Abstract
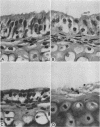
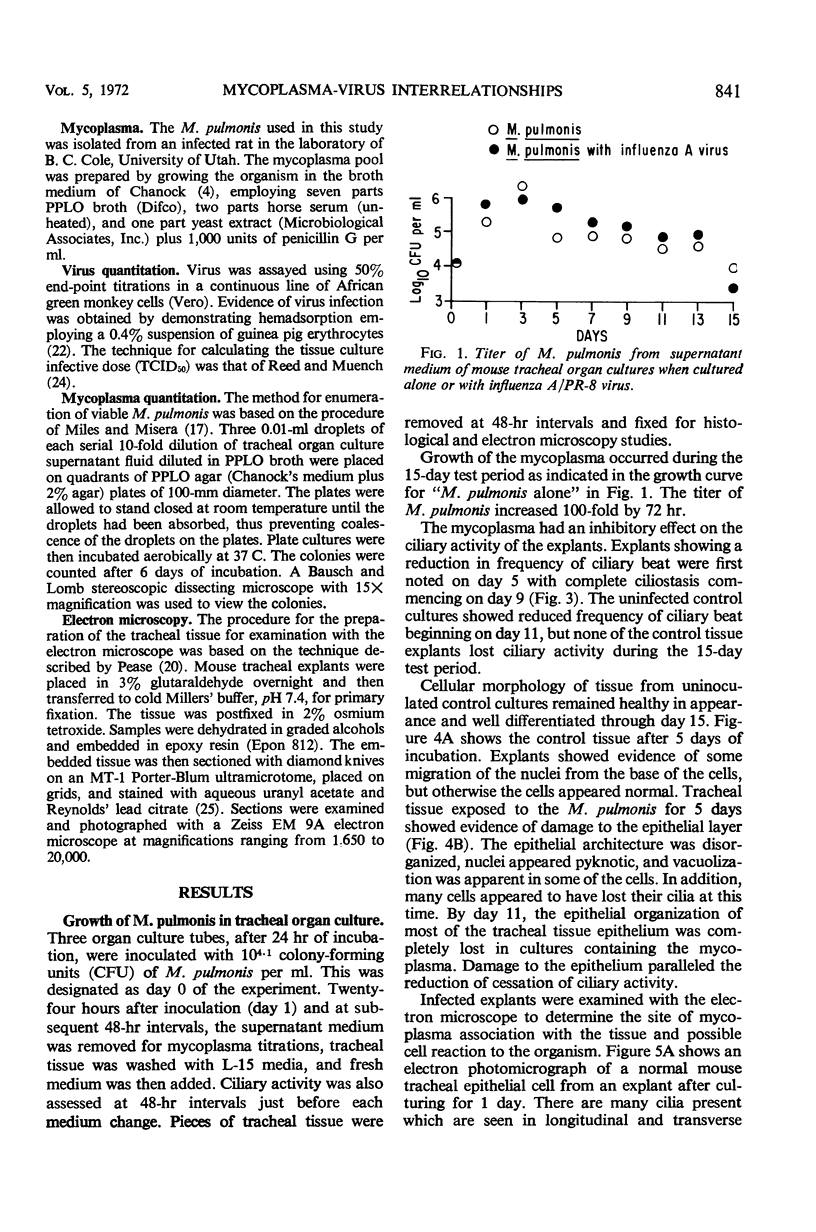
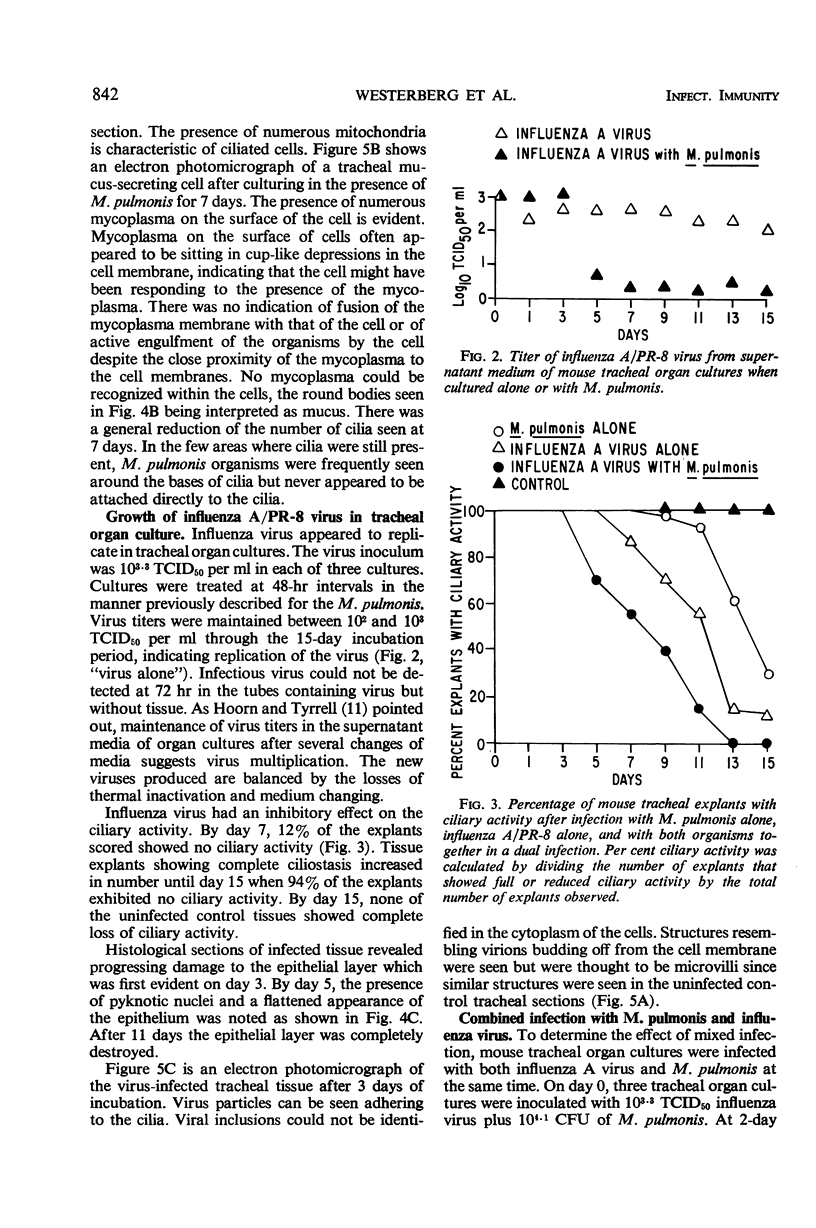
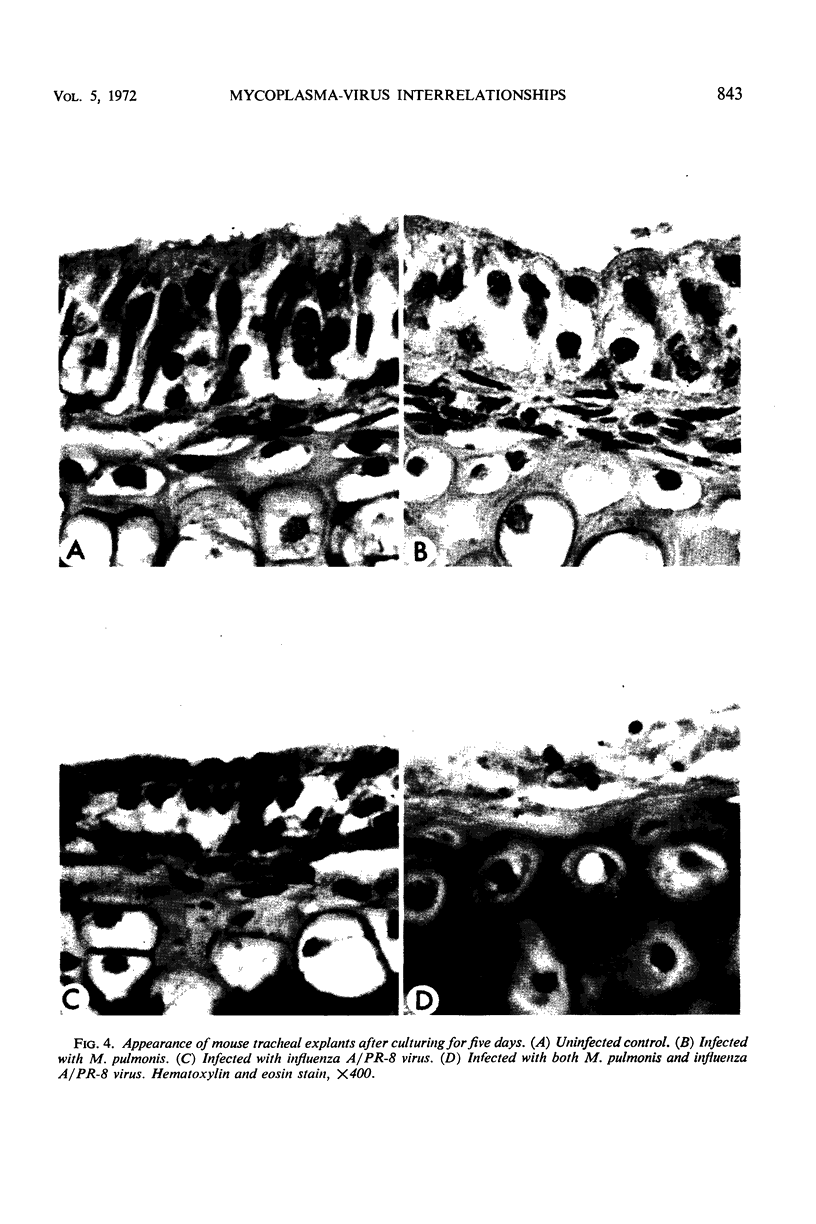
The effects produced by single and mixed infections with Mycoplasma pulmonis and influenza A/PR-8 virus were studied in mouse tracheal organ cultures. M. pulmonis multiplied in the tracheal organ cultures, producing inhibition of ciliary activity and histologic tissue damage. The organism grew in close association with the cell membranes but did not appear to attach directly to the membranes or the cilia. Influenza A virus also replicated in tracheal organ cultures, producing ciliary inhibition and more extensive cytopathologic changes. Virus particles were seen by electron microscopy to attach to and cause clumping of the cilia. Simultaneous infection of the organ cultures with mycoplasma and virus resulted in more rapid inactivation of ciliary activity and greater tissue damage than occurred when the cultures were infected with only mycoplasma or virus. Presence of the virus appeared to have no effect on the growth of the mycoplasma; however, the mycoplasma partially interfered with virus replication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANG F. B. Mucociliary function as protective mechanism in upper respiratory tract. Bacteriol Rev. 1961 Sep;25:228–236. doi: 10.1128/br.25.3.228-236.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTLER M., LEACH R. H. A MYCOPLASMA WHICH INDUCES ACIDITY AND CYTOPATHIC EFFECT IN TISSUE CULTURE. J Gen Microbiol. 1964 Feb;34:285–294. doi: 10.1099/00221287-34-2-285. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. D., Taylor-Robinson D. Growth and Pathogenesis of Mycoplasma mycoides var. capri in Chicken Embryo Tracheal Organ Cultures. Infect Immun. 1970 Oct;2(4):431–438. doi: 10.1128/iai.2.4.431-438.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Mycoplasma pneumoniae: hydrogen peroxide secretion and its possible role in virulence. Ann N Y Acad Sci. 1967 Jul 28;143(1):85–87. doi: 10.1111/j.1749-6632.1967.tb27648.x. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr, Denny F. W. Mycoplasma pneumoniae in hamster tracheal organ culture: immunofluorescent and electron microscopic studies. Proc Soc Exp Biol Med. 1971 Feb;136(2):569–573. doi: 10.3181/00379727-136-35313. [DOI] [PubMed] [Google Scholar]
- Heishman J. O., Olson N. O., Cunningham C. J. Transmission of Mycoplasma gallisepticum, Newcastle disease, infectious bronchitis, and combinations in a three-phase broiler house. Avian Dis. 1969 Feb;13(1):1–6. [PubMed] [Google Scholar]
- Hers J. F. Disturbances of the ciliated epithelium due to influenza virus. Am Rev Respir Dis. 1966 Mar;93(3 Suppl):162–177. doi: 10.1164/arrd.1966.93.3P2.162. [DOI] [PubMed] [Google Scholar]
- Hoorn B., Tyrrell D. A. Organ cultures in virology. Prog Med Virol. 1969;11:408–450. [PubMed] [Google Scholar]
- JANSSEN R. J., CHAPPELL W. A., GERONE P. J. SYNERGISTIC ACTIVITY BETWEEN PR8 INFLUENZA VIRUS AND STAPHYLOCOCCUS AUREUS IN THE GUINEA PIG. Am J Hyg. 1963 Nov;78:275–284. [PubMed] [Google Scholar]
- Lepper M. H. Potential interactions between multiple etiologies and pathogenesis of experimental diseases. Yale J Biol Med. 1968 Apr-Jun;40(5-6):541–549. [PMC free article] [PubMed] [Google Scholar]
- Loosli C. G. Synergism between respiratory viruses and bacteria. Yale J Biol Med. 1968 Apr-Jun;40(5-6):522–540. [PMC free article] [PubMed] [Google Scholar]
- Lutsky I. I., Organick A. B. Pneumonia due to mycoplasma in gnotobiotic mice. I. Pathogenicity of Mycoplasma pneumoniae, Mycoplasma salivarium, and Mycoplasma pulmonis for the lungs of conventional and gnotobiotic mice. J Bacteriol. 1966 Oct;92(4):1154–1163. doi: 10.1128/jb.92.4.1154-1163.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Sakamoto H. Modification of the growth of human mycoplasmas in tissue culture by infection with influenza virus and Japanese encephalitis (JE) virus. Proc Soc Exp Biol Med. 1969 Jun;131(2):343–348. doi: 10.3181/00379727-131-33874. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck F. M., Jr, Grumbles L. C., Hall C. F., Grimes J. E. Serology and gross lesions of turkeys inoculated with an avian influenza A virus, a paramyxovirus, and Mycoplasma gallisepticum. Avian Dis. 1970 Feb;14(1):54–65. [PubMed] [Google Scholar]
- Reed S. E. The interaction of mycoplasmas and influenza viruses in tracheal organ cultures. J Infect Dis. 1971 Jul;124(1):18–25. doi: 10.1093/infdis/124.1.18. [DOI] [PubMed] [Google Scholar]
- SOMERSON N. L., COOK M. K. SUPPRESSION OF ROUS SARCOMA VIRUS GROWTH IN TISSUE CULTURES BY MYCOPLASMA ORALE. J Bacteriol. 1965 Aug;90:534–540. doi: 10.1128/jb.90.2.534-540.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]