Abstract
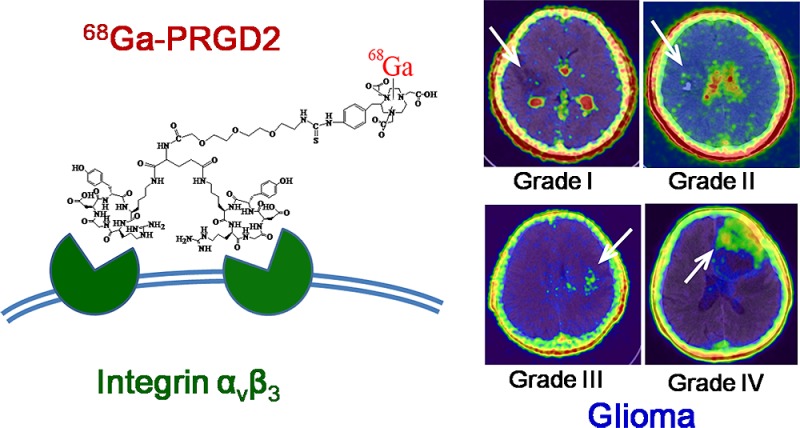
Integrin αvβ3 is overexpressed in both neovasculature and glioma cells. We aimed to evaluate 68gallium-BNOTA-PRGD2 (68Ga-PRGD2) as a new reagent for noninvasive integrin αvβ3 imaging in glioma patients. With informed consent, 12 patients with suspicious brain glioma, as diagnosed by enhanced magnetic resonance imaging (MRI) scanning, were enrolled to undergo 68Ga-PRGD2 PET/CT and 18F-FDG PET/CT scans before surgery. The preoperative images were compared and correlated with the pathologically determined WHO grade. Next, the expression of integrin αvβ3, CD34, and Ki-67 were determined by immunohistochemical staining of the resected brain tumor tissue. Our findings demonstrated that 68Ga-PRGD2 specifically accumulated in the brain tumors that were rich of integrin αvβ3 and other neovasculature markers, but not in the brain parenchyma other than the choroid plexus. Therefore, 68Ga-PRGD2 PET/CT was able to evaluate the glioma demarcation more specifically than 18F-FDG PET/CT. The maximum standardized uptake values (SUVmax) of 68Ga-PRGD2, rather than those of 18F-FDG, were significantly correlated with the glioma grading. The maximum tumor-to-brain ratios (TBRmax) of both tracers were significantly correlated with glioma grading, whereas 68Ga-PRGD2 seemed to be more superior to 18F-FDG in differentiating high-grade glioma (HGG) from low-grade glioma (LGG). Moreover, 68Ga-PRGD2 PET/CT showed different accumulation patterns for HGG of WHO grades III and IV. This is the first noninvasive integrin imaging study, to the best of our knowledge, conducted in preoperative patients with different grades of glioma, and it preliminarily indicated the effectiveness of this novel method for evaluating glioma grading and demarcation.
Keywords: integrin αvβ3, glioma, 68Ga, PET/CT
Introduction
Gliomas are the most common malignant brain tumors, characterized by extensive, diffuse infiltrative growth into the surrounding brain parenchyma and different degrees of neovascularization.1 Recent studies have demonstrated the diagnostic limitations of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET).2 The diagnostic accuracy of 18F-FDG PET is weakened by high physiologic glucose metabolism in the brain areas where glioma is prone to occur, such as the cerebral cortex, basal ganglia, and thalamus. This significantly limited the sensitivity for glioma detection and the specificity for border demarcation. The uses of 11C-methionine and 18F-fluorothymidine to image glioma showed better results but were still not enough for a final resolution.3 Thus, there is a need to develop a more specific radiotracer for PET to assess glioma.
Several researchers have demonstrated that the expression of the integrins αvβ3 and αvβ5, which are expressed in many glioma new-born vessels and glioma cells, generally increases with the grade of malignancy, and these integrins have been associated with poor prognosis,4,5 as replicated in a glioma animal model.6 Basic research showed that integrins could drive glioma progression7 and down-regulation of integrins or interference with integrin signaling pathways, which decreased migration and proliferation and improved survival in human glioblastoma cell lines.8,9 Cilengitide, an arginine-glycine-aspartic acid (RGD) pentapeptide integrin αvβ3 and αvβ5 inhibitor, was shown to improve glioblastoma multiforme (GBM, WHO grade IV) prognosis, based on preclinical and current clinical trials assessing angiogenesis, cell invasion, and migration, and it has been investigated in combination with standard therapy in several clinical trials in GBM patients.10−14
Glioma is known to be a highly heterogeneous disease, particularly regarding intratumoral heterogeneity, which contributes in part to drug resistance for some receptor inhibitors.15 Indeed, the premier research showed heterogeneous RGD uptake in GBM patients.16 With the advent of personalized medicine, understanding of intratumoral heterogeneity at different levels has become mandatory for improving clinical outcomes. Noninvasive RGD PET/CT imaging represents a more promising approach than 18F-FDG PET/CT for the specific visualization of integrin αvβ3 in preoperative glioma patients or for locating residual postoperative glioma; furthermore, this technique could potentially provide appropriate therapeutic guidance for anti-integrin targeted therapy and antiangiogenesis therapy, as well.
Until now, there have been only a small number of studies using RGD molecular imaging,6,17−20 including microPET studies, as well as studies using 99mTc-3P-RGD2 single-photon emission computed tomography/computed tomography (SPECT/CT) in subcutaneous tumor-bearing mice, MR relaxometry with RGD-labeled ultrasmall super paramagnetic iron oxide (USPIO), micro-SPECT/CT 99mTc-N2S2-Tat(49–57)-c(RGDyK) in cell lines and animal models, as well as 18F-RGD-K5 whole-body PET/CT in monkeys and human. Ji et al. demonstrated binding affinity against U87MG glioma cells by 99mTc-Galacto-RGD2, the tumor uptake of which was also in agreement with high integrin αvβ3 expression on glioma cells and in the neovasculature of nude mice bearing U87MG glioma xenografts.21
Schnell et al. reported the first clinical research about 18F-Galacto-RGD PET/CT scans in GBM patients.16 This study found that GBM demonstrated significant but heterogeneous RGD uptake, with the maximum uptake occurring in the highly proliferating and infiltrating areas of tumors, where αvβ3 expression was prominent in tumor microvessels, as well as in glial tumor cells. However, a limitation of this research was that it included not only newly diagnosed GBM but also recurrent GBM after external beam radiation or chemotherapy, thereby reducing its reliability.
In this prospective clinical study, we developed 68gallium as a new positron emitter to label small RGD peptide antagonists of integrin αvβ3, and we investigated this emitter in a prospective clinical cohort covering patients with different grades of newly diagnosed brain glioma. We hypothesized that the integrin αvβ3 expression shown in 68gallium-BNOTA-PRGD2 (68Ga-PRGD2) PET/CT scans could more precisely predict the preoperative glioma grading and could determine the demarcation more specifically than 18F-FDG PET/CT scans. The results were compared with those generated with 18F-FDG PET/CT through clinical case-by-case evaluations.
Materials and Methods
Patients
This study was approved by the Institute Review Boards of both Peking Union Medical College Hospital and Beijing Tiantan Hospital. It was conducted from November 2012 to March 2014. Written informed consent was obtained from all of the patients.
The inclusion criteria consisted of clinically based and magnetic resonance imaging (MRI)-based suspected newly diagnosed primary glioma; the patients were at least 18 years of age and had the ability to provide written and informed consent. The exclusion criteria were pregnancy, lactation, and inability to complete the needed examinations due to severe pain or claustrophobia. We evaluated all of the patients using preoperative brain 68Ga-PRGD2 PET/CT and 18F-FDG PET/CT scans within 3 days. The pathology was determined by two neuropathologists separately, and they reached in consensus by referring a third pathologist when there was any discrepancy. The criteria of pathology diagnosis are the 2007 edition of WHO classification.22 Low-grade glioma (LGG) includes grades I–II, and high-grade glioma (HGG) includes grades III–IV. This study was registered at www.clinicaltrials.gov under number NCT01801371.
68Ga-PRGD2 PET/CT Scanning
The cyclic RGD peptide was modified by PEGylated dimerization to form PEG3-E[c(RGDyK)]2 (PRGD2) and was chelated with S-2-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triyltriacetic acid (BNOTA).23−2568Ga-PRGD2 was synthesized on-site (immediately before injection) with radiochemical purity exceeding 97%. A Biograph 64 TruePoint TrueV PET/CT system (Siemens Medical Solutions, Erlangen, Germany) was used for scanning. For each patient, 1.85 MBq (0.05 mCi) of 68Ga-PRGD2 per kilogram of body weight was injected intravenously. After 30 min of rest, the patients underwent the examinations. After a low-dose CT scan (120 kV, 35 mA, 3 mm layer, 512 × 512 matrix, 70 cm FOV), brain PET acquisition was performed (1 bed position, 10 min in duration).
18F-FDG PET/CT Scanning
The patients underwent brain 18F-FDG PET/CT within 3 days of the 68Ga-PRGD2 PET/CT scan. 18F-FDG was produced on-site using Cyclotron RDS-111 (CTI, Knoxville, TN, USA). The same PET/CT system was used for scanning. Before the examinations, each patient was asked to fast for at least 4 h. The blood glucose levels of the patient were within normal limits (less than 6.4 mmol/L) before the 18F-FDG was injected at a dosage of 5.55 MBq (0.15 mCi) per kilogram of body weight. The patients rested quietly in a warm and dark room for approximately 1 h. Subsequently, the patients underwent the examinations, using the same parameters that had been employed for the 68Ga-PRGD2 PET/CT scan.
Semiquantitative Analysis
Three experienced nuclear medicine physicians read all of the images through consensus reading. The same nuclear medicine physician examined and measured the semiquantitative values for the final analysis. A Siemens MMWP workstation was used for postprocessing. For each patient, the volume of interest (VOI) was drawn, and the maximum standardized uptake values (SUVmax) and maximum tumor-to-brain ratios (TBRmax) were recorded. TBRmax was obtained by dividing the SUVmax of the VOI by that of the unaffected contralateral brain.
Immunofluorescence and Immunohistochemical Analysis
We conducted the fluorescence staining to investigate integrin αvβ3 expression and immunohistochemical staining to see the angiogenesis of the patients and corroborated their PET/CT findings. Frozen tumor tissue sections were incubated with hamster antimouse integrin αvβ3 antibody (1:100; clone BV3, Abcam, USA) and Abegrin (10 μg/mL), and then visualized by Cy3-conjugated donkey antihamster secondary antibody (1:200; Jackson ImmunoResearch Laboratories) and FITC-conjugated donkey antihuman secondary antibody (1:200) under the microscope (Carl Zeiss Axiovert 200M, Carl Zeiss, Thornwood, NY). Cryosections (4-μm thick) were obtained and fixed in 95% ethanol for 10 min. Subsequently, 3% hydrogen peroxide (H2O2) was added to quench endogenous peroxidase, and 10% goat serum (Zsgb Bio, Beijing, China) was used to block the remaining epitopes. Tissue slices were subsequently incubated at room temperature with one of the following monoclonal antibodies: CD34 (clone QBEnd/10, Leica Biosystems, Germany) at a dilution of 1:50 and Ki-67 (clone EP5, Epitomics, USA) at a dilution of 1:100. The samples were incubated with homologous secondary antibodies conjugated with horseradish peroxidase (HRP) for 60 min and with diaminobenzidine (DAB) (K4065, DAKO, USA) at a dilution of 1:20 under microscopic examination. Finally, the sections were counterstained with hematoxylin.
Statistical Analysis
All of the data are expressed as means and standard deviation (SD). Spearman’s correlation coefficient was calculated to assess the correlations between the SUVmax and TBRmax of 68Ga-PRGD2 and the grading of the glioma. All of the statistical analyses were performed using GraphPad Prism software (version 5.01, GraphPad Software, Inc., Ca, USA), and p < 0.05 was considered to be statistically significant.
Results
Patient Characteristics
The inclusion criteria were fulfilled by 12 patients (2 women, 10 men) with a mean age of 43 ± 13 years (range, 23–66 years). All of the patients presented with short histories of clinical symptoms or with newly diagnosed neurological deficits underwent MRI with gadolinium-DTPA enhancement suggesting a diagnosis of glioma and were last confirmed as glioma by postoperative pathology. The tumors were located in one brain lobe in 2 patients, in two lobes in 7 patients, in three lobes in 1 patient, in the thalamus in 1 patient, and in the pons in 1 patient (Table 1).
Table 1. Demographic Characteristics of the Enrolled Patients with Glioma.
| no. | gendera | age (years) | surgery | WHO grading | pathology | location |
|---|---|---|---|---|---|---|
| 1 | F | 23 | craniotomy | I | dysembryoplastic neuroepithelial tumor | putamen, globus pallidus, insula |
| 2 | M | 34 | craniotomy | I | neuronal-glial tumor, focal forms, rosette-forming glioneuronal tumor | tegmentum of pons |
| 3 | M | 40 | craniotomy | I, focal II | mixed neuronal-glial tumors (oligoastrocytoma, dysembryoplastic neuroepithelial tumor) | frontal, temporal lobe |
| 4 | M | 64 | stereotactic biopsy | II | diffuse astrocytoma | frontal lobe, insula |
| 5 | M | 51 | craniotomy | II, focal III | oligoastrocytoma, focal anaplastic | temporal, parietal lobe |
| 6 | F | 40 | craniotomy | II, focal III | oligoastrocytoma, focal anaplastic | frontal, temporal lobe, insula |
| 7 | M | 41 | craniotomy | II, focal III | astrocytoma, focal anaplastic | temporal, parietal, occipital lobe |
| 8 | M | 49 | craniotomy | III, focal IV | anaplastic oligoastrocytoma, focal glioblastoma | parietal, occipital lobe |
| 9 | M | 46 | craniotomy | III, focal IV | anaplastic oligoastrocytoma, focal glioblastoma | frontal lobe |
| 10 | M | 28 | craniotomy | IV | glioblastoma | temporal lobe |
| 11 | M | 66 | craniotomy | IV | glioblastoma | frontal, temporal lobe |
| 12 | M | 31 | craniotomy | IV | glioblastoma | thalamus |
M: male; F: female.
Comparison of 68Ga-PRGD2 and 18F-FDG PET/CT Scans
68Ga-PRGD2 did not accumulate in normal brain tissue, including the white matter and cortical gray matter, with the exception of the choroid plexus. Therefore, it could determine the boundary of glioma, compared to the clean background in patients’ brains (Figure 1I,J). In contrast, 18F-FDG uptake in some HGG was less than that in normal gray matter. Thus, HGG lesions were defined as low FDG uptake areas, surrounded by the relatively high 18F-FDG uptake of the normal brain tissue (Figure 1N). Therefore, 68Ga-PRGD2 PET/CT scans had much higher sensitivity for detecting glioma and higher specificity for determining tumor demarcation, compared to 18F-FDG PET/CT scans.
Figure 1.
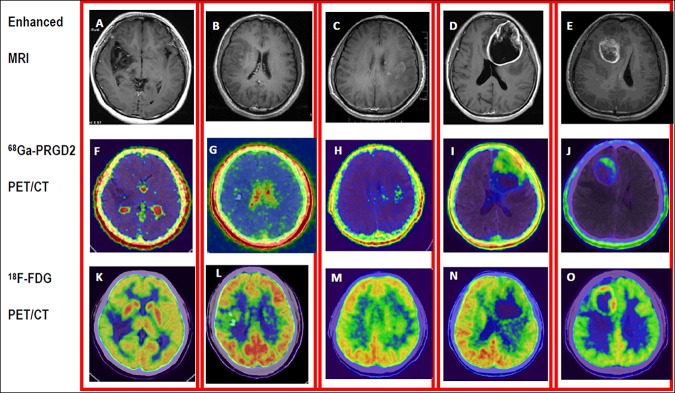
Demonstration of different WHO grade gliomas and comparison of the distribution of 68Ga-PRGD2 and 18F-FDG in the tumors. Enhanced MRI (upper row) of five patients was obtained after administration of gadolinium contrast agent. Low-grade gliomas (LGG) (A/F/K: F, 23 y, grade I. B/G/L: M, 40y, grade II) showed void to minimal accumulation of 68Ga-PRGD2 (middle row), whereas high-grade gliomas (HGG) (C/H/M: M, 41 y, grade III. D/I/N: M, 66 y, grade IV. E/G/O: M, 28 y, grade IV) showed moderate to intense uptake of 68Ga-PRGD2. The high-level cortical accumulation deteriorated the value of 18F-FDG PET/CT (lower row) in grading and demarcation of glioma, especially the LGG.
The 68Ga-PRGD2 accumulation pattern was different in grade III and IV glioma patients, compared with 18F-FDG uptake. For anaplastic astrocytoma (grade III), the 68Ga -PRGD2 expression had its own characteristic scattered focal area, resembling snowflakes (Figure 1H), while its uptake in GBM (grade IV) had much greater density, with a large bolus of PRGD2 accumulation (Figure 1I,J). In 18F-FDG PET/CT, only the necrotic center without tracer uptake was a clue to identify grade IV glioma (Figure 1N,O), while the tracer accumulation in the solid components of grades III and IV glioma may be similar (Figure 1M,N). Therefore, the accumulation shape and density could be clues for differentiating grade III and IV gliomas, which have completely different prognoses.
Correlation of PET/CT Images with Glioma Grading
As shown in Table 2, the SUVmax and TBRmax of 68Ga-PRGD2 were significantly correlated with the glioma grading (r = 0.67, p = 0.02, and r = 0.82, p = 0.001, respectively). In this group of patients, the TBRmax of 18F-FDG was significantly correlated with the glioma grading (r = 0.75, p = 0.005), whereas the SUVmax of 18F-FDG was not correlated with the glioma grading (r = 0.32, p = 0.30). The TBRmax of 68Ga-PRGD2 and 18F-FDG were both significantly correlated with the glioma grading (Table 2). However, for differentiating the HGG from the LGG, 68Ga-PRGD2 PET/CT reached 100% (8/8) sensitivity and 100% (4/4) specificity in this small group of patients when the threshold of TBRmax was set between 2.00 and 3.55, whereas the sensitivity, specificity, and accuracy of 18F-FDG PET/CT were 88% (7/8), 75% (3/4), and 83% (10/12), respectively, with the TBRmax threshold setting between 1.30 and 1.31.
Table 2. Correlation between the Glioma Grading and the Uptake of 18F-FDG and 68Ga-PRGD2a.
|
68Ga-PRGD2 |
18F-FDG |
||||
|---|---|---|---|---|---|
| no. | regarded WHO grading | SUVmax | TBRmax | SUVmax | TBRmax |
| 1 | I (LGG) | 0.79 | 0.80 | 5.90 | 0.57 |
| 2 | I (LGG) | 0.34 | 2.00 | 11.04 | 1.38 |
| 3 | II (LGG) | 0.09 | 0.90 | 10.70 | 1.30 |
| 4 | II (LGG) | 0.04 | 2.00 | 6.39 | 0.93 |
| 5 | III (HGG) | 0.75 | 8.33 | 7.73 | 1.31 |
| 6 | III (HGG) | 0.23 | 4.60 | 16.82 | 1.43 |
| 7 | III (HGG) | 0.44 | 4.40 | 6.24 | 1.11 |
| 8 | IV (HGG) | 2.31 | 3.55 | 15.76 | 1.36 |
| 9 | IV (HGG) | 1.9 | 10.56 | 8.87 | 2.07 |
| 10 | IV (HGG) | 1.21 | 11.00 | 7.69 | 1.77 |
| 11 | IV (HGG) | 0.88 | 4.63 | 37.18 | 4.77 |
| 12 | IV (HGG) | 0.5 | 10.00 | 10.43 | 3.02 |
| r | 0.67 | 0.82 | 0.32 | 0.75 | |
| p | 0.02* | 0.001* | 0.30 | 0.005* | |
Regarded WHO grading, the highest WHO grade of the total tumor when heterogeneity exists; SUVmax, maximal standardized uptake value; TBRmax, maximum tumor-to-background ratio; LGG, low-grade glioma; HGG, high-grade glioma; r, correlation coefficient to the regarded WHO grading. *p < 0.05 was considered to be significant.
Histopathological Features and Immunohistochemical Staining of Resected Tumor Tissue
Tumor pathology determination of each of 11 patients’ tumors was conducted on total resections or gross resections, whereas one patient missed the analysis because he received only stereotactic biopsy for the unresectable diffuse growth of the tumor.
We examined the histopathology and expression of integrin αvβ3 in the brain tumor tissue of 11 patients to corroborate the relevant findings with the 68Ga-PRGD2 PET/CT findings. In agreement with the intense 68Ga-PRGD2 accumulation in HGG (Figure 1), high levels of integrin αvβ3 receptor were selectively expressed on the tumor cells of HGG (Figure 3B,F), comparing to extremely low expression of integrin αvβ3 in LGG (Figure 2D). An extensive vascular network, with ongoing angiogenesis and proliferation, was observed in the HGG brain tumor tissue, as demonstrated by positive staining of CD34 (Figure 3C,G) and Ki-67 (Figure 3D,H).
Figure 3.
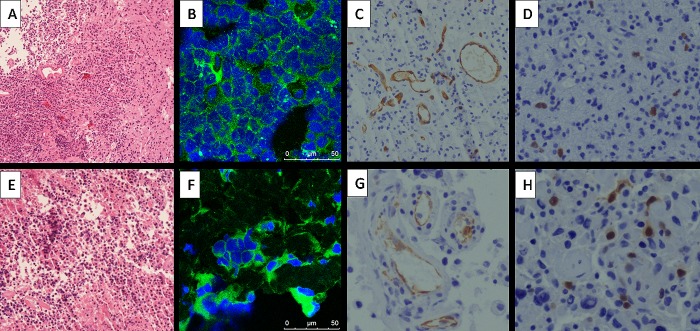
Immunohistochemical and immunofluorescence stains of the glioma of the patient (upper row A/B/C/D, patient no. 8 in Table 1; lower row E/F/G/H, patient no. 10 in Table 1; MRI and PET/CT images shown in Figure 1 E,G,O) with HGG. (A,E) Hematoxylin-eosin staining showed anaplastic oligoastrocytoma (WHO grade III) and GBM (WHO grade IV), respectively (magnification 100×). (B,F) High levels of expression of the integrin αvβ3 were observed in the tumor (magnification 200×). (C,G) The CD34 stains indicate more extensive vascular network in the tumors than that in Figure 2B (magnification 200×). (D,H) Positive nuclear expression of Ki-67 indicates active proliferation (magnification 200×).
Figure 2.
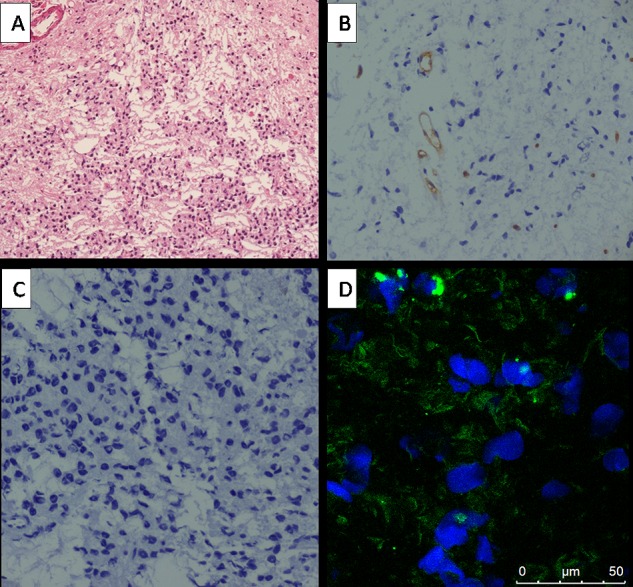
Immunohistochemical and immunofluorescence stains of the glioma of the patient (patient no. 1 Table 1, MRI and PET/CT images shown in Figure 1A,F,K) with LGG. (A) Hematoxylin-eosin staining showed WHO grade I glioma (magnification 100×). (B) The CD34 stains indicated few vascular networks in the tumors (magnification 200×). (C) Negative nuclear expression of Ki-67 indicated low proliferation (magnification 200×). (D) Very low expression of the integrin αvβ3 receptor were observed in the tumor or vascular endothelial cells.
Discussion
Over the past decade, numerous studies have demonstrated that 18F-FDG PET/CT is helpful for assessing glioma grading, determining the anaplastic transformation of LGG, differentiating recurrent glioma and radiation necrosis, and even predicting the survival of patients with recurrent glioma.3,26−28 However, the sensitivity of glioma detection by 18F-FDG PET/CT is relatively low, particularly for LGG, because 18F-FDG uptake in LGG is usually similar to that of normal white matter. Even in HGG, 18F-FDG uptake varied greatly.2918F-FDG uptake, by assessing only the mechanisms associated with elevated glucose metabolism, is nonspecific for the molecular characteristics of glioma.
68Ga-PRGD2 was specifically designed to target the endothelial cells of the neovasculature and glioma cells that express integrin αvβ3 at high levels in glioma. We demonstrated the 68Ga-PRGD2 PET/CT had higher sensitivity than 18F-FDG due to the clear lack of 68Ga-PRGD2 affinity of the normal brain.
SUVmax and TBR are both used to explain tumor PET/CT imaging, and they offer different advantages, with the former traditionally regarded as providing the representative value of each tumor. In this research, the SUVmax of 18F-FDG was not correlated with glioma grading; one reason for this finding was that different patients’ brains had different 18F-FDG uptake backgrounds. In previous research, different regions of the brain have been used for reference uptake as the index of TBR in semiquantitative analysis, including white matter and unaffected contralateral gray matter. One study demonstrated that cutoff levels of 1.5 for the tumor-to-white matter (T/WM) FDG uptake ratio and 0.6 for the tumor-to-cortex (T/C) ratio were useful in the differentiation of LGG from HGG, with sensitivity of 94% and specificity of 77%.28 However, TBRmax is more indicative of the most aggressive location in the tumor, and it can determine accurate grading for heterogeneous tumors. Additionally, it is easier and more reasonable to be used in clinical practice than the T/WM and T/C ratios because glioma can occur in both white matter and cortex, and it is sometimes difficult to distinguish the white matter and cortex on the CT images of PET/CT. Moreover, TBRmax has been increasingly used in new tracer PET/CT scans to assess glioma.30−32 The TBRmax values of 18F-FDG and 68Ga-PRGD2 PET/CT were both significantly correlated with glioma grading, but the former could not differentiate LGG from HGG, and it showed some overlap between these two groups of values. Therefore, 68Ga-PRGD2 PET/CT was superior to 18F-FDG in distinguishing LGG from HGG.
To the best of our knowledge, this was the first study conducted in humans that investigated the use of integrin imaging (specifically 68Ga-PRGD2 PET/CT) for preoperative, noninvasive assessment of glioma grading. We compared the findings of this technique with 18F-FDG PET/CT findings from the same patients. We provided histopathological confirmation showing that the different levels of expression of integrin αvβ3 on glioma cells corresponded to the WHO glioma grading.
There are some limitations of the present study. First, the number of enrolled patients with glioma was small, especially in the lower grade group. However, each patient underwent 68Ga-PRGD2 PET/CT and 18F-FDG PET/CT scanning, and the preliminary results of this study support a pilot proof-of-concept study. Second, the study lacked a sufficient number of control patients with other types of brain tumors. An additional study is required to recruit a broad variety of patients with brain-occupying lesions to determine the sensitivity, specificity, and accuracy of 68Ga-PRGD2 PET/CT in assessing glioma. Studies with more cases are needed to correlate the imaging findings related to post-treatment changes with the clinical responses and final prognoses of patients with glioma.
In conclusion, this prospective clinical study demonstrated that 68Ga-PRGD2 PET/CT is a specific method for identifying and assessing glioma neovasculature formation and glioma cells in patients with glioma. In contrast to 18F-FDG PET/CT, 68Ga-PRGD2 PET/CT is more specific for evaluating glioma demarcation. The SUVmax of 68Ga-PRGD2 is significantly correlated with glioma grading, and the TBRmax of 68Ga-PRGD2 is more superior to 18F-FDG for differentiating HGG from LGG. Therefore, 68Ga-PRGD2 PET/CT may be a useful tool for assessing glioma grading, demarcation, and neovasculature formation.
Acknowledgments
This work was supported, in part, by National Key Scientific Instrument and Equipment Development Project (2011YQ17006710), Major State Basic Research Development Program of China (973 Program) (Grant Nos. 2013CB733802 and 2014CB744503), the National Natural Science Foundation of China projects (81171370, 81271614, and 81371596), the Capital Special Project for Featured Clinical Application (Z121107001012119), a Beijing Key Laboratory of Brian Tumor Project, and the Intramural Research Program (IRP), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH). The authors are grateful to Guijun Jia, Ming Ni, Lin Qiao, and the other related staff at Beijing Tiantan Hospital, who helped in performing this study and collecting the data. Also we thank Junmei Wang, Yun Cui, and Peng Zhang for immunohistochemical analysis.
Author Contributions
⊥ These authors (D.L. and X.Z.) contributed equally to this work.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Plate K. H.; Scholz A.; Dumont D. J. Tumor angiogenesis and anti-angiogenic therapy in malignant gliomas revisited. Acta Neuropathol. 2012, 1246763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fougere C.; Suchorska B.; Bartenstein P.; Kreth F. W.; Tonn J. C. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro-Oncology 2011, 138806–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K.; Shinomiya A.; Okada M.; Hatakeyama T.; Kawai N.; Tamiya T. Usefulness of FDG, MET and FLT-PET studies for the management of human gliomas. J. Biomed. Biotechnol. 2012, 2012, 205818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittenhelm J.; Schwab E. I.; Sperveslage J.; Tatagiba M.; Meyermann R.; Fend F.; Goodman S. L.; Sipos B. Longitudinal expression analysis of alphav integrins in human gliomas reveals upregulation of integrin alphavbeta3 as a negative prognostic factor. J. Neuropathol. Exp. Neurol. 2013, 723194–210. [DOI] [PubMed] [Google Scholar]
- Schnell O.; Krebs B.; Wagner E.; Romagna A.; Beer A. J.; Grau S. J.; Thon N.; Goetz C.; Kretzschmar H. A.; Tonn J. C.; Goldbrunner R. H. Expression of integrin alphavbeta3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature. Brain Pathol. 2008, 183378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao G.; Zhou Y.; Wang F.; Liu S. Monitoring glioma growth and tumor necrosis with the U-SPECT-II/CT scanner by targeting integrin alphavbeta3. Mol. Imaging 2013, 12139–48. [PubMed] [Google Scholar]
- Holmes K. M.; Annala M.; Chua C. Y.; Dunlap S. M.; Liu Y.; Hugen N.; Moore L. M.; Cogdell D.; Hu L.; Nykter M.; Hess K.; Fuller G. N.; Zhang W. Insulin-like growth factor-binding protein 2-driven glioma progression is prevented by blocking a clinically significant integrin, integrin-linked kinase, and NF-kappaB network. Proc. Natl. Acad. Sci. U.S.A. 2012, 10993475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi Y.; Lakka S. S.; Chandrasekar N.; Yanamandra N.; Gondi C. S.; Mohanam S.; Dinh D. H.; Olivero W. C.; Gujrati M.; Tamiya T.; Ohmoto T.; Kouraklis G.; Aggarwal B.; Rao J. S. Down-regulation of integrin alpha(v)beta(3) expression and integrin-mediated signaling in glioma cells by adenovirus-mediated transfer of antisense urokinase-type plasminogen activator receptor (uPAR) and sense p16 genes. J. Biol. Chem. 2001, 2765047171–7. [DOI] [PubMed] [Google Scholar]
- Koutsioumpa M.; Polytarchou C.; Courty J.; Zhang Y.; Kieffer N.; Mikelis C.; Skandalis S.; Hellman U.; Iliopoulos D.; Papadimitriou E. Interplay between alpha v beta 3 integrin and nucleolin regulates human endothelial and glioma cell migration. J. Biol. Chem. 2012, 288, 343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonaco S. L.; Finniss S.; Xiang C.; Lee H. K.; Jiang W.; Lemke N.; Rempel S. A.; Mikkelsen T.; Brodie C. Cilengitide induces autophagy-mediated cell death in glioma cells. Neuro-Oncology 2011, 138857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M. R.; Kuhn J.; Lamborn K. R.; Lieberman F.; Wen P. Y.; Mehta M.; Cloughesy T.; Lassman A. B.; Deangelis L. M.; Chang S.; Prados M. Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03-02, a phase II trial with measures of treatment delivery. J. Neurooncol. 2012, 1061147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabors L. B.; Mikkelsen T.; Hegi M. E.; Ye X.; Batchelor T.; Lesser G.; Peereboom D.; Rosenfeld M. R.; Olsen J.; Brem S.; Fisher J. D.; Grossman S. A. A safety run-in and randomized phase 2 study of cilengitide combined with chemoradiation for newly diagnosed glioblastoma (NABTT 0306). Cancer 2012, 118225601–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaringi C.; Minniti G.; Caporello P.; Enrici R. M. Integrin inhibitor cilengitide for the treatment of glioblastoma: a brief overview of current clinical results. Anticancer Res. 2012, 32104213–23. [PubMed] [Google Scholar]
- MacDonald T. J.; Vezina G.; Stewart C. F.; Turner D.; Pierson C. R.; Chen L.; Pollack I. F.; Gajjar A.; Kieran M. W. Phase II study of cilengitide in the treatment of refractory or relapsed high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro-Oncology 2013, 15101438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson D. A.; Gini B.; Mottahedeh J.; Visnyei K.; Koga T.; Gomez G.; Eskin A.; Hwang K.; Wang J.; Masui K.; Paucar A.; Yang H.; Ohashi M.; Zhu S.; Wykosky J.; Reed R.; Nelson S. F.; Cloughesy T. F.; James C. D.; Rao P. N.; Kornblum H. I.; Heath J. R.; Cavenee W. K.; Furnari F. B.; Mischel P. S. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science 2014, 343616672–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell O.; Krebs B.; Carlsen J.; Miederer I.; Goetz C.; Goldbrunner R. H.; Wester H. J.; Haubner R.; Popperl G.; Holtmannspotter M.; Kretzschmar H. A.; Kessler H.; Tonn J. C.; Schwaiger M.; Beer A. J. Imaging of integrin alpha(v)beta(3) expression in patients with malignant glioma by [18F] Galacto-RGD positron emission tomography. Neuro-Oncology 2009, 116861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo-Garcia B. E.; Santos-Cuevas C. L.; De Leon-Rodriguez L. M.; Garcia-Becerra R.; Ordaz-Rosado D.; Luna-Guitierrez M. A.; Jimenez-Mancilla N. P.; Romero-Pina M. E.; Ferro-Flores G. Design and biological evaluation of (9)(9)mTc-N(2)S(2)-Tat(49-57)-c(RGDyK): a hybrid radiopharmaceutical for tumors expressing alpha(v)beta(3) integrins. Nucl. Med. Biol. 2013, 404481–7. [DOI] [PubMed] [Google Scholar]
- Kiessling F.; Huppert J.; Zhang C.; Jayapaul J.; Zwick S.; Woenne E. C.; Mueller M. M.; Zentgraf H.; Eisenhut M.; Addadi Y.; Neeman M.; Semmler W. RGD-labeled USPIO inhibits adhesion and endocytotic activity of alpha v beta3-integrin-expressing glioma cells and only accumulates in the vascular tumor compartment. Radiology 2009, 2532462–9. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Zhang X.; Xiong Z.; Cheng Z.; Fisher D. R.; Liu S.; Gambhir S. S.; Chen X. microPET imaging of glioma integrin {alpha}v{beta}3 expression using (64)Cu-labeled tetrameric RGD peptide. J. Nucl. Med. 2005, 46101707–18. [PubMed] [Google Scholar]
- Doss M.; Kolb H. C.; Zhang J. J.; Belanger M. J.; Stubbs J. B.; Stabin M. G.; Hostetler E. D.; Alpaugh R. K.; von Mehren M.; Walsh J. C.; Haka M.; Mocharla V. P.; Yu J. Q. Biodistribution and radiation dosimetry of the integrin marker 18F-RGD-K5 determined from whole-body PET/CT in monkeys and humans. J. Nucl. Med. 2012, 535787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S.; Czerwinski A.; Zhou Y.; Shao G.; Valenzuela F.; Sowinski P.; Chauhan S.; Pennington M.; Liu S. (99m)Tc-Galacto-RGD2: A novel (99m)Tc-labeled cyclic RGD peptide dimer useful for tumor imaging. Mol. Pharmaceutics 2013, 1093304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis D. N.; Ohgaki H.; Wiestler O. D.; Cavenee W. K.; Burger P. C.; Jouvet A.; Scheithauer B. W.; Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114297–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.; Liu Z.; Chen K.; Yan Y.; Watzlowik P.; Wester H. J.; Chin F. T.; Chen X. 18F-labeled galacto and PEGylated RGD dimers for PET imaging of alphavbeta3 integrin expression. Mol. Imaging Biol. 2010, 125530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang L.; Li W.; Guo N.; Ma Y.; Zhu L.; Kiesewetter D. O.; Shen B.; Niu G.; Chen X. Comparison study of [18F]FAl-NOTA-PRGD2, [18F]FPPRGD2, and [68Ga]Ga-NOTA-PRGD2 for PET imaging of U87MG tumors in mice. Bioconjugate Chem. 2011, 22122415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J.; Miller M.; Abend J.; Koide A.; Koide S.; Dewhurst S. Engineered fibronectin type III domain with a RGDWXE sequence binds with enhanced affinity and specificity to human alphavbeta3 integrin. J. Mol. Biol. 2003, 32651475–88. [DOI] [PubMed] [Google Scholar]
- Santra A.; Kumar R.; Sharma P.; Bal C.; Julka P. K.; Malhotra A. F-18 FDG PET-CT for predicting survival in patients with recurrent glioma: a prospective study. Neuroradiology 2011, 53121017–24. [DOI] [PubMed] [Google Scholar]
- Nihashi T.; Dahabreh I. J.; Terasawa T. Diagnostic accuracy of PET for recurrent glioma diagnosis: A meta-analysis. Am. J. Neuroradiol. 2012, 34, 944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbeke D.; Meyerowitz C.; Lapidus R. L.; Maciunas R. J.; Jennings M. T.; Moots P. L.; Kessler R. M. Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology 1995, 195147–52. [DOI] [PubMed] [Google Scholar]
- Chen W. Clinical applications of PET in brain tumors. J. Nucl. Med. 2007, 4891468–81. [DOI] [PubMed] [Google Scholar]
- Tateishi K.; Tateishi U.; Sato M.; Yamanaka S.; Kanno H.; Murata H.; Inoue T.; Kawahara N. Application of 62Cu-diacetyl-bis (N4-methylthiosemicarbazone) PET imaging to predict highly malignant tumor grades and hypoxia-inducible factor-1alpha expression in patients with glioma. Am. J. Neuroradiol. 2013, 34192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp M.; Heinzel A.; Galldiks N.; Stoffels G.; Felsberg J.; Ewelt C.; Sabel M.; Steiger H. J.; Reifenberger G.; Beez T.; Coenen H. H.; Floeth F. W.; Langen K. J. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J. Nucl. Med. 2013, 542229–35. [DOI] [PubMed] [Google Scholar]
- Jeong S. Y.; Lim S. M. Comparison of 3′-deoxy-3′-[18F]fluorothymidine PET and O-(2-[18F]fluoroethyl)-l-tyrosine PET in patients with newly diagnosed glioma. Nucl. Med. Biol. 2012, 397977–81. [DOI] [PubMed] [Google Scholar]


