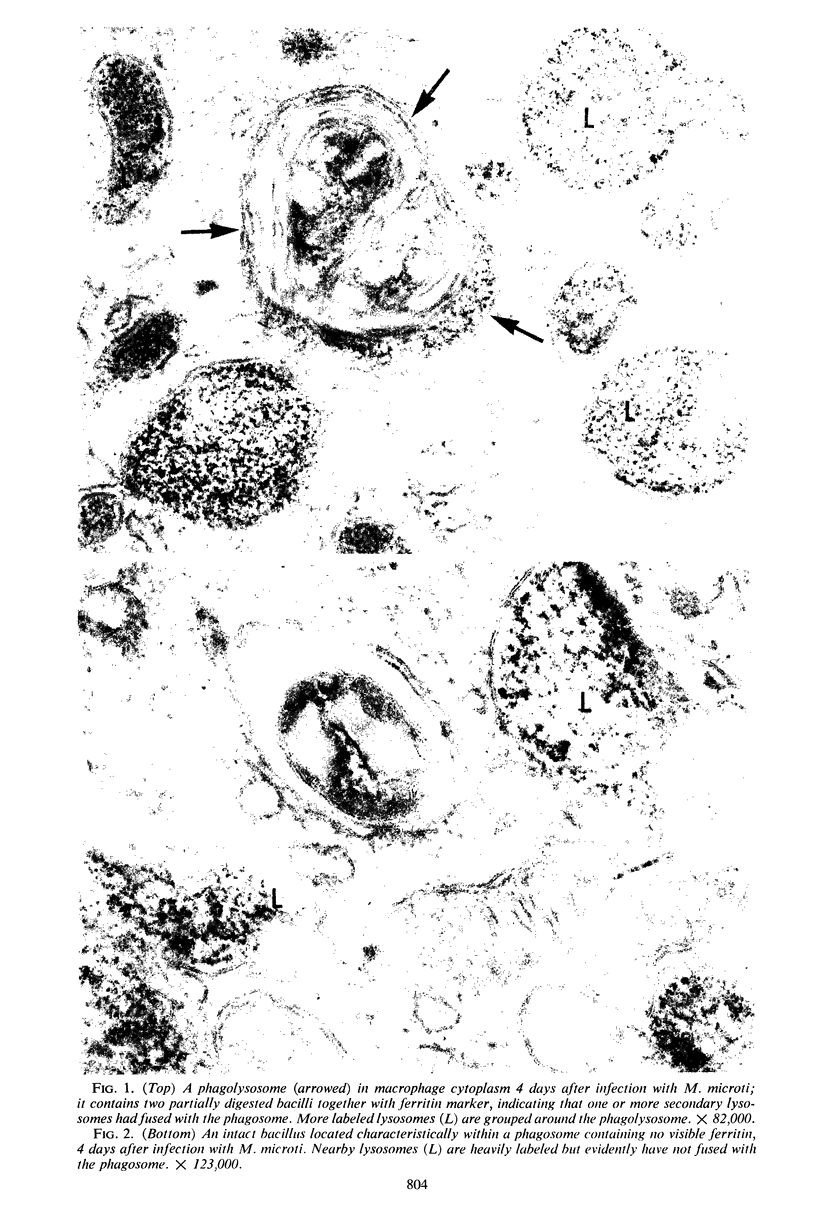
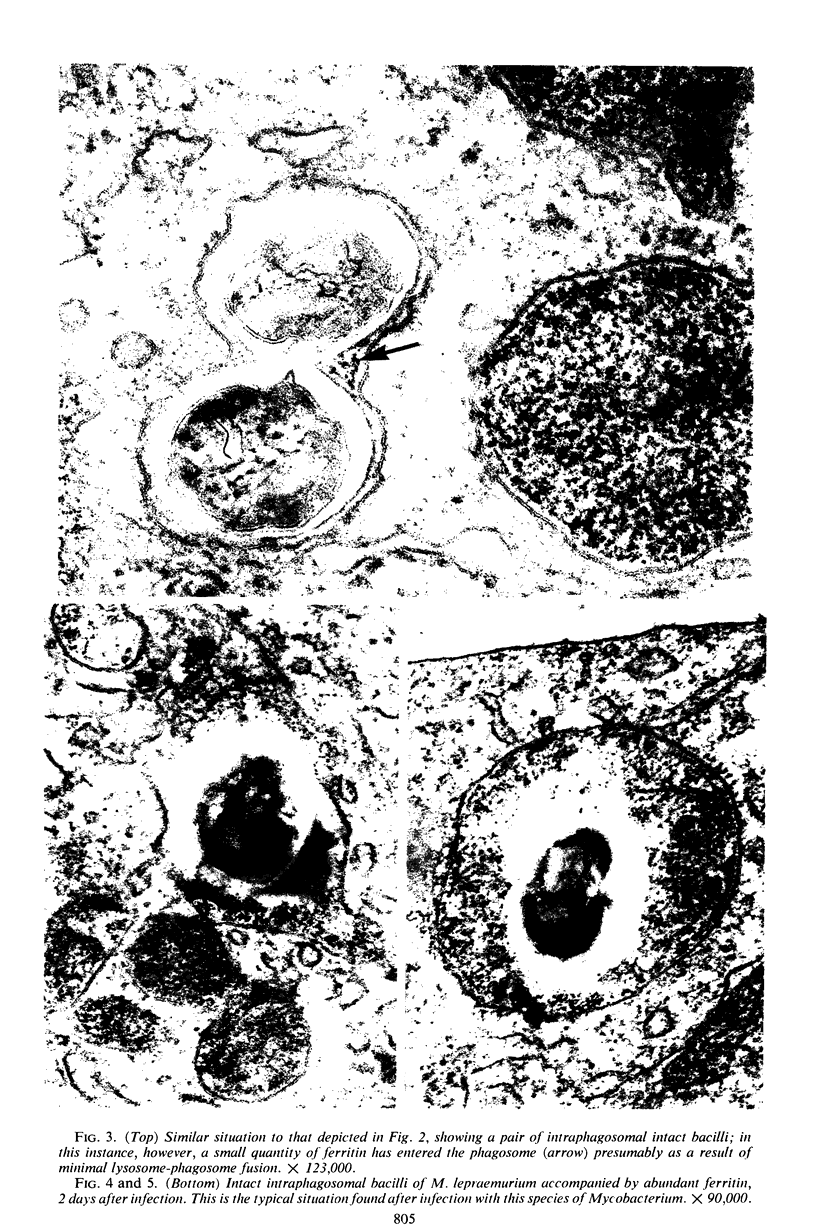
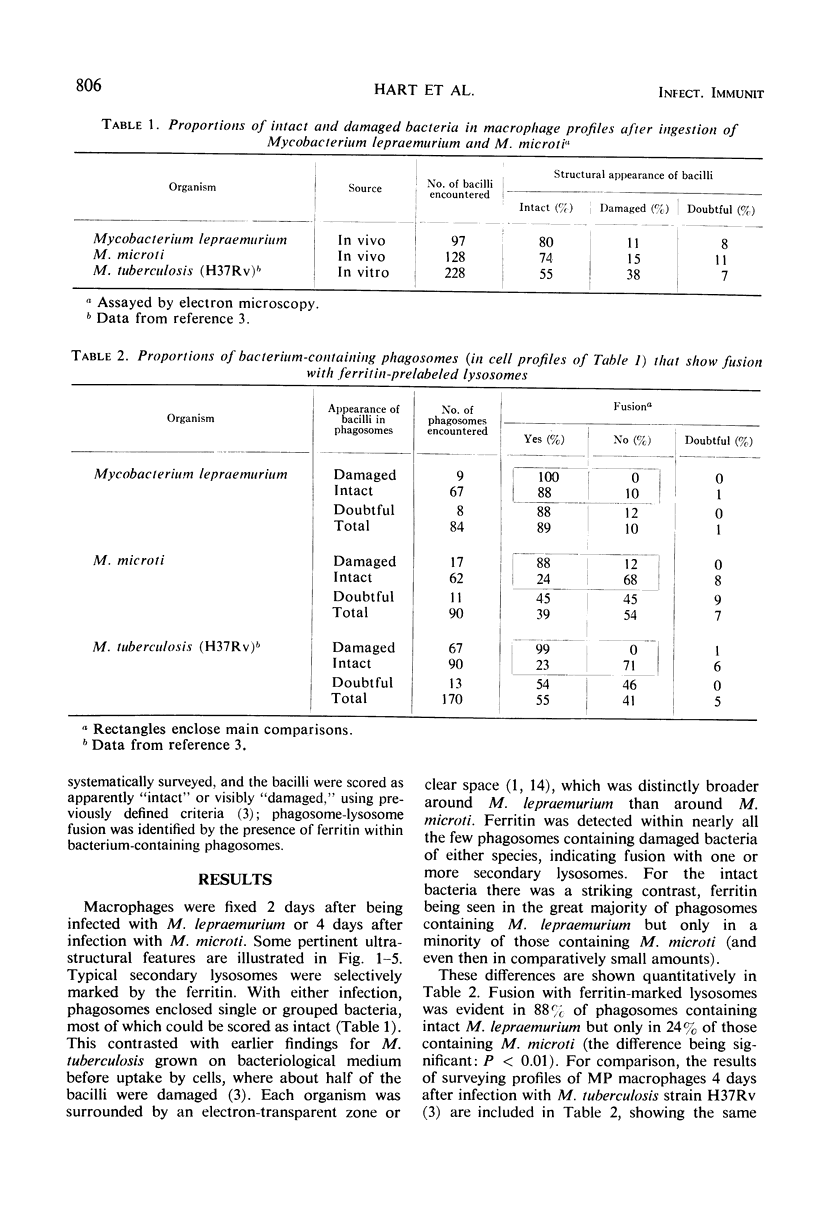
Mycobacterium lepraemurium and M. microti (causal agent of vole tuberculosis) were isolated from tissues of experimentally infected mice and used to infect normal mouse peritoneal macrophage cultures. The cellular response to these bacteria up to 4 days after infection was studied quantitatively by electron microscopy. Prelabeling with ferritin was used to facilitate observation of fusion between secondary lysosomes in the cells and phagosomes containing the bacteria. All bacteria were intraphagosomal, and a high proportion of them was morphologically “intact.” Nearly all phagosomes containing morphologically damaged (presumed nonviable) bacteria also contained ferritin, having fused with secondary lysosomes. Fusion of lysosomes had also occurred with most phagosomes containing intact M. lepraemurium but was infrequent with phagosomes containing intact M. microti. This tendency of multiplying mycobacteria of the tubercle type to avoid contact with lysosomal contents has already been reported for M. tuberculosis strain H37Rv. The different intracellular circumstances of the parasites may reflect different means of intracellular survival.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN J. M., BRIEGER E. M., REES R. J. ELECTRON MICROSCOPY OF THE HOST-CELL PARASITE RELATION IN MURINE LEPROSY. J Pathol Bacteriol. 1965 Jan;89:301–306. doi: 10.1002/path.1700890131. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Hart P. D. Potentiation by silica of the growth of Mycobacterium tuberculosis in macrophage cultures. Br J Exp Pathol. 1968 Oct;49(5):465–476. [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. A., Draper P. An electron-microscope study of rat fibroblasts infected with Mycobacterium lepraemurium. J Pathol. 1970 Sep;102(1):21–26. doi: 10.1002/path.1711020105. [DOI] [PubMed] [Google Scholar]
- CHANG Y. T. LONG-TERM CULTIVATION OF MOUSE PERITONEAL MACROPHAGES. J Natl Cancer Inst. 1964 Jan;32:19–35. [PubMed] [Google Scholar]
- Change Y. T., Andersen R. N., Vaituzis Z. Growth of Mycobacterium lepraemurium in cultures of mouse peritoneal macrophages. J Bacteriol. 1967 Mar;93(3):1119–1131. doi: 10.1128/jb.93.3.1119-1131.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper P., Rees R. J. Electron-transparent zone of mycobacteria may be a defence mechanism. Nature. 1970 Nov 28;228(5274):860–861. doi: 10.1038/228860a0. [DOI] [PubMed] [Google Scholar]
- GARBUTT E. W., REES R. J., BARR Y. M. Growth of Mycobacterium lepraemurium maintained in cultures of rat fibrolasts. J Gen Microbiol. 1962 Feb;27:259–268. doi: 10.1099/00221287-27-2-259. [DOI] [PubMed] [Google Scholar]
- HART P. D., REES R. J. Effect of macrocyclon in acute and chronic pulmonary tuberculous infection in mice as shown by viable and total bacterial counts. Br J Exp Pathol. 1960 Aug;41:414–421. [PMC free article] [PubMed] [Google Scholar]
- Hart P. D. Mycobacterium tuberculosis in macrophages: effect of certain surfactants and other membrane-active compounds. Science. 1968 Nov 8;162(3854):686–689. doi: 10.1126/science.162.3854.686. [DOI] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES R. J., VALENTINE R. C., WONG P. C. Application of quantitative electron microscopy to the study of Mycobacterium lepraemurium and M. leprae. J Gen Microbiol. 1960 Apr;22:443–457. doi: 10.1099/00221287-22-2-443. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., NISHIURA M., HARADA N., IMAEDA T. Electron microscopy of Mycobacterium leprae murium in ultra-thin sections of murine leprosy lesions. Int J Lepr. 1958 Apr-Jun;26(2):111–114. [PubMed] [Google Scholar]







