Abstract
Endemic species are assumed to have a high risk of extinction because their restricted geographic range is often associated with low abundance and high ecological specialization. This study examines the abundance of Chaetodon butterflyfishes at Lord Howe Island in the south-west Pacific, and compares interspecific differences in local abundance to the feeding behavior and geographic range of these species. Contrary to expected correlations between abundance and geographic range, the single most abundant species of butterflyfish was Chaetodon tricinctus, which is endemic to Lord Howe Island and adjacent reefs; densities of C. tricinctus (14.1 ± 2.1 SE fish per 200m2) were >3 times higher than the next most abundant butterflyfish (Chaetodon melannotus), and even more abundant than many other geographically widespread species. Dietary breadth for the five dominant butterflyfishes at Lord Howe Island was weakly and generally negative correlated with abundance. The endemic C. tricinctus was a distinct outlier in this relationship, though our extensive feeding observations suggest some issues with the measurements of dietary breadth for this species. Field observations revealed that all bites taken on benthic substrates by C. tricinctus were from scleractinian corals, but adults rarely, if ever, took bites from the benthos, suggesting that they may be feeding nocturnally and/or using mid-water prey, such as plankton. Alternatively, the energetic demands of C. tricinctus may be fundamentally different to other coral-feeding butterflyfishes. Neither dietary specialization nor geographic range accounts for interspecific variation in abundance of coral reef butterflyfishes at Lord Howe Island, while much more work on the foraging behavior and population dynamics of C. tricinctus will be required to understand its’ abundance at this location.
Keywords: Chaetodontidae, corallivore, disturbance, ecological function, endemism, schooling, selectivity, specialization
Introduction
Endemic species are an important component of biodiversity but are also considered to be disproportionately affected by disturbances, and more likely to go extinct because relatively moderate disturbances can simultaneously affect the entire population (McKinney 1997; Gaston 1998; Roberts et al. 2002). Moreover, geographic range is often correlated with abundance (e.g., Lawton 1993; Gaston 1994, 1996; Brown et al. 1995; McKinney 1997), further increasing the risk of extinction for restricted range species (Gaston et al. 1997; Gaston 1998). This double jeopardy of extinction risk may also be further compounded if small range size is associated with other traits (e.g., ecologically specialization and low dispersal: Gaston et al. 1997; Malcolm et al. 2006; Pimm et al. 2014), making these species even more vulnerable to extinction (Davies et al. 2004; Brook et al. 2008; Olden et al. 2008).
Ecological specialization (the extent to which species specialize in their use of prey or habitat resources) is increasingly considered alongside population size and geographical range as a key determinant of extinction risk (e.g., McKinney 1997; Davies et al. 2004; Dulvy et al. 2004; Koh et al. 2004; Brook et al. 2008). Ecological theory (e.g., Brown 1984) suggests that specialized species should have narrower geographic ranges and be less abundant than generalist counterparts, but empirical data (e.g., Gaston et al. 1997; Manne and Pimm 2001; Päivinen et al. 2005; Reif et al. 2006; Hobbs et al. 2010, 2011; Berkström et al. 2012) does not always support the theory. An alternative explanation is that extinction filtering promotes persistence of species with compensatory relationships between range size, ecological specialization and population size that reduce the risk of extinction (e.g., Johnson 1998; Williams et al. 2006).
Despite the importance of ecological specialization for the biology, ecology and evolution of animals (e.g., Futuyma and Moreno 1988), ecological specialization is either rarely or poorly quantified (Devictor et al. 2010). Coral-feeding butterflyfishes (Chaetodon; Chaetodontidae) are an ideal group to study ecological specialization because their feeding behavior and dietary composition is easily measured, as is the differential availability of alternative prey (e.g., Berumen et al. 2005; Blowes et al. 2013; Noble et al. 2014). This enables direct estimates of dietary specialization across gradients of prey availability (e.g., Lawton et al. 2012), clearly distinguishing species that display distinct preferences regardless of prey availability (fundamental or obligate specialists) versus those that vary in their patterns of prey use simply to make use of locally abundant prey types (realized or facultative specialists). Moreover, sympatric butterflyfishes often exhibit significant variation in dietary selectivity, ranging from species that feed almost exclusively on just one coral species (e.g., Chaetodon trifascialis, Pratchett 2005; Pratchett et al. 2013a) to species that feed on >50 coral species, often in direct accordance with their relative abundance (e.g., Chaetodon lunulatus, Pratchett 2005).
Butterflyfishes are among the best-studied group of coral reef fishes (Pratchett 2014), owing partly to their inherent reliance on live coral for food and associated vulnerability to significant and widespread declines in live coral cover (e.g., Wilson et al. 2006, 2014). Pratchett et al. (2008, 2011) showed that interspecific differences in the vulnerability of butterflyfishes to coral loss are greatest among species for which corals represent >80% of total food intake (termed obligate corallivores, Cole et al. 2008). However, even among obligate coral-feeding fishes, responses to coral loss vary depending upon the extent to which species are more or less specialized in their use of different coral prey (Pratchett et al. 2008). There is, therefore, a definite need to better understand the specific foraging behavior and ecological specialization of coral reef butterflyfishes, especially among those species that are geographically restricted and exposed to local coral depletion (Lawton et al. 2012).
In this study, we explore the abundance, diversity and feeding behavior of Chaetodon butterflyfishes Lord Howe Island, and assess whether local abundance of individual species is related to their dietary specialization and/or geographic range. Lord Howe Island is the world’s southernmost coral reef, with fish faunas comprising a mix of both tropical and temperate species (Zann 2000), and a relatively high number of endemics (Randall 1976). Previous studies conducted within tropical coral-dominated environments have revealed that specialist coral-feeding species tend to dominate butterflyfish assemblages (Emslie et al. 2010; Pratchett et al. 2013a), but coral-feeding fishes are under-represented at some marginal or peripheral coral reef locations (e.g., Pratchett et al. 2013b). Given high cover of corals across much of the reef habitat at Lord Howe Island (Hoey et al. 2011), we would expect to find a high abundance of coral-feeding butterflyfishes, though the isolation and extreme latitude may moderate the abundance of some species. In this study, direct feeding observations were used to quantify both feeding rates and diet (or feeding substrata) of dominant butterflyfishes. Notably, this is the first study on the feeding habits of the three-striped butterflyfish (Chaetodon tricinctus), which is endemic to Lord Howe Island and nearby reefs (Hobbs et al. 2009; van der Meer et al. 2013).
Methods
Field surveys
Lord Howe Island (31°32′S, 159°04′E) is located 630 km east of the Australian mainland in the Tasman Sea (Fig. 1A). The western side of the island is dominated by an extensive lagoon with a high cover (ca. 30%), but low diversity, of scleractinian corals (e.g., Hoey et al. 2011). Sampling for this study was undertaken at three sites (North Bay, Stephen’s Hole and Potholes) equally spaced along the lagoon in areas of distinct platform reef <2 m depth, separated by deeper (4–6 m) sandy areas (Fig. 1B).
Figure 1.

Map showing (A) geographic location of Lord Howe Island, and (B) the location of the three study sites on Lord Howe Island (North Bay, Stephen’s Hole, and Potholes) used to quantify butterflyfish assemblages.
Butterflyfish abundance was quantified using underwater visual census (UVC) along haphazardly placed 50 × 4 m belt transects (n = 12 replicates per site) in December 2011. Butterflyfishes were surveyed while simultaneously deploying a 50-m transect tape to delineate transect length. All butterflyfishes 2 m either side of the transect midline were then recorded to species, as well as estimating their total length (TL, to nearest cm) and recording group size. Coral cover and benthic composition were quantified using point-intercept transects (following Pratchett et al. 2004, 2011) to record the specific substratum type underlying uniformly spaced points (0.5 m apart) along the length of each 50 m transect. Scleractinian (hard) corals, alcyonacean (soft) corals, and macroalgae (>5 mm) were identified to genus (and Acropora hard corals were further defined to tabulate or arborescent growth forms), with other substratum types categorized as sand/rubble or pavement.
Feeding observations
To characterize and compare the feeding rates and diets of butterflyfishes at Lord Howe Island, the range of prey types, and the proportional use of different prey types by each species of butterflyfish (use was defined as an observed bite by the individual on a prey type), was quantified using replicate 3-min feeding observations following Pratchett (2005). Feeding observations were conducted during a similar time of year in each two consecutive years, May 2010 and June 2011. Feeding observations only commenced after the focal individual had taken their first bite, or 3-min after the observation started to allow fish to acclimate to observer presence. Observations were aborted if the focal individual fled or sought shelter from the observer. During each feeding observation, the total number of bites taken from different genera of hard coral, soft coral or any other noncoral macroinvertebrate was recorded. For the dominant coral genera, Acropora, we also distinguished between tabular (e.g., Acropora glauca), and arborescent (e.g., Acropora yongei) colonies. The number of bites taken from other reef substrata (i.e., consolidated reef pavement, coral rubble, or sand) that were not obviously occupied by corals or macroinvertebrates was also recorded. A minimum of 20 feeding observations were conducted for each of the five most common butterflyfish species recorded at Lord Howe Island: C. lunulatus, Chaetodon melannotus, Chaetodon plebeius, C. tricinctus, and C. trifascialis. Increased sampling effort was applied to the endemic C. tricinctus (186 of 419 feeding observation) due to apparent size-based differences in feeding behavior (discussed below).
Data analyses
Spatial variation in the abundance and composition of Chaetodon butterflyfishes and categories of reef substratum were examined across the three sample sites (North Bay, Stephen’s Hole, and Potholes) using permutational multivariate analysis of variance (PERMANOVA). PERMANOVAs were conducted with 9999 permutations of the raw data constructed into resemblance matrices for the Chaetodon assemblages using a modified Gower Log10 measure (Anderson et al. 2006), and for the reef substratum categories using a Bray-Curtis similarity measure on square-root transformed data for the 36 transects (Anderson et al. 2008). Ordinations were used to visualize structure within the reef substratum and Chaetodon assemblages via principal coordinates analysis (PCO) on the same resemblance matrices. Pairwise PERMANOVA was used to further explore differences between sites. PCOs were optimized with vector overlays of raw Pearson correlations (limited to r > 0.4) and bubble plots to explore key Chaetodon species and substratum categories underlying spatial structure in this reef assemblage.
The extent to which spatial differences in Chaetodon assemblages could be explained by reef habitat composition was explored by distance-based linear models (DISTLM), which were based on the same resemblance matrices above, and used Akaike Information Criteria for finite samples (AICc) to select the “best” models with a range of settings (models with either 1, 2, 3, or 4 substratum categories incorporated) from all of the possible combinations of habitat predictor variables (Anderson et al. 2008). As recommended by Anderson et al. (2008), we checked for multicollinearity among possible habitat predictor variables using draftsman plots. This led to exclusion of abiotic substratum categories (sand/rubble, pavement) from the DISTLM analysis, as they were strongly (negatively) correlated with biotic categories (chiefly scleractinian corals). All analyses and ordinations were performed in PRIMER (version 6.1.16) with PERMANOVA+ (version 1.0.6).
To compare dietary composition and feeding selectivity among Chaetodon butterflyfishes, forage ratios were calculated following Manly et al. (2002), which illustrate the use of each prey category (number of bites taken) relative to the availability of each prey type across the three study sites. Bonferroni-corrected 95% confidence limits were calculated for each prey category used by each butterfly- fish species to establish the significance of prey selectivity. Selection ratios −95% CI that were >1 indicate that prey that were used significantly more than expected based on their availability (i.e., preferred), while ratios +95% CI that were <1 indicate prey that were used disproportionately less than expected (i.e., avoided).
Variation in both bite rates and diet breadth were analyzed using two-way ANOVAs to detect differences among species (C. lunulatus, C. melannotus, C. plebeius, C. tricinctus, and C. trifascialis) and among locations (North Bay, Stephen’s Hole, and Potholes), and Tukey’s post hoc test was used to reveal major differences among species. Raw data on the number of bites taken by each individual butterflyfish were square-root transformed prior to analyses to reduce the influence of occasional very large values. Replicate estimates of diet breadth were based on the number of distinct coral types that were consumed by each individual during the 3-min feeding observation; specialist species are expected to concentrate feeding on only 1–2 coral species, whereas generalists may feed on predominant or preferred prey while actively foraging across a range of different prey types (Pratchett 2014). One-way ANOVA was used to test for size-related differences in feeding rates for C. tricinctus, comparing among individuals with an estimated TL of <5 cm, 5–10 cm, and >10 cm. It was apparent during feeding observations that bite rates were highest among the smallest size classes and tended to decline with increasing size, so a minimum of 20 feeding observations were conducted for each size class. Similar analyses were not performed for other Chaetodon butterflyfishes, mainly because there was much less variation in the size of fishes, and so most feeding observations were of larger (presumably adult) individuals.
After accounting for spatial variation in abundance of different butterflyfishes, overall abundance of each species was determined by averaging across all sites. This aggregate measure of individual abundance was then used to examine whether interspecific differences in local abundance are related to geographic range (across all species present) and diet breadth (for subset of species for which dietary composition was measured). To compare geographic range among butterflyfishes, we used published estimates of maximal area of occurrence (Jones et al. 2002). Diet breadth was calculated as described above.
Results
A total of 13 species of Chaetodon butterflyfish were recorded across the three lagoonal reef sites at Lord Howe Island, although six of these species (Chaetodon citrinellus, Chaetodon vagabundus, Chaetodon speculum, Chaetodon ephippium, Chaetodon guentheri, and Chaetodon pelewensis) were rare (Fig. 2). Butterflyfish assemblages were significantly different among sites (PERMANOVA: pseudo-F2,33 = 2.98, P = 0.003), largely due to significant differences between North Bay and the other sites (pseudo-t22 = 1.79, P = 0.009 and pseudo-t22 = 2.30, P = 0.001 pairwise comparisons with Potholes and Stephen’s Hole, respectively), with no significant difference between Potholes and Stephen’s Hole (pseudo-t22 = 0.73, P = 0.760). Ordination revealed that spatial variation in Chaetodon assemblages was largely due to variation in abundance of five abundant species: C. tricinctus, C. melannotus, C. plebeius, C. lunulatus, and C. trifascialis (Fig. 3A). Densities of both C. tricinctus and C. melannotus were 2–3 times higher at North Bay (average = 23.0 and 7.42 fishes per 200 m2, respectively) compared to Stephen’s Hole and Potholes.
Figure 2.
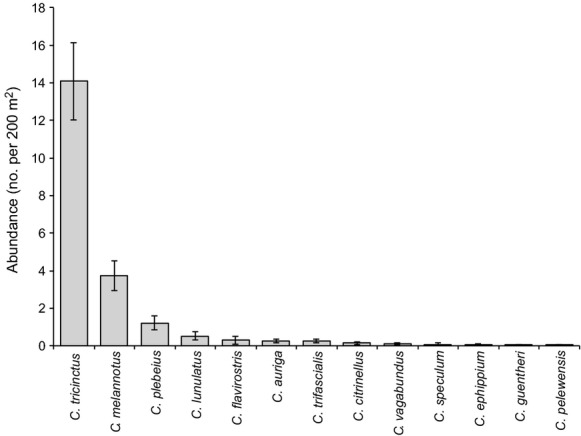
Mean (±SE) abundance of all Chaetodon butterflyfishes recorded at Lord Howe Island. Data are pooled across all sites to highlight relative abundance of different species.
Figure 3.
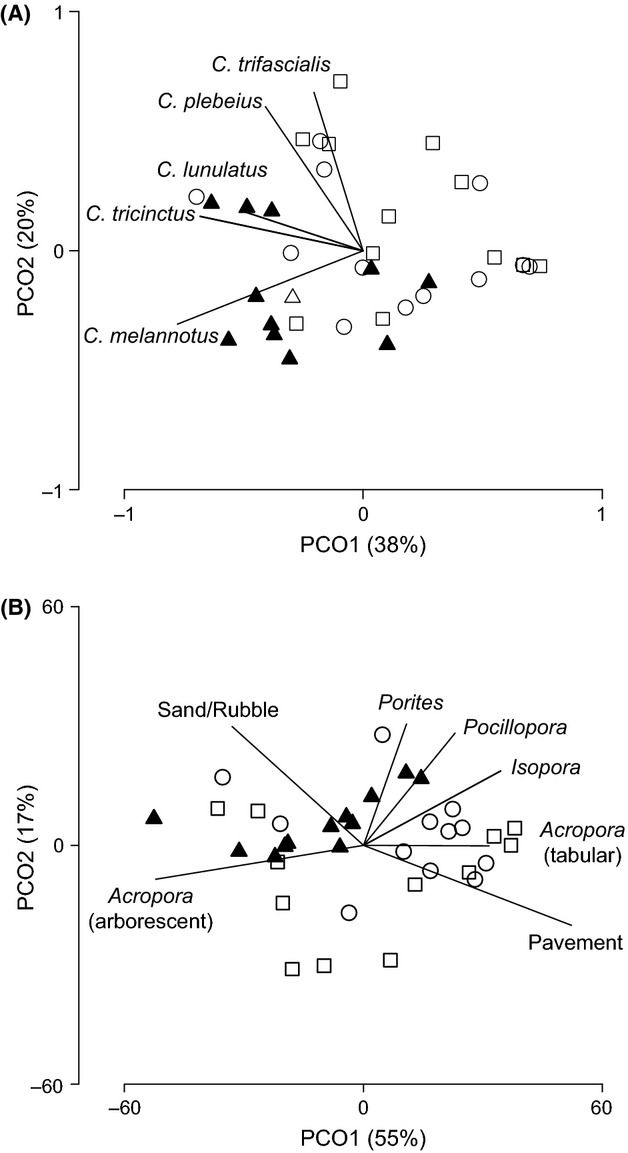
Principal coordinates analysis (PCO) of spatial variation in the abundance and composition of the (A) Chaetodon assemblage, and (B) coral reef habitat for 36 transects spread across three sites (North Bay = filled triangles, Potholes = open circles, Stephen’s Hole = open squares) at Lord Howe Island. Vectors are variables (Chaetodon species and substratum categories, respectively) most correlated (Pearson’s correlation coefficient, r > 0.4) with the PCO axes.
Similarly, reef substratum composition was significantly different among sites (pseudo-F2,33 = 3.34, P = 0.009), particularly between North Bay and the other two sites (pseudo-t22 = 2.33, P = 0.004 and pseudo-t22 = 1.87, P = 0.034), but not between Potholes and Stephen’s Hole (pseudo-t22 = 1.27, P = 0.176). Spatial variation in reef habitat structure was largely attributable to seven benthic categories: sand/rubble, pavement, Acropora (arborescent), Acropora (tabular), Pocillopora, Isopora and Porites (Fig. 3B). Cover of scleractinian corals was much higher at North Bay (43.4%) compared to Stephen’s Hole (38.7%) and Potholes (30.3%), mostly because of higher cover of arborescent Acropora (32.1%), which was the dominant coral at North Bay (comprised 73.9% of all coral). DISTLM marginal tests indicated scleractinian corals accounted for 46.0% of variation in Chaetodon assemblages, followed by abiotic substratum types (sand/rubble and pavement, 20.4%), soft coral and macroalgae (<0.1% each, Table 1A). Porites, Acropora (arborescent), Pocillopora, and/or Cyphastrea appear to provide the best explanatory habitat variables in distance-based linear models of spatial variation in the Lord Howe Island Chaetodon assemblage (Table 1B). While proportional abundances for each of the above five Chaetodon species tended to be highest in areas characterized by some of these types of coral (Fig. 4), considerable variation remains unexplained in these habitat-based DISTLMs (i.e., all r2 < 0.28, Table 1B).
Table 1.
Summary of (A) marginal tests and (B) distance-based linear model (DISTLM) selection, based upon Akaike Information Criteria for finite samples (AICc) to select “best” model combinations of habitat variables (i.e., best solutions for models with 1, 2, 3, or 4 variables) to explain spatial variation in Chaetodon assemblages at Lord Howe Island. Marginal tests are for higher groupings of substratum variables to explore overall trends in multivariate variation (following Anderson et al. 2008). Abiotic categories (sand/rubble and pavement) were excluded from DISTLM selection due to strong (negative) correlations with biotic categories (following Anderson et al. 2008)
| (A) Marginal tests | ||||||
|---|---|---|---|---|---|---|
| Group | SS | Residual df | Regression df | % variation | Pseudo-F | P |
| Scleractiniancoral | 7.035 | 23 | 13 | 46.0 | 1.64 | 0.008 |
| Sand/rubble/pavement | 3.123 | 33 | 3 | 20.4 | 4.24 | 0.001 |
| Soft coral | 0.903 | 34 | 2 | 0.06 | 2.14 | 0.063 |
| Macroalgae | 0.649 | 34 | 2 | 0.04 | 1.51 | 0.145 |
| (B) Best DISTLM solutions | |||
|---|---|---|---|
| Habitat variables | AICc | SS (resid.) | r2 |
| Porites | 29.16 | 14.05 | 0.08 |
| Porites + Acropora (arborescent) | 31.05 | 12.60 | 0.18 |
| Porites + Acropora (arborescent) + Pocillopora | 30.93 | 11.78 | 0.23 |
| Porites + Acropora (arborescent) + Pocillopora + Cyphastrea | 30.24 | 11.14 | 0.27 |
Figure 4.
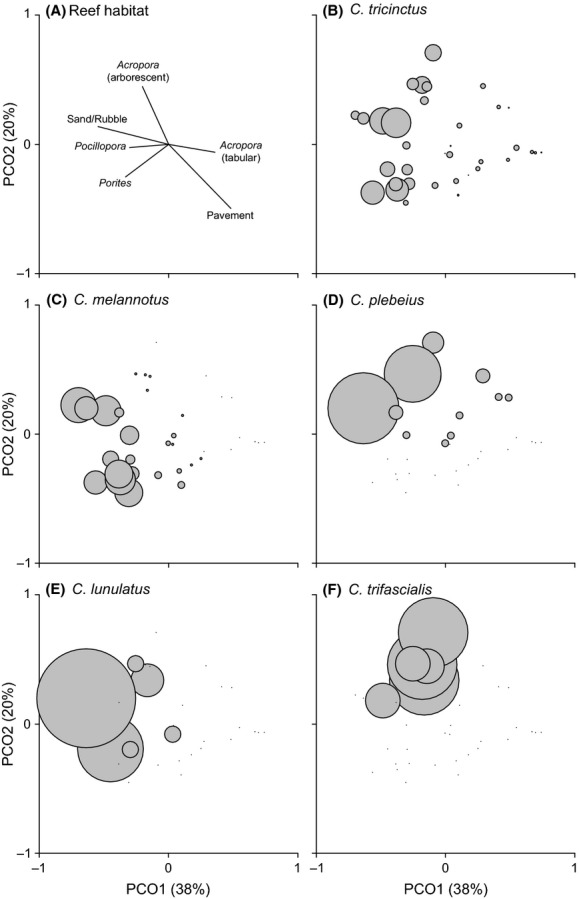
Optimized principal coordinate analysis (PCO) of spatial variation in Chaetodon abundance and composition across 36 transects at Lord Howe Island. (A) Reef habitat variables most correlated (Pearson’s correlation coefficient, r > 0.4) with the PCO axes. Bubble sizes indicate proportional abundance of (B) Chaetodon tricinctus, (C) Chaetodon melannotus, (D) Chaetodon plebeius, (E) Chaetodon lunulatus, and (F) Chaetodon trifascialis in areas characterized by tabular Acropora and/or pavement (indicated on panel A as transects toward bottom right quadrants of each panel), arborescent Acropora (top left quadrants) or Porites, Pocillopora and sand/rubble (bottom left quadrants).
The three stripe butterflyfish, C. tricinctus was by far the most abundant Chaetodon species at all locations, accounting for 67.7% of all individuals (Fig. 2). The mean abundance of C. tricinctus was 14.08 ± 2.05 (SE) fish per 200 m2, compared to 3.72 ± 0.78 SE fish per 200 m2 for the next most abundant species, C. melannotus (Fig. 2). Most C. tricinctus (374 of 640 individuals) occurred in schools of up to 42 individuals, with only 16% of individuals (n = 142) recorded in pairs, and 14% of individuals (n = 124) observed on their own. Larger aggregations of C. tricinctus tended to be found in interreefal habitats (over sand), but in close proximity to colonies of arborescent Acropora (Fig. 4B). Abundance of C. trifascialis was also highest where there was high arborescent Acropora (Fig. 4F), while abundance of C. lunulatus was highest where there was high cover of Pocillopora (Fig. 4E).
Feeding behavior
Feeding rates (number of bites taken per 3-min) varied greatly within and among the butterflyfishes considered during this study (C. lunulatus, C. plebeius, C. tricinctus, and C. trifascialis). Notably, a large proportion of C. melannotus (35 of 67) and C. tricinctus (43 of 141) were not seen to take any bites throughout an entire 6-min observation period (i.e., when including the 3-min acclimation period), in contrast to very few (0–2) instances of nonfeeding in the other species. Accordingly, mean bites rates of C. melannotus and C. tricinctus were markedly lower than C. lunulatus, C. plebeius, and C. trifascialis, with mean bite rates (averaged across all sites) varying by a factor of six among these species (Table 2). Bite rates varied significantly among species, but also varied among sites (Table 3), whereby the feeding rates for all but C. trifascialis were higher at Stephen’s Hole than at North Reef or Potholes. For C. lunulatus, bite rates recorded at Stephen’s Hole (27.00 bites per 3-min ± 7.22 SE) were twice those recorded at Potholes (12.56 bites per 3-min ± 1.66 SE). For C. melannotus, bite rates recorded at Stephen’s Hole (5.36 bites per 3-min ± 2.51 SE) were three times higher than recorded at Potholes (1.72 bites per 3-min ± 0.71 SE) or North Bay (1.78 bites per 3-min ± 0.67 SE). For C. trifascialis, bite rates were consistently high across all sites, but were highest at North Bay (19.9 bites per 3-min ± 1.02 SE). Even after accounting for those individuals that did not feed at all, the mean number of bites taken by C. melannotus (5.78 bites per 3-min ± 1.69 SE) and C. tricinctus (11.98 bites per 3-min ± 0.84 SE) were much lower than for the other three species (Table 2).
Table 2.
Bite rates, coral use, and feeding selectivity of five Chaetodon butterflyfishes at Lord Howe Island, ordered according to increasing selectivity. Significance of prey selection was assessed using forage selection ratios and Bonferroni corrected 95% confidence intervals (“=“indicates prey that were used in proportion to availability, “+” indicates prey used significantly more than expected, “−” indicates prey used less than expected, and “0” indicates prey that were not used at all). Overall significance of feeding selectivity was tested using total forage ratios, comparing relative use of different prey categories to their availability across the three study sites (Manly et al. 2002)
| Species | n | Bite rate | Hard corals (%) | Arborescent Acropora | Tabular Acropora | Isopora | Pocillopora | Porites | Soft corals | Total Forage Ratio | Sig. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chaetodon melannotus | 67 | 2.85 | 6.28 | 3.14% (−) | 1.05% (−) | 0.52% (−) | 1.05% (−) | 0.52% (−) | 45.03% (+) | 1797.53 | <0.001 |
| Chaetodon lunulatus | 51 | 16.69 | 99.76 | 30.55% (−) | 6.46% (+) | 3.06% (=) | 29.38% (+) | 20.92% (+) | 0.00% (0) | 2849.47 | <0.001 |
| Chaetodon plebeius | 65 | 15.85 | 99.90 | 23.20% (−) | 16.21% (+) | 26.70% (+) | 19.42% (=) | 9.81% (+) | 0.00% (0) | 3499.17 | <0.001 |
| Chaetodon tricinctus | 141 | 8.33 | 100 | 51.57% (=) | 22.38% (+) | 1.96% (−) | 19.23% (=) | 0.68% (−) | 0.00% (0) | 3940.70 | <0.001 |
| Chaetodon trifascialis | 73 | 17.63 | 100 | 55.71% (=) | 38.54% (+) | 0.39% (−) | 3.89% (−) | 0.23% (−) | 0.00% (0) | 4552.50 | <0.001 |
Table 3.
Two-way factorial ANOVAs testing for differences in (A) bite rates and (B) the range of prey types consumed among species (see Table 2 for details) and among the three distinct study locations (North Bay, Stephen’s Hole and Potholes). Given that both the total number of bites and the number of distinct prey types consumed within a 3-min period is highly constrained, data were square-root transformed prior to analyses
| A) Bite rate | |||||
|---|---|---|---|---|---|
| Source | SS | df | MS | F | Sig. |
| Species | 518.74 | 4 | 129.68 | 58.95 | <0.001 |
| Sites | 35.37 | 2 | 17.68 | 8.04 | <0.001 |
| Species × sites | 23.20 | 8 | 2.90 | 1.32 | 0.23 |
| Error | 840.40 | 382 | 2.20 | ||
| Total | 4534.00 | 396 | |||
| B) Range of prey types | |||||
|---|---|---|---|---|---|
| Source | SS | df | MS | F | Sig. |
| Species | 6.58 | 4 | 1.64 | 16.56 | <0.001 |
| Sites | 0.29 | 2 | 0.15 | 1.49 | 0.23 |
| Species × sites | 0.63 | 8 | 0.08 | 0.79 | 0.61 |
| Error | 30.20 | 382 | 0.10 | ||
| Total | 545.00 | 396 | |||
For C. tricinctus, feeding rates differed significantly among fishes in different size classes (ANOVA, F2,138 = 1434.25, P < 0.001), being highest for the smallest fishes (14.60 bites per 3-min ± 2.67 SE) and declining with increasing TL (Fig. 5). All individuals <5 cm TL remained in close proximity to the benthos feeding almost continually on scleractinian corals throughout feeding observations. Among C. tricinctus of 6–10 cm TL, 20 individuals (of 86 in total) did not feed; larger individuals that did feed under observation exhibited sustained feeding on scleractinian corals, taking a mean of 11.12 bites per 3-min (±1.05 SE). For individuals >10 cm, only 2 (of 25) individuals were seen to feed on benthic substrata and these fishes took only 1 and 2 bites, respectively, throughout a 3-min observation. For the most part, all individuals >10 cm TL remained in schools in mid-water and rarely approached or searched the substratum during our diurnal observations. While it is possible that they were opportunistically feeding on passing plankton, as they did occasionally open and close their mouths, they tended to move very slowly rather than making any darting movements to actively seek out planktonic prey.
Figure 5.
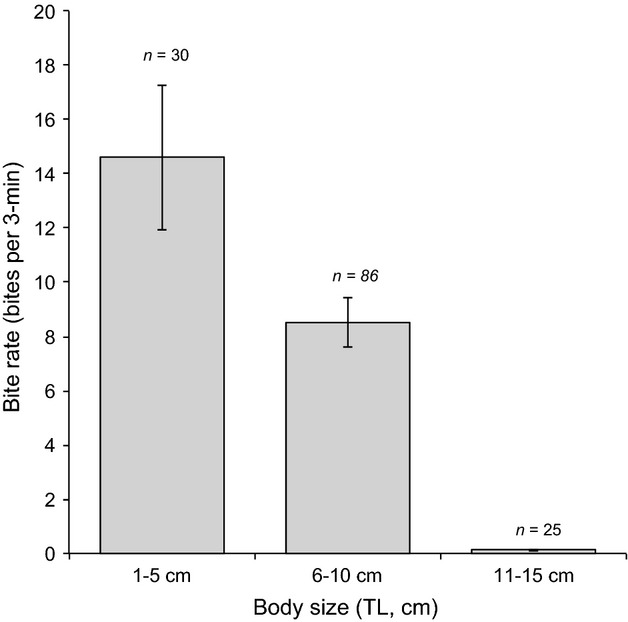
Size-based variation in mean (±SE) bites rates of Chaetodon tricinctus. Total length (TL) was visually estimated (to the nearest cm) for all fishes for which 3-min feeding observations were conducted. Data were pooled across site, and the number of fishes within each size class (n) is shown.
Four (of five) dominant Chaetodon butterflyfishes (C. lunulatus, C. plebeius, C. tricinctus, and C. trifascialis) at Lord Howe Island were classified as obligate corallivores (following Cole et al. 2008) due to them taking almost 100% of recorded bites from the surface of live corals (Table 2). The exception was C. melannotus, which took only 6.28% of bites from the surface of scleractinian corals, with most of their bites taken on soft corals. All of the obligate corallivore species fed predominantly on Acropora (Table 2), which was prevalent across all sites. However, all four species of butterflyfishes clearly distinguished between different types of Acropora, consuming tabular Acropora disproportionately more than expected based on availability across the three sites, while they consumed arborescent Acropora in lower or equal proportions to availability (Table 2).
All butterflyfishes exhibited significant levels of dietary selectivity (Table 2), consuming some corals disproportionately to their availability. Chaetodon melannotus avoided all scleractinian corals in preference for soft corals (Table 2), but still consumed an average of 1.90 different coral types per 3-min observation (Fig. 6B). Chaetodon lunulatus was the least selective of the four obligate corallivores, consuming an average of 2.25 different coral genera per 3-min observation (Fig. 6B). While most bites were taken from arborescent Acropora, C. lunulatus preferentially consumed tabular Acropora, Porites, and Pocillopora (Table 2). Chaetodon plebeius exhibited intermediate levels of dietary selectivity, consuming an average of 2.19 different coral genera per 3-min observation (Fig. 6B) and preferentially consumed preferentially consumed tabular Acropora, Isopora, and Porites. Chaetodon tricinctus and C. trifascilis were the most specialized coral feeders (Table 2), generally consuming only 1–2 different coral genera during feeding observations. Both species took most bites from arborescent Acropora, but preferred tabular Acropora to the exclusion of most other coral prey (Table 2), while most strongly avoiding Isopora and Porites.
Figure 6.
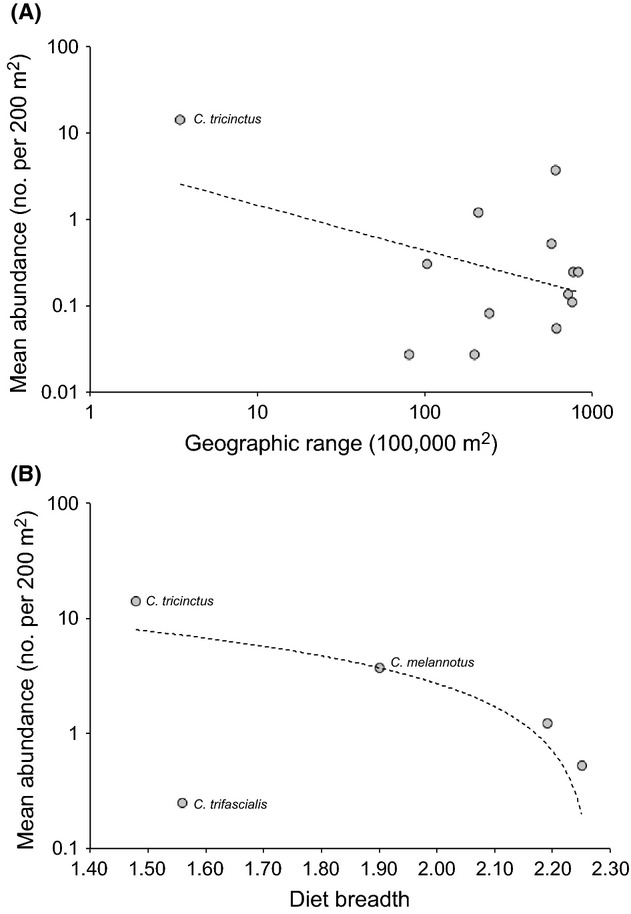
Correlations of (A) geographic range and (B) dietary breadth versus mean abundance (averaged across all sites) for Chaetodon butterflyfishes at Lord Howe Island. Abundance and geographic range are shown on a log-scale. Dietary breadth was estimated only for the five most abundant butterflyfishes at Lord Howe Island.
Correlates of species abundance
Chaetodon tricinctus was the dominant butterflyfish at all study sites, and while their abundance varied, it tended to be >3 times more abundant than any other butterflyfish species present. Found only at Lord Howe Island, nearby Elizabeth and Middleton Reefs and Norfolk Island, C. tricinctus geographic range is <5% of the next smallest range species, C. uentheri. The most widespread species recorded at Lord Howe Island, Chaetodon auriga and C. trifascialis, have widespread geographic ranges that extend across the entire Indo-Pacific and are >200 times larger than that of C. tricinctus, but both these widespread species are rare at Lord Howe Island (especially compared to C. tricinctus). Mean abundance of coral reef butterflyfishes at Lord Howe Island (averaged across the three sites) was weakly negatively correlated (r = −0.40, n = 13, P = 0.18) with geographic range (Fig. 6A). This relationship appeared to be driven by the high abundance and limited geographic range of C. tricinctus. Indeed excluding C. tricinctus from the analysis resulted in no relationship between abundance and geographic range (r = 0.09, n = 12, P = 0.78).
Regardless of the metric, C. trifascialis and C. tricinctus have the most specialized diets at Lord Howe Island. Notwithstanding the apparent lack of feeding among larger individuals, C. tricinctus used available coral prey in very similar proportions to C. trifascialis, feeding predominantly on arborescent Acropora, but selectively targeting tabular Acropora (Table 2). The main difference was that C. trifascialis avoided eating Pocillopora corals, whereas C. tricinctus consumed Pocillopora in approximate accordance with its’ availability. Despite similarities in their selectivity and dietary composition, C. tricinctus was >50 times more abundant than C. trifascialis, being the most and least abundant (respectively) of the five species for which dietary composition was analyzed. Other coral-feeding butterflyfishes (C. melannotus, C. plebeius, and C. lunulatus) were less selective and less abundant compared to C. tricinctus, suggesting that if there was any relationship between mean abundance and diet breadth it would be negative (Fig. 6B). However, the actual relationship based on these five species was nonsignificant (r = −0.34, n = 5, P = 0.58).
Discussion
The extent to which patterns of local abundance in coral reef fishes can be related to ecological specialization and/or geographical range size is uncertain, given the wide variety of relationships detected among taxonomic groups and locations (e.g., Hawkins et al. 2000; Bean et al. 2002; Hobbs et al. 2010; Berkström et al. 2012). Here, we reveal that marked interspecific variations in the local abundance of coral reef butterflyfishes at Lord Howe Island are weakly correlated to the geographic range size of species, but unrelated to levels of feeding specialization. Much of this range–abundance relationship hinges upon the most abundant species, C. tricinctus, which is a regional endemic with >3 times higher abundance than any other butterflyfish species at Lord Howe Island, and is the dominant species across all of our study sites. While high local abundances are often thought to be linked to high levels of preferred resource availability (Brown 1984; Brown et al. 1995; Gregory and Gaston 2000), in C. tricinctus we find unusual foraging behavior that is, unlike any other butterfly-fish classed as an obligate corallivore (Cole et al. 2008).
Despite their vulnerability to coral loss (e.g., Pratchett et al. 2006), butterflyfish assemblages are often dominated by obligate coral-feeding species (reviewed by Pratchett 2014). At Lord Howe Island, obligate coral-feeding species (including C. tricinctus) accounted for 77.43% of all butterflyfishes (580 of 749), and three of four of the most abundant species were all obligate coral-feeding species. Obligate corallivores also dominate butterflyfish assemblages at many other locations throughout the Indo-Pacific (Emslie et al. 2010; Pratchett et al. 2013a; Cole and Pratchett 2014), but it is less clear to what extent specialist versus generalist corallivores dominate butterflyfish assemblages.
Highly specialized species are expected to be much less abundant than generalist counterparts because they are assumed to be more constrained by a narrower range of possible resources (Brown 1984; Gaston et al. 1997). While such trends have been recorded in some coral reef fishes (Hawkins et al. 2000; Bean et al. 2002), the relative abundance of generalist versus specialists species within a specific location will depend upon the availability of different resources (Munday 2004); consequently, specialist species may be more abundant where their preferred resources are also abundant (Brown 1984; Emslie et al. 2010; Pratchett et al. 2013a). At Lord Howe Island, four species of obligate coral-feeding butterflyfishes (C. lunulatus, C. plebeius, C. tricinctus, and C. trifascialis) all consumed tabular Acropora disproportionately to its availability, as shown elsewhere (Berumen and Pratchett 2006; Cole et al. 2012; Pratchett et al. 2013a). Given that proportional consumption of tabular Acropora was highest for the two most specialized species, C. tricinctus and C. trifascialis (Table 2), it may be that a predominance of Acropora corals at Lord Howe Island (which accounted for up to 94% of coral recorded on individual transects) confounds the expected negative relationship between dietary specialization and abundance. While it is clear that specialist butterflyfishes are numerically dominant in some locations (e.g., Pratchett et al. 2013a), this is not necessarily the case at Lord Howe Island. The dominant species, C. tricinctus, does feed on a relatively restricted range of different corals, but it is not altogether clear how this species derives sufficient energy, especially as adults.
While it has long been assumed that C. tricinctus consumes mainly scleractinian corals (Kuiter 1996), which is consistent with its’ abundance in coral-rich habitats (Lieske and Myers 2001; Hobbs et al. 2009; Hoey et al. 2014), this is the first detailed study of their foraging behavior. Based on phylogenetically conserved patterns of feeding (e.g., Bellwood et al. 2010) one would assume C. tricinctus is an obligate corallivore. Bellwood et al. (2010) showed that C. tricinctus is within a clade containing all obligate hard-coral-feeding butterflyfishes. Clearly, when C. tricinctus feeds on corals (e.g., as juveniles) it is very selective, and preferentially targets Acropora and Pocillopora. Bite rates of small (<5 cm TL) C. tricinctus (14.60 bites per 3-min ± 2.67 SE) are also consistent with bite rates recorded for other obligate coral-feeding butterflyfishes (Gregson et al. 2008). However, the adult foraging behavior is very different to other obligate coral-feeding butterflyfishes. Obligate coral-feeding butterflyfishes typically exhibit sustained high levels of diurnal feeding upon hard corals (Gregson et al. 2008), which is attributed to physical constraints on the amount of coral tissue that can be effectively removed with each bite (Tricas 1989). It is possible that cooler water temperatures at this high-latitude coral reef may be reducing metabolic rates and altering the energetic budgets of these tropical fishes (Beamish 1981; Harmelin-Vivien 2002; Pörtner 2002), which may manifest as different types of foraging behaviors among these butterflyfish species (Clarke 2003). Size-based declines in feeding rates have been recorded among other functional groups of fishes (e.g., van Rooij et al. 1996; Bonaldo et al. 2006), and may reflect declines in energetic requirements among large and mature individuals, whereas juveniles invest substantially into growth and development (Harmelin-Vivien 2002). It is also possible that adult C. tricinctus feed mainly at night, as has been suggested for some other coral-feeding butterflyfishes (Zekeria et al. 2002). Alternatively, C. tricinctus may fundamentally alter its foraging behavior with ontogeny, as shown for some coral-feeding wrasses (Cole 2010).
The schooling behavior of C. tricinctus is also very unique, especially among corallivorous butterflyfishes. Aside from Lord Howe Island, we know that C. tricinctus is also very abundant and often forms large schools at Elizabeth and Middleton Reefs (Hobbs et al. 2009; Hoey et al. 2014), but is generally rare and occurs singly or in pairs at Norfolk Island (van der Meer et al. 2013). In reviewing the social organization of butterflyfishes, Hourigan (1989) reported that schooling is restricted to planktivorous butterflyfishes, whereas obligate corallivores tend to form pairs that aggressively maintain distinct feeding territories (Hourigan 1989; Roberts and Ormond 1992). Schooling behavior among coral reef fishes is generally considered to be a strategy to decrease search times for patchily distributed resources, provide increased protection from predators, and/or save on the energetic costs of locomotion (Ward et al. 2002; Liao 2007; Pereira and Ferreira 2013). Without further evidence (e.g., observations of nocturnal behavior) it is difficult to conclude whether this behavior plays a role in driving the extreme abundance of C. tricinctus at Lord Howe Island (especially, compared to other butterflyfishes).
Aside from resource use and availability, interspecific differences in abundance of coral reef fishes may be explained by contrasting population dynamics and key demographic rates. In particular, the relative abundance of different fishes is fundamentally dependent upon species-specific rates of recruitment (e.g., Schroeder 1987; Doherty and Williams 1988; Doherty 1991; Caselle and Warner 1996) and this is likely to be even more important at relatively isolated locations, such as Lord Howe Island. Small and isolated coral reefs, like islands, often contain a high proportion of endemic species (Jones et al. 2002; Allen 2008). Moreover, endemic marine fishes are often more (not less) abundant than their widespread counterparts (e.g., Hourigan and Reese 1987; Randall 1998; Jones et al. 2002; DeMartini 2004; DeMartini and Friedlander 2004; Hobbs et al. 2010, 2011). One obvious explanation for this pattern is that restricted range species have reproductive strategies that minimize dispersal and advection of larvae away for their natal reefs, thereby limiting the capacity for range expansion, but also ensuring effective self-recruitment (e.g., DeMartini 2004; DeMartini and Friedlander 2004; Eble et al. 2009; Hobbs et al. 2011). Consistent with this hypothesis, we recorded few (if any) very small (<5 cm TL) individuals, assumed to represent new recruits, for any species, except C. tricinctus. Moreover, van der Meer et al. (2013) showed that there are very high rates of self-recruitment at each of the reefs (Lord Howe Island, Elizabeth and Middleton Reefs) where C. tricinctus is the predominant butterflyfish species. However, interspecific comparisons of recruitment rates will require systematic surveys over multiple recruitment seasons, as well detailed demographic studies to account for possible interspecific differences in growth rates.
There is increasing evidence that terrestrial macroecological relationships between abundance and range size do not necessarily apply to coral reef fishes (e.g., Hobbs et al. 2010, 2011, 2012; Berkström et al. 2012). Contrary to expectations, the most abundant species of butterflyfish at Lord Howe Island, C. tricinctus, is a restricted range endemic and also appears to be among the most specialized of butterflyfishes recorded at this location. Endemic species may predominate at isolated locations because they are uniquely adapted to the local conditions (Blackburn et al. 1997; Thiollay 1997; Reif et al. 2006). Similarly, highly specialized species may be particularly abundant at locations with very high availability of their preferred habitat and/or food resources. Chaetodon tricinctus, however, remains an enigmatic species that contradicts much of the established understanding of coral-feeding butterflyfishes. Future research needs to consider whether the energetic demands (metabolic rates) of C. tricinctus are fundamentally different from that of other coral-feeding butterflyfishes, or how adult fishes derive necessary energy despite infrequent bouts of benthic feeding. This research is necessary to clearly establish the vulnerability of C. tricinctus to increasing degradation of coral reef environments. Specialist coral-feeding butterflyfishes are extremely vulnerable to sustained and ongoing coral loss (Pratchett et al. 2008) that is, occurring on reefs throughout the world (Hughes et al. 2003), but flexible foraging (Noble et al. 2014) and highly resilient population dynamics may help to buffer against species extinctions (Lawton et al. 2011).
Acknowledgments
We thank I. Kerr and S.-A. Gudge from the New South Wales Marine Park Authority, and B. Busteed and the crew from Howea Divers, for advice and logistical support during this project. Funding was provided by ARC Centre of Excellence for Coral Reef Studies.
Conflict of Interest
None declared.
References
- Allen GR. Conservation hotspots of biodiversity and endemism for Indo-Pacific coral reef fishes. Aquat. Conserv. 2008;18:541–556. [Google Scholar]
- Anderson MJ, Ellingsen KE. McArdle BH. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006;9:683–693. doi: 10.1111/j.1461-0248.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Gorley RN. Clarke KR. PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth: PRIMER-E; 2008. [Google Scholar]
- Beamish FWH. Swimming performance and metabolic rate of three tropical fishes in relation to temperature. Hydrobiologia. 1981;83:245–254. [Google Scholar]
- Bean K, Jones G. Caley M. Relationships among distribution, abundance and microhabitat specialization in a guild of coral reef triggerfish (family Balistidae) Mar. Ecol. Prog. Ser. 2002;233:263–272. [Google Scholar]
- Bellwood DR, Klanten S, Cowman PF, Pratchett MS, Konow N. van Herwerden L. Evolutionary history of the butterflyfishes (f: Chaetodontidae) and the rise of coral feeding fishes. J. Evol. Biol. 2010;23:335–349. doi: 10.1111/j.1420-9101.2009.01904.x. [DOI] [PubMed] [Google Scholar]
- Berkström C, Jones G, McCormick M. Srinivasan M. Ecological versatility and its importance for the distribution and abundance of coral reef wrasses. Mar. Ecol. Prog. Ser. 2012;461:151–163. doi: 10.3354/meps09788. [Google Scholar]
- Berumen ML. Pratchett MS. 2006. Effects of resource availability on the competitive behaviour of butterflyfishes (Chaetodontidae). Proc 10th Int Coral Reef Symp 2004, Okinawa, Japan.
- Berumen ML, Pratchett MS. McCormick MI. Within-reef differences in diet and body condition of coral-feeding butterflyfishes (Chaetodontidae) Mar. Ecol. Prog. Ser. 2005;287:217–227. [Google Scholar]
- Blackburn TM, Gaston KJ, Quinn RM, Arnold H. Gregory RD. Of mice and wrens: the relation between abundance and geographic range size in British mammals and birds. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 1997;352:419–427. [Google Scholar]
- Blowes SA, Pratchett MS. Connolly SR. Heterospecific aggression and dominance in a guild of coral-feeding fishes: the roles of dietary ecology and phylogeny. Am. Nat. 2013;182:157–168. doi: 10.1086/670821. [DOI] [PubMed] [Google Scholar]
- Bonaldo RM, Krajewski JP, Sazima C. Sazima I. Foraging activity and resource use by three parrotfish species at Fernando de Noronha Archipelago, tropical West Atlantic. Mar. Biol. 2006;149:423–433. [Google Scholar]
- Brook BW, S Sodhi N. Bradshaw CJ. Synergies among extinction drivers under global change. Trends Ecol. Evol. 2008;23:453–460. doi: 10.1016/j.tree.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Brown JH. On the relationship between abundance and distribution of species. Am. Nat. 1984;124:255–279. [Google Scholar]
- Brown JH, Mehlman DW. Stevens GC. Spatial variation in abundance. Ecology. 1995;76:2028–2043. [Google Scholar]
- Caselle JE. Warner RR. Variability in recruitment of coral reef fishes: the importance of habitat at two spatial scales. Ecology. 1996;77:2488–2504. [Google Scholar]
- Clarke A. Costs and consequences of evolutionary temperature adaptation. Trends Ecol. Evol. 2003;18:573–581. [Google Scholar]
- Cole AJ. Cleaning to corallivory: ontogenetic shifts in feeding ecology of tubelip wrasse. Coral Reefs. 2010;29:125–129. [Google Scholar]
- Cole AJ. Pratchett MS. Diversity in diet and feeding behaviour of butterflyfishes; reliance on reef corals versus reef habitats. In: Pratchett MS, Berumen ML, Kapoor B, editors; Biology of butterflyfishes. Boca Raton, FL: CRC Press; 2014. pp. 107–139. [Google Scholar]
- Cole AJ, Pratchett MS. Jones GP. Diversity and functional importance of coral-feeding fishes on tropical coral reefs. Fish Fish. 2008;9:286–307. doi: 10.1111/j.1467-2979.2008.00290.x. [Google Scholar]
- Cole AJ, Lawton RJ, Wilson SK. Pratchett MS. Consumption of tabular acroporid corals by reef fishes: a comparison with plant–herbivore interactions. Funct. Ecol. 2012;26:307–316. [Google Scholar]
- Davies KF, Margules CR. Lawrence JF. Asynergetic effect puts rare, specialized species at greater risk of extinction. Ecology. 2004;85:265–271. [Google Scholar]
- DeMartini EE. Habitat and endemism of recruits to shallow reef fish populations: selection criteria for no-take MPAs in the NWHI Coral Reef Ecosystem Reserve. Bull. Mar. Sci. 2004;14:185–205. [Google Scholar]
- DeMartini EE. Friedlander AM. Spatial patterns of endemism in shallow-water reef fish populations of the Northwestern Hawaiian Islands. Mar. Ecol. Prog. Ser. 2004;271:281–296. [Google Scholar]
- Devictor V, Clavel J, Julliard R, Lavergne S, Mouillot D, Thuiller W, et al. Defining and measuring ecological specialization. J. Appl. Ecol. 2010;47:15–25. [Google Scholar]
- Doherty PJ. Spatial and temporal patterns in recruitment. In: Sale PF, editor. The ecology of fishes in coral reefs. San Diego, CA: Academic Press; 1991. pp. 261–293. [Google Scholar]
- Doherty PJ. Williams DM. The replenishment of coral reef fish populations. Oceanogr. Mar. Biol. Ann. Rev. 1988;26:447–551. [Google Scholar]
- Dulvy NK, Ellis JR, Goodwin NB, Grant A, Reynolds JD. Jennings S. Methods of assessing extinction risk in marine fishes. Fish Fish. 2004;5:255–276. [Google Scholar]
- Eble JA, Toonen RJ. Bowen BW. Endemism and dispersal: comparative phylogeography of three surgeonfishes across the Hawaiian Archipelago. Mar. Biol. 2009;156:689–698. [Google Scholar]
- Emslie MJ, Pratchett MS, Cheal AJ. Osborne K. Great Barrier Reef butterflyfish community structure: the role of shelf position and benthic community type. Coral Reefs. 2010;29:705–715. [Google Scholar]
- Futuyma DJ. Moreno G. The evolution of ecological specialization. Annu. Rev. Ecol. Syst. 1988;19:207–233. [Google Scholar]
- Gaston KJ. Rarity. UK: Chapman and Hall, London; 1994. [Google Scholar]
- Gaston KJ. Species-range-size distributions: patterns, mechanisms and implications. Trends Ecol. Evol. 1996;11:197–201. doi: 10.1016/0169-5347(96)10027-6. [DOI] [PubMed] [Google Scholar]
- Gaston KJ. Species-range size distributions: products of speciation, extinction and transformation. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 1998;353:219–230. [Google Scholar]
- Gaston KJ, Blackburn TM. Lawton JH. Interspecific abundance-range size relationships: an appraisal of mechanisms. J. Anim. Ecol. 1997;66:579–601. [Google Scholar]
- Gregory RD. Gaston KJ. Explanations of commonness and rarity in British breeding birds: separating resource use and resource availability. Oikos. 2000;88:515–526. [Google Scholar]
- Gregson M, Pratchett M, Berumen M. Goodman B. Relationships between butterflyfish (Chaetodontidae) feeding rates and coral consumption on the Great Barrier Reef. Coral Reefs. 2008;27:583–591. [Google Scholar]
- Harmelin-Vivien ML. Energetics and fish diversity on coral reefs. In: Sale PF, editor. Coral reef fishes: dynamics and diversity in a complex ecosystem. San Diego, CA: Academic Press; 2002. pp. 265–274. [Google Scholar]
- Hobbs JP, Neilson J. Gilligan JJ. 2009. Distribution, abundance, habitat association and extinction risk of marine fishes endemic to the Lord Howe Island region. Report to Lord Howe Island Marine Park.
- Hobbs JP-A, Jones GP. Munday PL. Rarity and extinction risk in coral reef angelfishes on isolated islands: interrelationships among abundance, geographic range size and specialisation. Coral Reefs. 2010;29:1–11. [Google Scholar]
- Hobbs J-PA, Jones GP. Munday PL. Extinction risk in endemic marine fishes. Conserv. Biol. 2011;25:1053–1055. doi: 10.1111/j.1523-1739.2011.01698.x. [DOI] [PubMed] [Google Scholar]
- Hobbs J-PA, Jones GP, Munday PL, Connolly SR. Srinivasan M. Biogeography and the structure of coral reef fish communities on isolated islands. J. Biogeogr. 2012;39:130–139. [Google Scholar]
- Hoey AS, Pratchett MS. Cvitanovic C. High macroalgal cover and low coral recruitment undermines the potential resilience of the world’s southernmost coral reef assemblages. PLoS ONE. 2011;6:e25824. doi: 10.1371/journal.pone.0025824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey AS, Pratchett MS, Johansen J. Hoey J. 2014. 2014 marine ecological survey of Elizabeth and Middleton reefs, Lord Howe Commonwealth marine reserve. Report to the Department of the Environment, Canberra, ACT, Australia.
- Hourigan TF. Environmental determinants of butterflyfish social systems. Environ. Biol. Fishes. 1989;25:61–78. [Google Scholar]
- Hourigan TF. Reese ES. Mid-ocean isolation and the evolution of Hawaiian reef fishes. Trends Ecol. Evol. 1987;2:187–191. doi: 10.1016/0169-5347(87)90018-8. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Bellwood DR. Connolly SR. Biodiversity hotspots, centres of endemicity, and the conservation of coral reefs. Ecol Lett. 2002;5:775–784. [Google Scholar]
- Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, et al. Climate change, human impacts and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- Johnson CN. Species extinction and the relationship betweendensity and distribution. Nature. 1998;394:272–274. [Google Scholar]
- Jones GP, Caley MJ. Munday PL. Rarity in coral reef fish communities. In: Sale PF, editor; Coral reef fishes dynamics and diversity in a complex ecosystem. San Diego, CA: Academic Press; 2002. pp. 81–101. [Google Scholar]
- Koh LP, Sodhi NS. Brook BW. Ecological correlates of extinction proneness in tropical butterflies. Conserv. Biol. 2004;18:1571–1578. [Google Scholar]
- Kuiter RH. Guide to sea fishes of Australia. Sydney, NSW: New Holland; 1996. [Google Scholar]
- Lawton JH. Range, population abundance and conservation. Trends Ecol. Evol. 1993;8:409–413. doi: 10.1016/0169-5347(93)90043-O. [DOI] [PubMed] [Google Scholar]
- Lawton RJ, Messmer V, Bay LK. Pratchett MS. High gene flow across large geographic scales reduces extinction risk for highly specialised coral feeding butterflyfishes. Mol. Ecol. 2011;17:3584–3598. doi: 10.1111/j.1365-294X.2011.05207.x. [DOI] [PubMed] [Google Scholar]
- Lawton RJ, Cole AJ, Berumen ML. Pratchett MS. Geographic variation in resource use by specialist versus generalist butterflyfishes. Ecography. 2012;35:566–576. [Google Scholar]
- Liao JC. A review of fish swimming mechanics and behaviour in altered flows. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2007;362:1973–1993. doi: 10.1098/rstb.2007.2082. doi: 10.1098/rstb.2007.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieske E. Myers R. Collins pocket guide: coral reef fishes. London: Collins; 2001. [Google Scholar]
- Malcolm JR, Liu C, Neilson RP, Hansen L. Hannah L. Global warming and extinctions of endemic species from biodiversity hotspots. Conserv. Biol. 2006;20:538–548. doi: 10.1111/j.1523-1739.2006.00364.x. doi: 10.1111/j.1523-1739.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- Manly BF, McDonald L, Thomas DL, McDonald TL. Erickson WP. Resource selection by animals: statistical design and analysis for field studies. Dordrecht, the Netherlands: Springer; 2002. [Google Scholar]
- Manne LL. Pimm SL. Beyond eight forms of rarity: which species are threatened and which will be next? Anim. Conserv. 2001;4:221–229. doi: 10.1017/S1367943001001263. [Google Scholar]
- McKinney ML. How do rare species avoid extinction? A paleontological view. In: Kunin WE, Gaston KJ, editors. The biology of rarity. London: Chapman and Hall; 1997. pp. 110–129. [Google Scholar]
- van der Meer MH, Horne JB, Gardner MG, Hobbs J-PA, Pratchett MS. van Herwerden L. Limited contemporary gene flow and high self-replenishment drives peripheral isolation in an endemic coral reef fish. Ecol. Evol. 2013;3:1653–1666. doi: 10.1002/ece3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL. Habitat loss, resource specialization, and extinction on coral reefs. Glob. Chang. Biol. 2004;10:1642–1647. [Google Scholar]
- Noble MM, Pratchett MS, Coker DJ, Cvitanovic C. Fulton CJ. Foraging in corallivorous butterflyfish varies with wave exposure. Coral Reefs. 2014;33:351–361. doi: 10.1007/s00338-014-1140-7. [Google Scholar]
- Olden JD, LeRoyPoff N. Bestgen KR. Trait synergisms and the rarity, extirpation, and extinction risk of desert fishes. Ecology. 2008;89:847–856. doi: 10.1890/06-1864.1. [DOI] [PubMed] [Google Scholar]
- Päivinen J, Grapputo A, Kaitala V, Komonen A, Kotiaho JS, Saarinen K, et al. Negative density–distribution relationship in butterflies. BMC Biol. 2005;3:1–13. doi: 10.1186/1741-7007-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira PHC. Ferreira BP. Effects of life phase and schooling patterns on the foraging behaviour of coral-reef fishes from the genus Haemulon. J. Fish Biol. 2013;82:1226–1238. doi: 10.1111/jfb.12054. [DOI] [PubMed] [Google Scholar]
- Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science. 2014;344:1246752-1–1246752-10. doi: 10.1126/science.1246752. doi: 10.1126/science.1246752. [DOI] [PubMed] [Google Scholar]
- Pörtner HO. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2002;132:739–761. doi: 10.1016/s1095-6433(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Pratchett MS. Dietary overlap among coral-feeding butterflyfishes (Chaetodontidae) at Lizard Island, northern Great Barrier Reef. Mar. Biol. 2005;148:373–382. [Google Scholar]
- Pratchett MS. Feeding preferences and dietary specialization among obligate coral-feeding butterflyfishes. In: Pratchett MS, Berumen ML, Kapoor B, editors. Biology of butterflyfishes. Boca Raton, FL: CRC Press; 2014. pp. 140–179. [Google Scholar]
- Pratchett MS, Hoey AS, Wilson SK, Messmer V. Graham NAJ. Changes in the biodiversity and functioning of reef fish assemblages following coral bleaching and coral loss. Diversity. 2011;3:424–452. [Google Scholar]
- Pratchett MS, Wilson SK, Berumen ML. McCormick MI. Sublethal effects of coral bleaching on an obligate coral feeding butterflyfish. Coral Reefs. 2004;23:352–356. [Google Scholar]
- Pratchett MS, Wilson SK. Baird AH. Declines in the abundance of Chaetodon butterflyfishes following extensive coral depletion. J. Fish Biol. 2006;69:1269–1280. [Google Scholar]
- Pratchett MS, Munday PL, Wilson SK, Graham NAJ, Cinner JE, Bellwood DR, et al. Effects of climate-induced coral bleaching on coral-reef fishes: ecological and economic consequences. Oceanogr. Mar. Biol. Ann. Rev. 2008;46:251–296. [Google Scholar]
- Pratchett MS, Graham NAJ. Cole AJ. Specialist corallivores dominate butterflyfish assemblages in coral-dominated reef habitats. J. Fish Biol. 2013a;82:1177–1191. doi: 10.1111/jfb.12056. [DOI] [PubMed] [Google Scholar]
- Pratchett MS, Hoey AS, Feary DA, Bauman AG, Burt JA. Riegl B. Functional composition of Chaetodon butterflyfishes at a peripheral and extreme coral reef location, the Persian Gulf. Mar. Pollut. Bull. 2013b;72:333–341. doi: 10.1016/j.marpolbul.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Randall JE. 1976. The endemic shore fishes of the Hawaiian Islands, Lord Howe Island and Easter Island. ColloqueCommerson 1973 O.R.S.T.O.M. Travauxet Documents No. 47:49–73.
- Randall JE. Zoogeography of shore fishes of the Indo-Pacific region. Zool. Stud. 1998;37:227–268. [Google Scholar]
- Reif J, Horak D, Sedlacek O, Riegert J, Pesata M, Hrazsky K, et al. Unusual abundance-range size relationship in an Afromontane bird community: the effect of geographical isolation? J. Biogeogr. 2006;33:1959–1968. [Google Scholar]
- Roberts CM. Ormond RFG. Butterflyfish social behaviour, with special reference to the incidence of territoriality: a review. Environ. Biol. Fishes. 1992;34:79–93. [Google Scholar]
- Roberts CM, McClean CJ, Veron JEN, Hawkins JP, Allen GR, McAllister DE, et al. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science. 2002;295:1280–1284. doi: 10.1126/science.1067728. [DOI] [PubMed] [Google Scholar]
- van Rooij JM, de Jong E, Vaandrager F. Videler JJ. Resource and habitat sharing by the stoplight parrotfish, Sparisomaviride, a Caribbean reef herbivore. Environ. Biol. Fishes. 1996;47:81–91. [Google Scholar]
- Schroeder RE. Effects of patch reef size and isolation on coral reef fish recruitment. Bull. Mar. Sci. 1987;41:441–451. [Google Scholar]
- Thiollay JM. Distribution and abundance patterns of bird community and raptor populations in the Andaman archipelago. Ecography. 1997;20:67–82. [Google Scholar]
- Tricas TC. Prey selection by coral-feeding butterfly-fishes: strategies to maximize the profit. Environ. Biol. Fishes. 1989;25:171–185. [Google Scholar]
- Ward JF, Austin RM. Macdonald DW. A simulation model of foraging behaviour and the effect of predation risk. J. Anim. Ecol. 2002;69:16–30. [Google Scholar]
- Williams YM, Williams SE, Alford RA, Waycott M. Johnson CN. Niche breadth and geographical range: ecological compensation for geographical rarity in rainforest frogs. Biol. Lett. 2006;2:532–535. doi: 10.1098/rsbl.2006.0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SK, Graham NAJ, Pratchett MS, Jones GP. Polunin NVC. Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Glob. Change Biol. 2006;12:2220–2234. [Google Scholar]
- Wilson SK, Graham NAJ. Pratchett MS. Susceptibility of butterflyfish to habitat disturbance: do ‘chaets’ ever prosper? In: Pratchett MS, Berumen ML, Kapoor B, editors; Biology of butterflyfishes. Boca Raton, FL: CRC Press; 2014. pp. 226–245. [Google Scholar]
- Zann LP. The eastern Australian region: a dynamic tropical/temperate biotone. Mar. Pollut. Bull. 2000;41:188–203. [Google Scholar]
- Zekeria ZA, Dawit Y, Ghebremedhin S, Naser M. Videler JJZ. Resource partitioning among four butterflyfish species in the Red Sea. Mar. Freshw. Res. 2002;53:63–168. [Google Scholar]


