Abstract
Purpose.
Sjögren's syndrome (SS) is a common autoimmune disease that can cause aqueous-deficient dry eye and the aberrant differentiation of ocular mucosal epithelial cells toward a lineage that is pathologically keratinized and skin-like. PAX6 is the master regulator of corneal lineage commitment. Recently, we showed a functional role for PAX6 in preventing ocular surface damage induced by the proinflammatory cytokine, IL-1β, in a mouse model of SS. Here, we examine PAX6's potential as a clinical biomarker that predicts ocular surface disease in SS patients.
Methods.
Impression cytology specimens isolated from the bulbar conjunctiva of control (n = 43) and SS patients (n = 43) were used to evaluate the relative abundance of PAX6, IL-1β, and pathologic keratinization marker, small proline-rich protein (SPRR1B) by TaqMan qPCR. Transcript expression was examined relative to clinical data, including the ocular staining score (OSS), tear breakup time (TBUT), Schirmer tear test, serum autoantibody results, and the labial salivary gland focus score.
Results.
PAX6 expression was significantly reduced in SS patients (P = 0.010, Wilcoxon rank sum test), and highly correlated with OSS (Spearman ρ = 0.239, 95% CI 0.02–0.43; P = 0.027). The extent to which PAX6 predicted SPRR1B was largely dependent on IL-1β expression (R2 = 0.28, P < 0.01) and elevated IL-1β predicted reduced TBUT (R2 = 0.24, P = 0.035), low tear secretion (R2 = 0.30, P = 0.011), and focus score (R2 = 0.21, P = 0.002).
Conclusions.
Downregulation of PAX6 in SS patients was highly associated with ocular surface damage and largely dependent on the level of inflammation. Restoration of PAX6 may provide a clinical approach to manage dry eye in SS patients.
Keywords: Sjögren's syndrome, PAX6, dry eye, ocular surface, squamous metaplasia
Loss of master corneal regulator, PAX6, in response to chronic inflammation provokes pathological keratinization of the ocular surface. We provide the first evidence of PAX6 loss in patients with the ocular component of SS that is highly associated with ocular surface damage and local inflammation.
Introduction
Sjögren's syndrome (SS) is a systemic disorder characterized by dryness that is attributed to lymphocytic infiltration of the lacrimal and salivary glands. Despite powerful immunosuppressive and immunomodulatory therapy, autoimmune-mediated dry eye disease, known clinically as keratoconjunctivitis sicca (KCS), is highly recalcitrant to treatment and there is no cure. In addition to the lack of effective treatment for KCS, another major obstacle is the lack of early diagnostic tools and diagnostic criteria. A devastating, end-stage consequence of many autoimmune diseases that cause aqueous-deficient dry eye is squamous metaplasia (SQM). In diseases like Stevens-Johnson syndrome, ocular cicatricial pemphigoid, and SS, the moist, ocular muco-epithelium is gradually transformed to a dry, keratinized, “skin-like” epithelium that can progress to corneal opacification and vision loss in the most severe cases.1–4 Clinical diagnosis of SQM is limited to impression cytology, introduced by Nelson and Wright in 1984,5 with diagnostic criteria established by Tseng in 1985.4 These criteria are largely based on cytological classification of goblet cells (GCs) and non-GC conjunctival epithelial cells, without direct assessment of vision-threatening keratinization. Previously, we established keratin envelope protein, SPRR1B, as a biomarker of pathologic keratinization that is characteristic of SQM development. SPRR1B is upregulated in SS patients and a mouse model of autoimmune KCS that predicts the presence of ocular epithelial damage and local expression of proinflammatory mediator, IL-1β. Yet, as part of the keratin envelope, SPRR1B is detected only after keratinization has occurred. Thus, we seek to identify novel biomarkers that play a functional role in SQM development and are altered in the earliest stages of disease.
During embryogenesis, the ocular surface epithelium develops from a highly specialized surface ectoderm, via a constellation of induction and regulatory transcription factors. Paired-box protein 6 (PAX6) is the master regulator of corneal lineage commitment that instructs corneal progenitor cells to differentiate into nonkeratinized epithelial cells characteristic of the ocular mucosal surface. PAX6 is universally expressed throughout the entire differentiated, stratified ocular surface epithelium of the adult eye (i.e., from cornea, limbus to conjunctiva).6 Although the postnatal function of PAX6 in ocular mucosal biology is not fully understood, the corneal phenotype is affected in both overlapping and distinct ways when the expression of PAX6 is increased or decreased.7–9 For example, overexpression of PAX6 in the FVB/N genetic background shared features with the PAX+/− mouse, including defective epithelial differentiation, neovascularization, and immune cell invasion.10 In humans, a decline of ocular surface PAX6 expression has been observed in a number of ocular surface diseases, ranging from immune disorders like Stevens-Johnson syndrome, to genetic defects, like aniridia.2
We hypothesized that altered ocular mucosal epithelial differentiation, the hallmark of SQM in dry eye disease, may result from the loss of the regulatory function provided by PAX6. To explore the potential role of PAX6 loss in the pathogenesis of autoimmune-mediated dry eye in vivo, we characterized a novel murine model of SS-like KCS and SQM.11–13 Mice deficient in the autoimmune regulator gene (Aire KO) spontaneously developed a T cell–mediated exocrinopathy,14,15 which provoked aqueous tear deficiency and SQM as early as postnatal week six. Interestingly, we noted loss of PAX6 expression from mucosal epithelial cells lining the ocular surface of Aire KO mice and a similar decrease was also noted in a small number of human patients with chronic KCS. By performing adoptive transfer studies, we determined that loss of PAX6 in Aire KO mice occurred as a direct consequence of autoreactive CD4+ T cell infiltration and the subsequent activation of IL-1R1 on resident cells of the ocular surface. Using adenovirus to force PAX6 expression, we restored the ocular mucosal phenotype in Aire KO mice, thus defining a functional role for PAX6 dysregulation in SQM development. Whether the functional significance of PAX6 loss could predict SQM development in human patients with autoimmune-mediated KCS has yet to be determined.
Here, we examined the hypothesis that PAX6 downregulation represented an early event leading to dysregulation of corneal differentiation in human patients with SS. Using impression cytology and quantitative PCR (qPCR), we quantified ocular surface expression of PAX6 in patients with SS, and found reduced levels were highly associated with ocular surface damage and SQM development. Moreover, in agreement with mechanistic studies in Aire KO mice, the extent to which PAX6 predicted SQM development was largely dependent on the level of local inflammation. These studies extended the use of impression cytology as a diagnostic tool and suggested significant clinical implications of maintaining or restoring ocular mucosal PAX6 to prevent the consequences of SQM that accompany chronic, autoimmune-mediated dry eye.
Methods
Human Subject Recruitment and Sample Collection
All aspects of the human subject studies presented in this manuscript were approved by the University of California San Francisco Committee for Human Research. Clinical data and impression cytology specimens were obtained from patients with SS (n = 43) and age-matched controls (n = 43) randomly selected from the Sjögren's International Collaborative Clinical Alliance (SICCA) Registry and Biorepository. Briefly, criteria for enrollment in the SICCA Registry cohort required all participants to have at least one of the following: symptoms of dry eyes or dry mouth, previous suspicion or diagnosis of SS, elevated serum anti-nuclear autoantibody (ANA), positive rheumatoid factor (RF), or anti-SSA/B, enlarged salivary glands, a recent increase in dental caries, or a possible diagnosis of secondary SS. Informed consent was obtained in writing from all subjects before participation. Subjects were presented with a written document describing the study objectives and sample collection procedures. Clinical examination included a quantitative assessment of KCS severity using fluorescein and lissamine green dyes to determine the ocular staining score (OSS).16 The OSS ranged from 0 to 12. A score of “0” indicated no corneal or conjunctival staining with fluorescein or lissamine green dye, respectively. A score of 12 indicated severe staining of the cornea and conjunctiva with confluent and central fluorescein staining, the presence of corneal filaments, and confluent areas of lissamine green of the bulbar conjunctiva of greater than or equal to 4 mm2. An OSS greater than or equal to 3 was considered positive for KCS. Consistent with established American College of Rheumatology criteria, participants were defined as SS if they tested positive for at least two of the following three primary outcome variables: (1) an OSS of 3 or greater (as defined in Whitcher et al.16), (2) a labial salivary gland (LSG) biopsy exhibiting focal lymphocytic sialadenitis with a focus score greater than or equal to 1 focus/4 mm2 (as defined by Daniels et al.17,18), and (3) serology positive for autoantibody anti-SSB/La or anti-SSA/Ro or both ANA titer (≥1:320) and positive RF.19 Control subjects were defined as those with no history of ocular surface disease or surgery in either eye; an OSS lower than 3; unanesthetized Schirmer greater than 5; LSG focus score lower than 1; and serology negative for SSA, SSB, ANA, and RF. Exclusion criteria included known diagnosis of hepatitis C virus, HIV, sarcoidosis, amyloidosis, active tuberculosis, graft-versus-host disease, past head and neck radiation treatment, current treatment with daily eye drops for glaucoma, corneal surgery in the past 5 years to correct vision, cosmetic eyelid surgery in the past 5 years, or physical or mental condition interfering with successful participation in the study. For the purposes of our investigation, we excluded all participants with a confirmed diagnosis of another autoimmune connective tissue disease that may confound findings associated with SS. Contact lens wearers were asked to discontinue wear for 7 days before the SICCA examination.
Transcriptional Profiling of PAX6, IL-1β, and SPRR1B Using Taqman PCR
Impression cytology specimens were isolated from filters using Qiagen RNeasy Plus Micro Kit (Qiagen, Inc., Valencia, CA, USA) according to the manufacturer's instructions with the following modification. In brief, impression cytology filters, previously frozen in RNAlater (Thermo Fisher Scientific, Waltham, MA, USA) and stored at −80°C were transferred into 350 μL Buffer RLT (Qiagen, Inc.) with 1% beta mercaptoethanol (BME). After vortexing for 30 seconds, filters were crushed using a microfuge tube pestle. The lysate and filter were then transferred to a QIAshredder column (Qiagen, Inc.), and centrifuged for 5 minutes at 12,000g with the flow-through collected into a 2-mL tube. Total RNA was then extracted using the Qiagen RNeasy Plus Micro Kit protocol. RNA was eluted from mini columns with 14 μL RNase-free water. RNA concentrations were determined by NanoDrop (Wilmington, DE, USA). For cDNA synthesis, we used a SuperScript VILO cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA) according to manufacturer's instructions starting with 20 ng total RNA. cDNA was stored at −20°C until use.
To compare the relative abundance of PAX6, IL-1β, and SPRR1B transcripts, we used TaqMan Gene Expression Assays (Life Technologies, Carlsbad, CA, USA) (gene assay ID: Hs00240871_m1, Hs99999029_m1, Hs00234164_m1, respectively) which consist of a pair of unlabeled PCR primers and a TaqMan probe with a fluorogenic label on the 5′ end and minor groove binder and nonfluorescent quencher on the 3′ end. One microliter of cDNA was used in a 20-μL reaction mix per well. Real-time PCR was performed using thermal cycling conditions at 95°C for 10 minutes, followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C using an ABI Prism 7500 Real Time PCR System (Applied Biosystems, Fort City, CA, USA). All assays were performed and compared in three technical replicates with a housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (gene assay ID: Hs99999905_m1). GAPDH was chosen as the reference gene based on published work demonstrating that its expression is consistently maintained in inflamed corneal tissues20,21 as well as in our own studies of Aire KO mice following various interventions.11,13,22 Threshold cycle (Ct) values were derived from triplicate measures of all cDNA specimens. The amplification efficiency of primers was obtained by constructing a standard curve for each primer. The relative quantity of gene expression was calculated using analysis software provided by Applied Biosystems.
Statistical Methods
Both linear and logistic regression and the Spearman rank correlation were used to assess association between continuous variables (with confidence intervals [CIs] derived using the bootstrap method). Continuous outcomes were compared between two groups using the Wilcoxon rank sum statistic. All comparisons were two-sided and conducted with an α of 0.05.
Results
Impression cytology specimens and clinical data were obtained from 43 control patients and 43 patients with SS from the SICCA Registry and Biorepository. Demographic data and ocular characteristics of each group are summarized in Table 1. Using diagnostic criteria established by SICCA to define our study group,19 we tested the hypothesis that reduced expression of PAX6 would predict the severity of ocular surface disease in patients with SS. Each participant with SS tested positive for at least two of the three primary outcome variables: (1) an OSS greater than or equal to 3,16 (2) a focus score greater than or equal to 1 focus/4 mm2,17,18 and (3) presence of autoantibody anti-SSB/La or anti-SSA/Ro or both ANA titer (≥1:320) and RF. A Venn diagram summarizing the SS cohort revealed that 49% had all three characteristics of SS; 19% had an abnormal OSS and an abnormal focus score; 26% had an abnormal OSS and positive serology; and 1% had positive serology, an abnormal focus score, and an OSS less than 3 in the eye from which the impression cytology specimen was obtained (Fig. 1). Additional outcome variables included, ocular surface transcript levels of proinflammatory cytokine IL-1β (a measure of local ocular inflammation), cornified envelope protein, SPRR1B (a measure of pathologic keratinization of the ocular surface), tear breakup time (TBUT; a measure of tear film stability), and Schirmer tear testing (a measure of tear secretion).
Table 1.
Patient Characteristics
|
Control,
n
= 43 |
SS,
n
= 43 |
|
| Age, mean ± SD | 53.7 ± 4.4 | 53.8 ± 13.4 |
| Sex, n (%) | ||
| Male | 8 (18.6) | 8 (18.6) |
| Female | 35 (81.4) | 35 (81.4) |
| Schirmer, mean ± SD | 14.5 ± 7.3 | 10.5 ± 8.7 |
| Tear film breakup time, mean ± SD | 8.7 ± 2.9 | 4.9 ± 3.7 |
| OSS, total mean ± SD | 1.0 ± 0.8 | 7.0 ± 3.6 |
| Nasal LG, mean ± SD | 0.21 ± 0.4 | 2.1 ± 1.2 |
| Corneal, mean ± SD | 0.49 ± 0.7 | 2.6 ± 1.9 |
| Temporal LG, mean ± SD | 0.30 ± 0.5 | 2.3 ± 1.0 |
| LSG focus score, mean ± SD | 0.65 ± 0.3 | 3.2 ± 2.9 |
| Serology, n (%) | ||
| SSA | 0 | 29 (67) |
| SSB | 0 | 19 (44) |
| ANA | 0 | 33 (77) |
| RF | 0 | 23 (53) |
| Meibomitis, n (%) | 0 | 9 (21) |
| Blepharitis, n (%) | 0 | 5 (12) |
LG, lissamine green.
Figure 1.
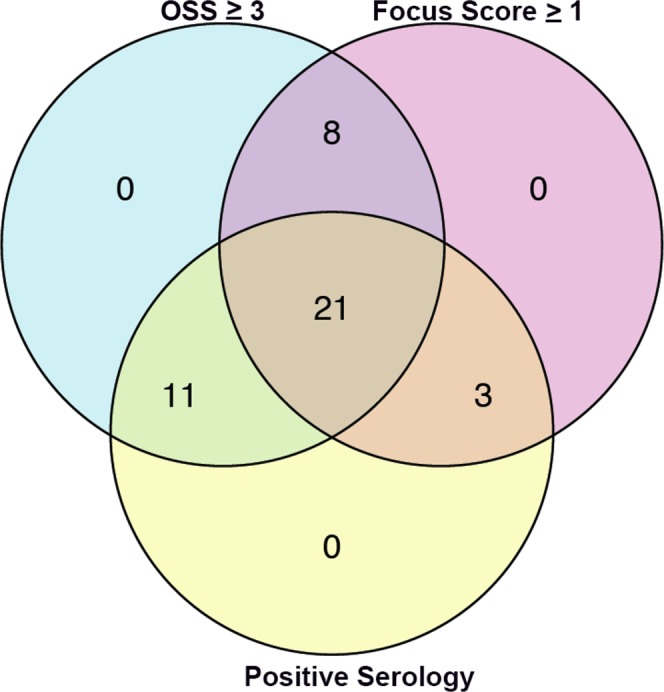
Venn diagram summarizing the diagnostic criteria met by study participants, where a positive result for at least two of the three characteristics was considered diagnostic for SS.
PAX-6 Transcript Levels Are Reduced in Patients With SS
As the universal master control gene for eye development and ocular mucosal epithelial cell differentiation, we hypothesized that loss of PAX6 expression in the setting of chronic inflammation would provoke a shift in the ocular mucosal epithelium from a moist, mucus-secreting surface to one that is pathologically keratinized and skin-like. Using impression cytology specimens to collect epithelial cells from the ocular surface, we found expression of PAX6 transcript was significantly reduced in SS patients when compared with age- and sex-matched controls (P = 0.010, Wilcoxon rank sum test). This difference persisted even after controlling for age (P = 0.014, logistic regression). Thus, PAX6 was significantly reduced in patients with the diagnosis of SS (Table 2).
Table 2.
qPCR Data
|
Control,
n
= 43 |
SS,
n
= 43 |
|
| IL-1β, mean ± SD | ||
| RQ | 5.2 ± 18.8 | 20.8 ± 68.6 |
| ΔCt | 7.8 ± 3.0 | 6.9 ± 3.2 |
| PAX6, mean ± SD | ||
| RQ | 1.1 ± 0.5 | 0.93 ± 0.5* |
| ΔCt | 0.27 ± 0.6 | 0.58 ± 0.6* |
| SPRR1B, mean ± SD | ||
| RQ | 4.4 ± 5.8 | 18.8 ± 34.4* |
| ΔCt | 4.4 ± 2.4 | 3.0 ± 2.6* |
RQ, relative quantification.
Control versus SS comparison, P < 0.05.
PAX6 Loss Predicts Ocular Surface Epithelial Disease
We hypothesized that downregulation of PAX6 and loss of corneal homeostasis may provoke ocular surface staining and altered corneal differentiation; both characteristic of SS-associated dry eye. Examining the relationship(s) among PAX6 loss, disruption of ocular surface integrity, and altered differentiation, we found that the Spearman rank correlation between the OSS (a measure of epithelial disease severity) and PAX6 was 0.239 (95% CI 0.02–0.43, bootstrap method; P = 0.027). This association was strengthened when we controlled for differences in the local expression of IL-1β. As previously described, OSS is derived from measures of both fluorescein and lissamine green staining, with each contributing 6 points to disease severity on the 12-point scale.16 We hypothesized that lissamine green staining of the bulbar conjunctiva would be a better predictor of ocular surface disease in SS patients than fluorescein staining of the cornea. Although fluorescein staining of the cornea by itself had a weaker association than lissamine green staining alone, further analysis provided no evidence of a significant difference between lissamine green and fluorescein in predicting PAX6 (95% CI for the difference between the Spearman correlation of lissamine green and PAX6 and the Spearman correlation of corneal staining and PAX6: −0.08 to 0.29, bootstrap method; P > 0.05).
PAX6 Loss and Pathologic Keratinization Are Dependent on Local Inflammation
Using SPRR1B gene expression as a readout for pathologic keratinization, we confirmed its ocular surface expression was significantly increased in SS patients compared with controls (P = 0.009, Wilcoxon rank sum test) (Table 2). Moreover, increased levels of SPRR1B were highly correlated to elevated expression of IL-1β on the ocular surface (Spearman rank correlation: 0.48, P < 0.0001) and increased ocular staining (Spearman rank correlation: 0.24, P = 0.026). The significance of these associations was in agreement with previous human studies13 and provided additional support for animal studies where we demonstrated an essential functional role for IL-1β in mediating ocular surface damage and SQM development in the pathogenesis of SS-associated KCS.11,13
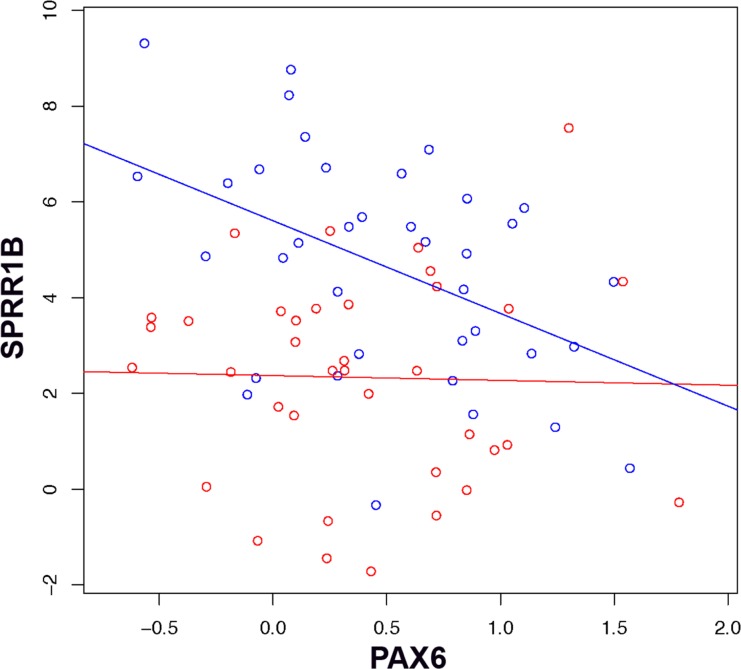
Next, we tested the hypothesis that loss of PAX6, an indicator of altered ocular surface homeostasis, would predict the development of pathologic keratinization in SQM. Controlling for differences in the local expression of IL-1β, we found loss of master regulator, PAX6, was statistically associated with increased expression of keratinization marker, SPRR1B (P = 0.02, linear regression, cases bootstrap). We examined the linear regression of SPRR1B on PAX6 for observations below the median level of IL-1β and above the median (Fig. 2). We found no evidence of a relationship between PAX6 and SPRR1B when IL-1β levels were low (P = 0.88, linear regression, represented by red points and associated regression line), but for values of IL-1β above the median, we found a substantial negative relationship between PAX6 and SPRR1B expression (P = 0.004, linear regression, represented by blue points and associated regression line). This suggested that the relationship between SQM development (increased SPRR1B) and altered corneal differentiation (loss of PAX6) was highly dependent on the level of ocular surface inflammation (IL-1β).
Figure 2.
Scatterplot of SPRR1B (vertical axis) versus PAX6 ΔCT (horizontal axis). Points below the median IL-1 ΔCT (high expression of IL-1β) are represented in blue; points above the median (low expression of IL-1β) in red. The regression line of SPRR1B versus PAX6 for each group is indicated by a blue or red line.
Local Inflammation, But Not PAX6, Predicts Altered Tear Film Quality and Quantity in SS
Using a similar strategy, we explored the relationship(s) between PAX6 and routine clinical tests used in the assessment of dry eye. Specifically, we quantified tear film stability and tear secretion using the TBUT and unanesthetized Schirmer tear test, respectively. Although PAX6 was not a good predictor of the TBUT or Schirmer test (P = 0.83, Wilcoxon rank sum test; P = 0.48; Spearman rank correlation, respectively), elevated expression of IL-1β, when considered alone, was highly predictive of TBUT (Spearman correlation 0.24, P = 0.035) and reduced tear secretion (Spearman correlation 0.30, P = 0.011). Thus, the local inflammatory response was a strong predictor of altered tear stability and secretion, whereas the altered state of epithelial differentiation was not.
Relationship Between PAX6 and Established Diagnostic Criteria for SS
The criteria used to diagnose SS have evolved as our understanding of the disease pathogenesis has progressed. The LSG focus score and presence of SS-associated autoantibodies in serum have remained key diagnostic criteria for SS. For the purpose of our study, we used the focus score as a surrogate biomarker to define the severity of exocrinopathy, where an increased number of lymphocytic foci is indicative of immune-mediated destruction of acinar cells. Although the extent to which salivary and lacrimal glands are affected in SS may differ, the two are clearly related and, thus, we hypothesized that the inflammatory state of the gland may be associated with that of the ocular surface. Furthermore, we used the presence of autoantibodies in the blood as a measure of systemic disease, where the presence of SSA or SSB or both ANA and RF was considered positive for SS. With the focus score and serology results providing readouts for exocrine and systemic disease, respectively, we examined their relationship with PAX6. Interestingly, we found neither serology nor LSG focus score to be a significant predictor of PAX6; however, the focus score was highly correlated with ocular surface expression of IL-1β (Spearman ρ = 0.4, 95% bootstrap CI 0.12–0.60, P = 0.005). Thus, inflammation of the exocrine gland predicted increased expression of proinflammatory cytokine, IL-1β, at the ocular surface. In contrast, there was no evidence of a relationship between the presence of SS-associated autoantibodies in serum and ocular surface expression of IL-1β (P = 0.71, Wilcoxon rank sum test), or any of the other clinical readouts of dry eye.
Loss of PAX6 and SQM Development Are Not Associated With Inflammatory Diseases of the Lid Margin (e.g., Meibomitis and Blepharitis)
We found no evidence of a relationship between PAX6 expression and either meibomitis (P = 0.15, Wilcoxon rank sum) or blepharitis (P = 0.39, Wilcoxon rank sum) in patients with SS. Nor did we find relationships between IL-1β and meibomitis (P = 0.45, Wilcoxon rank sum) or IL-1β and blepharitis (P = 0.37, Wilcoxon rank sum). Interestingly, we did find modest evidence of a relationship between meibomitis and the expression of SQM marker, SPRR1B (P = 0.047, Wilcoxon rank sum); however, this relationship was no longer statistically significant when accounting for multiple comparisons.
Discussion
The PAX6 has long been recognized as the universal master control gene for eye morphogenesis.23,24 Correct PAX6 dosage postnatally is imperative for developing a normal corneal epithelial phenotype and for maintaining corneal homeostasis. Overexpression of PAX6 in the mouse cornea produces an abnormal phenotype,10 whereas downregulation has been associated with abnormal epidermal differentiation in surgically removed corneoscleral tissues from patients with severe ocular surface diseases, like Stevens-Johnson syndrome, chemical burn, aniridia, and recurrent pterygium.2 Consistent with these associations, we have used a mouse model of SS-associated dry eye to demonstrate a functional role for PAX6 loss in mediating abnormal ocular mucosal epithelial cell differentiation and the development of SQM in response to chronic inflammation. In Aire KO mice, PAX6 loss occurred as a direct consequence of autoreactive CD4+ T-cell infiltration and local activation of IL-1/IL-1R1 signaling via resident cells of the ocular mucosa.25 Thus, we showed for the first time that universal master corneal control gene, PAX6, which maintains corneal epithelial phenotype during homeostasis, is downregulated in response to IL-1–mediated inflammation in SS-associated dry eye.
In the current study, we translate the results of our animal studies to the clinical setting by demonstrating PAX6 loss in superficial cells lining the ocular surface of patients with the ocular component of SS. Although ocular surface damage and the blinding consequences of SQM tend to be less severe in SS patients compared with Aire KO mice, reduced expression of ocular PAX6 in SS patients was significantly correlated with the level of ocular surface staining. A similar relationship was noted for keratinization biomarker SPRR1B, which was significantly elevated in SS patients and strongly associated with ocular surface damage. Thus, PAX6 loss and elevated SPRR1B expression provided readouts for epithelial disease severity and SQM development even in the absence of marked opacification, vascularization, and/or clinically significant keratinization. These findings support the consideration of PAX6 reintroduction as a novel therapeutic to restore ocular mucosal phenotype, maintain homeostasis, and prevent SQM in the inflamed eye. Finally, it is important to note that, based on criteria for enrollment in the SICCA Registry, all participants in this study had at least one characteristic consistent with the diagnosis of SS. Specifically, among the 43 participants classified as controls, 6 (~14%) had a self-reported history of rheumatoid arthritis or SS, 33 (~77%) were referred by a clinician suspicious of SS, 38 (~88%) suffered from symptoms of dry eye, 39 (~91%) suffered from symptoms of dry mouth, 10 (~23%) reported an enlarged salivary gland, and 18 (~42%) reported frequent dental caries. Thus, all control patients had at least one characteristic consistent with a diagnosis of SS, suggesting the significance of the associations reported in this study may be underestimated.
Histologically, PAX6 loss and SQM development are a consequence of chronic inflammation and the altered immune response has long been recognized as the critical driving force in dry eye pathogenesis.26 Although multiple cellular and soluble immune mediators are enhanced in the chronically inflamed dry eye, our work has consistently shown a foremost role for the proinflammatory cytokine, IL-1β.11,13,27,28 Interleukin-1β released by resident cells of the ocular surface has proven essential for SQM development by altering the glycosylation of conjunctival goblet cells, as well as the integrity, morphology, and differentiation of mucosal epithelial cells. Accordingly, in patients with SS we found the expression level of ocular surface IL-1β was a key factor in establishing the link between PAX6 loss and increased expression of SQM biomarker, SPRR1B. In fact, the strongest association between PAX6 loss and elevated SPRR1B was noted when expression of IL-1β was high. Increased expression of IL-1β was also a strong predictor of routine clinical tests used to assess dry eye patients. For example, high levels of IL-1β predicted ocular surface staining, low TBUT, and reduced tear secretion.
Notably, there was also a strong association between labial salivary gland focus score and ocular surface IL-1β. The focus score is one of three important diagnostic criteria for SS that specifically reflects the level of exocrinopathy. Unlike the lacrimal gland, multiple labial salivary glands are easily accessible along the inner surface of the lower lip and there is minimal risk of scaring from the surgical procedure. Thus, the focus score generated from LSG biopsy tissue has become the mainstay of assessing whether inflammation is present in SS patients and, if so, its type and severity. Although the extent to which SS affects the salivary and lacrimal glands may differ, for the purposes of this study, we chose to use the focus score as a surrogate readout for exocrine gland damage. Its positive association with ocular surface IL-1β supported the concept that immune-mediated dysfunction of the exocrine gland in SS-associated dry eye is strongly linked to chronic inflammation and destruction of other organs targeted by autoreactive T cells during the disease process. This association is consistent with an existing paradigm in dry eye pathogenesis that suggests desiccating and osmotic stress resulting from reduced tear secretion sets off an ocular surface inflammatory response that initiates the release of proinflammatory mediators from the mucosal epithelial cells, including IL-1β.29 Collectively, our animal and human studies suggest that chronic infiltration of autoreactive CD4+ effector T cells and ongoing production of IL-1β provokes the loss of PAX6, which plays an essential, functional role in promoting defective cell growth and differentiation characteristic of SS-associated SQM.
The current study provides evidence to support animal studies highlighting the essential role of PAX6 in maintaining ocular mucosal homeostasis and, thus, preventing SQM development in autoimmune-mediated dry eye. We have revealed a direct, functional link between local inflammation, PAX6 loss, and SQM development in dry eye pathogenesis in mice, and, here, we extend these findings to the clinical setting. The important parallel between human and animal studies underscores the potential significance of forced PAX6 expression to restore homeostasis in the chronically inflamed dry eye. Moreover, the foremost role of IL-1β as an essential mediator of local inflammation in dry eye has proven to serve as a robust predictor of ocular staining, tear instability, and reduced tear secretion. Collectively, human and animal studies support the therapeutic potential of PAX6 rescue25 combined with local inhibition of IL-1/IL-1R1 signaling27,30,31 as a novel approach to manage the recalcitrant ocular surface disease that accompanies autoimmune-mediated dry eye.
Acknowledgments
Supported by the National Institutes of Health, National Institute for Dental and Craniofacial Research, National Eye Institute, and Office of Research on Women's Health; Contract N01-DE32636; and National Eye Institute Grant EY016203 (NAM).
Disclosure: N.A. McNamara, None; M. Gallup, None; T.C. Porco, None
References
- 1. Li W, Hayashida Y, Chen YT, et al. Air exposure induced squamous metaplasia of human limbal epithelium. Invest Ophthalmol Vis Sci. 2008; 49: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li W, Chen YT, Hayashida Y, et al. Down-regulation of Pax6 is associated with abnormal differentiation of corneal epithelial cells in severe ocular surface diseases. J Pathol. 2008; 214: 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun TT. Altered phenotype of cultured urothelial and other stratified epithelial cells: implications for wound healing. Am J Physiol Renal Physiol. 2006; 291: F9–F21. [DOI] [PubMed] [Google Scholar]
- 4. Tseng SC. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology. 1985; 92: 728–733. [DOI] [PubMed] [Google Scholar]
- 5. Nelson JD, Wright JC. Conjunctival goblet cell densities in ocular surface disease. Arch Ophthalmol. 1984; 102: 1049–1051. [DOI] [PubMed] [Google Scholar]
- 6. Koroma BM, Yang JM, Sundin OH. The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1997; 38: 108–120. [PubMed] [Google Scholar]
- 7. Davis J, Duncan MK, Robison WG Jr, Piatigorsky J. Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J Cell Sci. 2003; 116: 2157–2167. [DOI] [PubMed] [Google Scholar]
- 8. Ramaesh T, Collinson JM, Ramaesh K, Kaufman MH, West JD, Dhillon B. Corneal abnormalities in Pax6+/− small eye mice mimic human aniridia-related keratopathy. Invest Ophthalmol Vis Sci. 2003; 44: 1871–1878. [DOI] [PubMed] [Google Scholar]
- 9. Dora N, Ou J, Kucerova R, Parisi I, West JD, Collinson JM. PAX6 dosage effects on corneal development, growth, and wound healing. Dev Dyn. 2008; 237: 1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis J, Piatigorsky J. Overexpression of Pax6 in mouse cornea directly alters corneal epithelial cells: changes in immune function, vascularization, and differentiation. Invest Ophthalmol Vis Sci. 2011; 52: 4158–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen YT, Lazarev S, Bahrami AF, et al. Interleukin-1 receptor mediates the interplay between CD4(+) T cells and ocular resident cells to promote keratinizing squamous metaplasia in Sjogren's syndrome. Lab Invest. 2012; 92: 556–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen YT, Li S, Nikulina K, Porco T, Gallup M, McNamara N. Immune profile of squamous metaplasia development in autoimmune regulator-deficient dry eye. Mol Vis. 2009; 15: 563–576. [PMC free article] [PubMed] [Google Scholar]
- 13. Chen YT, Nikulina K, Lazarev S, et al. Interleukin-1 as a phenotypic immunomodulator in keratinizing squamous metaplasia of the ocular surface in Sjogren's syndrome. Am J Pathol. 2010; 177: 1333–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeVoss J, Hou Y, Johannes K, et al. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006; 203: 2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeVoss JJ, LeClair NP, Hou Y, et al. An autoimmune response to odorant binding protein 1a is associated with dry eye in the aire-deficient mouse. J Immunol. 2010; 184: 4236–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whitcher JP, Shiboski CH, Shiboski SC, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjogren's Syndrome International Registry. Am J Ophthalmol. 2010; 149: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daniels TE. Salivary histopathology in diagnosis of Sjogren's syndrome. Scand J Rheumatol Suppl. 1986; 61: 36–43. [PubMed] [Google Scholar]
- 18. Daniels TE, Cox D, Shiboski CH, et al. Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjogren's syndrome among 1,726 registry participants. Arthritis Rheum. 2011; 63: 2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shiboski S, Shiboski C, Criswell L, et al. American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance Cohort. Arthritis Care Res (Hoboken). 2012; 64: 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bian F, Qi H, Ma P, et al. An immunoprotective privilege of corneal epithelial stem cells against Th17 inflammatory stress by producing glial cell-derived neurotrophic factor. Stem Cells. 2010; 28: 2172–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoon KC, De Paiva CS, Qi H, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007; 48: 2561–2569. [DOI] [PubMed] [Google Scholar]
- 22. Li S, Nikulina K, DeVoss J, et al. Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease. Invest Ophthalmol Vis Sci. 2008; 49: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Collinson JM, Hill RE, West JD. Different roles for Pax6 in the optic vesicle and facial epithelium mediate early morphogenesis of the murine eye. Development. 2000; 127: 945–956. [DOI] [PubMed] [Google Scholar]
- 24. Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995; 121: 1433–1442. [DOI] [PubMed] [Google Scholar]
- 25. Chen YT, Chen FY, Vijmasi T, Stephens DN, Gallup M, McNamara NA. Pax6 downregulation mediates abnormal lineage commitment of the ocular surface epithelium in aqueous-deficient dry eye disease. PLoS One. 2013; 8: e77286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDermott AM, Perez V, Huang AJ, et al. Pathways of corneal and ocular surface inflammation: a perspective from the Cullen symposium. Ocul Surf. 2005; 3: S131–S138. [DOI] [PubMed] [Google Scholar]
- 27. Vijmasi T, Chen FY, Chen YT, Gallup M, McNamara N. Topical administration of interleukin-1 receptor antagonist as a therapy for aqueous-deficient dry eye in autoimmune disease. Mol Vis. 2013; 19: 1957–1965. [PMC free article] [PubMed] [Google Scholar]
- 28. Li S, Gallup M, Chen YT, McNamara N. Molecular mechanism of proinflammatory cytokine-mediated squamous metaplasia in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010; 51: 2466–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li T, Lu Z, Lu L. Pax6 regulation in retinal cells by CCCTC binding factor. Invest Ophthalmol Vis Sci. 2006; 47: 5218–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amparo F, Dastjerdi MH, Okanobo A, et al. Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. JAMA Ophthalmol. 2013; 131: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okanobo A, Chauhan SK, Dastjerdi MH, Kodati S, Dana R. Efficacy of topical blockade of interleukin-1 in experimental dry eye disease. Am J Ophthalmol. 2012; 154: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]