Abstract
The MALDI-LTQ-Orbitrap XL mass spectrometer is a high performance instrument capable of high resolution and accurate mass (HRAM) measurements. The maximum m/z of 4000 precludes the MALDI analysis of proteins without generating multiply charged ions. Herein, we present the study of HRAM laserspray ionization mass spectrometry (MS) with MS/MS and MS imaging capabilities using 2-nitrophloroglucinol (2-NPG) as matrix on a MALDI-LTQ-Orbitrap XL mass spectrometer. The optimized conditions for multiply charged ion production have been determined and applied to tissue profiling and imaging. Biomolecules as large as 15 kDa have been detected with up to five positive charges at 100K mass resolution (at m/z 400). More importantly, MS/MS and protein identification on multiply charged precursor ions from both standards and tissue samples have been achieved for the first time with an intermediate-pressure source. The initial results reported in this study highlight potential utilities of laserspray ionization MS analysis for simultaneous in situ protein identification, visualization and characterization from complex tissue samples on a commercially available HRAM MALDI MS system.
Introduction
Matrix-assisted laser desorption/ionization (MALDI) [1] is a soft ionization technique that predominantly generates singly charged ions from solid state analytes under vacuum, intermediate pressure [2], and atmospheric pressure (AP) [3]. The capability of ionizing solid-state analytes from surfaces makes MALDI mass spectrometry (MS) an ideal tool for biological tissue analysis. The development of MALDI MS imaging (MSI) to map molecular spatial distributions greatly diversified the utility of MALDI MS [4–6].
The limited mass range of high performance mass analyzers, inefficient fragmentation of singly charged ions, and difficulties in protein identification directly from tissue are three challenges facing MALDI analysis. MALDI is often coupled with a time-of-flight (TOF) mass analyzer, which theoretically has an unlimited mass range. However, the mass resolution and accuracy of TOF instruments are often limited, especially at high m/z. In contrast, the MALDI-LTQ-Orbitrap mass spectrometer combines the high mass accuracy (<3ppm with external calibration) and high resolution (>100K at m/z 400) of an orbitrap with the fast scan rates and MS/MS capabilities (collisional induced dissociation (CID)) of a linear ion trap [7]. The addition of a high-energy collision dissociation (HCD) cell provides significant flexibility to MS/MS experiments. However, the mass range for this instrument is limited to m/z 50–4000. Therefore, producing multiply charged ions is critical for intact protein analysis directly from tissue by this instrument platform.
Laserspray ionization (LSI) is a newer technique that utilizes laser ablation of a solid matrix/analyte mixture off a surface to produce multiply charged ions similar to those observed in electrospray ionization (ESI) [8]. It is a subset of the ionization techniques that produce multiply charged ions via matrix-assisted ionization (MAI) [9]. LSI was initially performed in transmission geometry, but has expanded to reflection geometry [10], intermediate-vacuum [11], and high-vacuum [12]. It has brought intact proteins larger than 60 kDa into the operational mass range of a linear ion-trap [13]. However, MAI-like multiply charged ions have not previously been reported on an LTQ-Orbitrap mass spectrometer with an intermediate-vacuum MALDI source. At lower source pressures, multiply charged ion generation with laser ablation becomes less straightforward and typically requires instrument-specific tuning parameters.
The goal of this study is to maximize multiply charged ion production for enhanced protein and peptide analysis on a MALDI-LTQ-Orbitrap system. We use a common LSI matrix, 2-nitrophloroglucinol, first reported by Trimpin and coworkers [12]. For the first time, CID and HCD MS/MS analyses of multiply charged ions have been achieved, demonstrating the utility and application of multiply charged ions in high resolution MALDI MS for in situ protein identification, visualization and characterization.
Experimental
Reagents and sample preparation
All reagents and standards were used without additional purification. For peptide and protein analyses, bradykinin, insulin and lysozyme standards were prepared. For tissue analysis, animal experiments were conducted following institutional guidelines (UW-Madison IACUC). Rat brain tissue was embedded in gelatin, snap frozen, cryosectioned into 12 μm slices and thaw mounted onto microscopic slides. Further details can be found in the SI.
Optimization of multiply charged ion production
Parameters that were optimized for multiply charged ion profiling and imaging on rat brain included 2-NPG concentration (5, 10, 12.5, 15 and 20 mg/mL), formic acid percentage (0%, 0.025%, 0.1% and 1%), solvent composition (30% acetonitrile (ACN), 50% ACN, 70% ACN, 30% methanol, 50% methanol and 70% methanol) and laser energy (5, 10, 15, 20, 25μJ). The laser energy refers to the value indicated in the instrument control software, which is the energy at the laser head. The laser energy at the source is significantly lower, theoretically by a factor of 10 after the two neutral density filters. Fifteen consecutive 12 μm rat brain sections from the same rat brain were used for all optimization tests in order to minimize variability. For each matrix combination, three spots of 0.5μL 2-NPG were applied onto the midbrain area of rat brain section. Different laser energies were used to analyze each matrix spot.
Multiply charged ion profiling and imaging
All MS experiments were performed on a MALDI-LTQ-Orbitrap XL (Thermo Scientific, Bremen, Germany) equipped with 60 Hz 337 nm N2 laser. Full MS experiments for standards, tissue profiling and imaging were performed in FTMS mode under positive polarity with a mass range of 900–4000 (m/z 2000–4000 for lysozyme) and a mass resolution of 100,000 (at m/z 400). The step size for imaging experiments was set to be 300μm. MS/MS experiments were performed by CID and HCD in both standard and tissue analyses. Collisional energy was optimized for each parent ion. Xcalibur (Thermo Scientific, Bremen, Germany) was used for spectrum processing. ImageQuest (Thermo Scientific, Bremen, Germany) and MSiReader [14] (NC State University, North Carolina, U.S.A) were used for MS image data processing. The mass window for each peak imaged was set to 10ppm.
Results and Discussion
Optimization of laserspray ionization conditions for tissue sections
LSI conditions for generating multiply charged ions in the MALDI source were optimized on consecutive rat brain sections in order to account for the biological and chemical complexity encountered in tissue samples. Each condition was evaluated by the largest molecule, highest charge state, and percentage of multiply charged peaks detected (Figure 1, Supplementary Figure S1). The production of multiply charged signals was most significantly affected by laser energy (Figure 1a and b) and solvent composition (Figure 1c and d). 25 μJ laser energy produced the highest abundance of multiply charged ions, with low abundance doubly charged ions being detected at 20μJ and 30μJ. The largest molecule detected with 25μJ was 8564.67 Da. The dependence on laser energy is consistent with previously reported observations from intermediate- and high-vacuum LSI [11–12]. For solvent composition, significantly more multiply charged ions were produced with 70% ACN compared to all other conditions (Figure 1c and 1d), as high organic solvent content tends to extract peptide and protein analytes better. Additionally, 70% ACN may facilitate the formation of loosely-packed crystals thought to give rise to high yields of multiply charged LSI ions [11]. The optimal 2-NPG concentration and formic acid composition was 12.5 mg/mL and 0.025%, respectively (Supplementary Figure S1).
Figure 1.
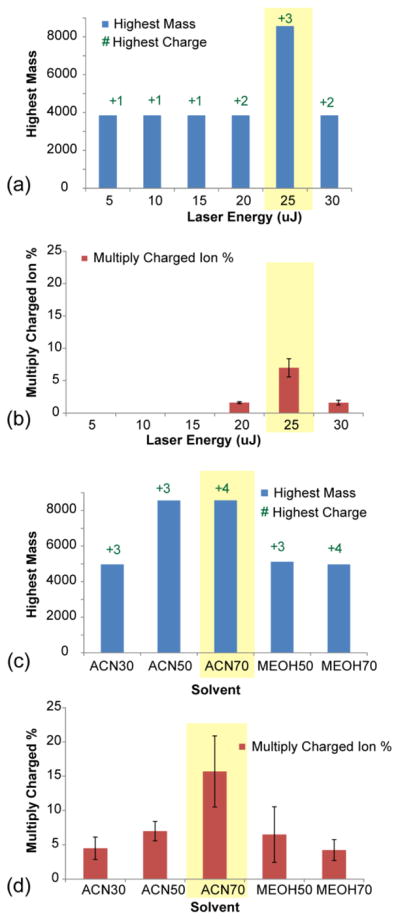
Optimization of LSI conditions for generating multiply charged ions in the MALDI source. (a) Highest mass and highest charge state detected at different laser energies (5,10,15,20 and 25μJ), (b) Percentage of multiply charged ion produced among all peaks with S/N larger than 3 at different laser energies (5,10,15,20 and 25μJ). (c) Highest mass and highest charge state detected with different solvent compositions (30% ACN, 50% ACN, 70% ACN, 50% methanol and 70% methanol), (d) Percentage of multiply charged ions produced among all peaks with S/N larger than 3 with different solvent compositions. All measurements are in triplicates. The error bar represents the standard deviation at each condition. The best conditions are highlighted in yellow shading.
HRAM analysis of peptide and protein standards with optimized conditions
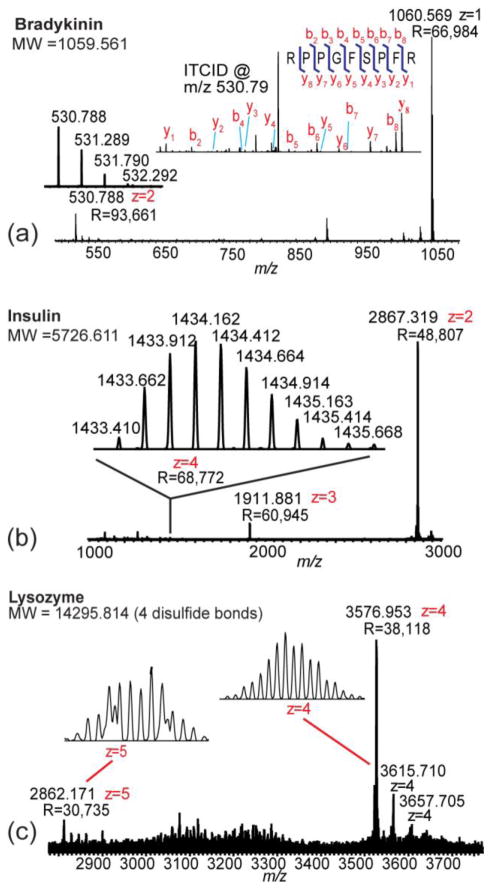
Peptide and protein standards (bradykinin, insulin and lysozyme) were analyzed under the optimized conditions with 100K mass resolution (at m/z 400) (Figure 2).
Figure 2.
Peptide and protein standards analyzed under the optimized multiply charged MALDI condition. (a) Full MS and MS/MS analyses of 10μg/mL bradykinin peptide standard. MS/MS analysis of the doubly charged ion was performed by CID. (b) MS analysis of 10μg/mL insulin protein standard. (c) MS analysis of 214μg/mL lysozyme protein standard. Some other +4 charged ions, which were impurities, were also observed near the +4 lysozyme peak.
With the optimized conditions, both singly and doubly charged ions were detected for bradykinin with 0.4ppm mass error (Figure 2a). MS/MS analyses were performed on the doubly charged bradykinin ion by CID and HCD. All b and y ions except b1 were detected. To illustrate the difference in ion production from common MALDI matrices, peptide standards were also analyzed with 2,5-dihydroxybenzoic acid (DHB), α-cyano-4-hydroxycinnamic acid (CHCA), and sinapinic acid (SA) matrices. Doubly charged ions were only produced by DHB.
The masses of singly charged insulin and lysozyme exceed the detection range of the instrument. With the optimized LSI conditions developed in this study, multiply charged insulin and lysozyme ions were detected. 1.75μM insulin yielded +2 (R=48,807), +3 (R=60,945), and +4 (R=68,772) ions in the MS spectrum (Figure 2b). MS/MS spectra were acquired by HCD. For 15μM lysozyme, +4 (R=38,118) and +5 (R=30,735) charged ions were observed (Figure 2c). Additional +4 charged ions were also observed near the main lysozyme peak due to the presence of impurities from the standard. The largest detected ion had a molecular weight of 14930.86 Da.
In situ protein characterization and visualization by full MS, MS/MS and MS imaging
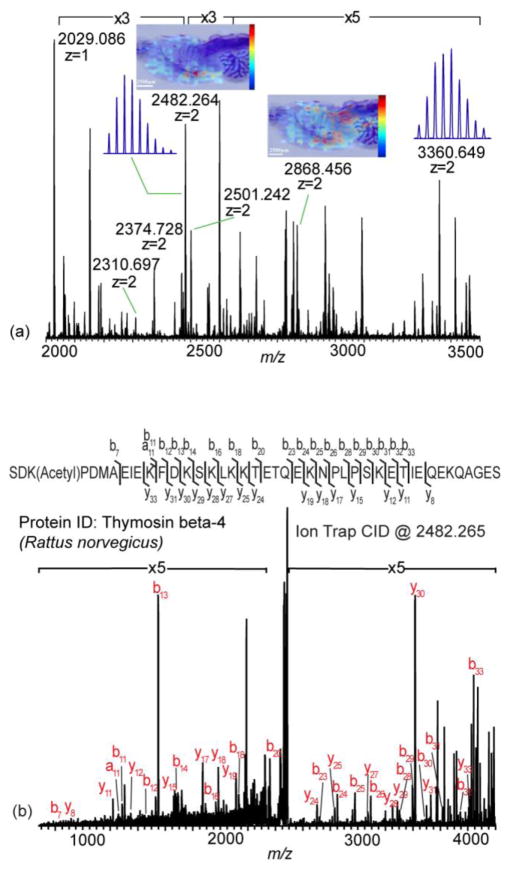
DHB was compared with 2-NPG for the ability to generate multiply charged ions on tissue using optimized LSI conditions. Only singly charged ions were observed on tissue sections prepared with DHB (Supplementary Figure S2). For 2-NPG, high resolution spectra with singly, doubly and triply charged ions were acquired (Figure 3a, Supplementary Figure S2b and c). Several peptides and proteins could be putatively identified by accurate mass matching with sub ppm mass error, such as myelin basic protein fragment 2–19, thymosin beta-4 and cytochrome c oxidase subunit 7c (Supplementary Figure S2c). MS imaging experiments were performed on rat brain tissue sections by applying 2-NPG using an airbrush. The distributions of doubly charged proteins (m/z 2482.264 and m/z 2868.456) were mapped in the MS imaging experiments (Figure 3a). However, due to the volatile nature of 2-NPG and the relatively slow acquisition rate of the MALDI orbitrap XL instrument, the step size was compromised to be 300μm.
Figure 3.
Multiply charged MALDI MS tissue profiling and imaging analysis. Full MS profiling, MS/MS and MS imaging analyses on rat brain tissue sections were performed with the optimized condition. (a) Full MS profiling and imaging analyses on rat brain tissue section. Multiply charged ions were detected. Spatial distributions of doubly charged signals at m/z 2482.264 and m/z 2868.456 were mapped by MS imaging. (b) CID MS/MS analysis at m/z 2482.265. This protein was identified to be thymosin beta-4 (Rattus norvegicus).
MS/MS was used to further confirm protein identity. Doubly charged ions at m/z of 2482.265 were fragmented and sequenced (Figure 3b). b and y ions were produced with high abundance and good sequence coverage. By manual de novo sequencing and in-house database searching, this protein was identified to be thymosin beta-4. The combination of HRAM full MS scan with CID MS/MS scan enabled confident protein identification. The ability to perform MS/MS also provides mechanistic insights into LSI [8] and MAI [9]. Previous intermediate-pressure and high vacuum LSI studies were unable to perform MS/MS, possibly due to ion liberation after the instrument’s isolation stage. Here, declustered multiply charged ions are formed before the ion trap enabling MS/MS fragmentation.
Conclusions
For the first time, multiply charged full MS, MS/MS and MSI analyses have been achieved on a commercially available, intermediate-vacuum, ultra-high performance MALDI-LTQ-Orbitrap XL system. Molecules as large as 15 kDa have been detected with up to five positive charges at 100K mass resolution (at m/z 400). By expanding the mass range using multiply charged MALDI, MALDI-LTQ-Orbitrap provides an attractive platform that enables in situ analysis of both peptides and proteins in a single experiment.
Supplementary Material
Acknowledgments
The authors are thankful to Dr. Robert Thorne’s laboratory at UW-Madison for providing rat brain tissue samples and Dr. Sarah Trimpin at the Wayne State University for helpful suggestions. This work is supported in part by the National Institutes of Health grants (1R01DK071801, 1R56DK071801). We would like to acknowledge NIH shared instrument program for funding the instrument purchase (S10 RR029531). C.B.L. acknowledges an NIH-supported Chemistry Biology Interface Training Program Predoctoral Fellowship (grant number T32-GM008505) and an NSF Graduate Research Fellowship (DGE-1256259). L. Li acknowledges an H.I. Romnes Faculty Research Fellowship.
References
- 1.Tanaka K, Waki H, Ido Y, Akita S, Yoshida Y, Yoshida T, Matsuo T. Protein and polymer analyses up to m/z 100 000 by laser ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 1988:151–153. [Google Scholar]
- 2.Garrett TJ, Yost RA. Analysis of intact tissue by intermediate-pressure MALDI on a linear ion trap mass spectrometer. Anal Chem. 2006;78:2465–2469. doi: 10.1021/ac0522761. [DOI] [PubMed] [Google Scholar]
- 3.Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Detection Technologies. Ambient mass spectrometry. Science. 2006;311:1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- 4.Ye H, Gemperline E, Li L. A vision for better health: mass spectrometry imaging for clinical diagnostics. Clin Chim Acta. 2013;420:11–22. doi: 10.1016/j.cca.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoeckli M, Chaurand P, Hallahan DE, Caprioli RM. Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues. Nat Med. 2001;7:493–496. doi: 10.1038/86573. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Ouyang Z. Mass spectrometry imaging for biomedical applications. Anal Bioanal Chem. 2013;405:5645–5653. doi: 10.1007/s00216-013-6916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strupat K, Kovtoun V, Bui H, Viner R, Stafford G, Horning S. MALDI Produced Ions Inspected with a Linear Ion Trap-Orbitrap Hybrid Mass Analyzer. J Am Soc Mass Spectrom. 2009;20:1451–1463. [Google Scholar]
- 8.Trimpin S, Inutan ED, Herath TN, McEwen CN. Laserspray ionization, a new atmospheric pressure MALDI method for producing highly charged gas-phase ions of peptides and proteins directly from solid solutions. Mol Cell Proteomics. 2010;9:362–367. doi: 10.1074/mcp.M900527-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trimpin S, Inutan ED. Matrix assisted ionization in vacuum, a sensitive and widely applicable ionization method for mass spectrometry. J Am Soc Mass Spectrom. 2013;24:722–732. doi: 10.1007/s13361-012-0571-z. [DOI] [PubMed] [Google Scholar]
- 10.Inutan ED, Richards AL, Wager-Miller J, Mackie K, McEwen CN, Trimpin S. Laserspray ionization, a new method for protein analysis directly from tissue at atmospheric pressure with ultrahigh mass resolution and electron transfer dissociation. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.000760. M110 000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inutan ED, Wang B, Trimpin S. Commercial intermediate pressure MALDI ion mobility spectrometry mass spectrometer capable of producing highly charged laserspray ionization ions. Anal Chem. 2011;83:678–684. doi: 10.1021/ac102779e. [DOI] [PubMed] [Google Scholar]
- 12.Trimpin S, Ren Y, Wang B, Lietz CB, Richards AL, Marshall DD, Inutan ED. Extending the laserspray ionization concept to produce highly charged ions at high vacuum on a time-of-flight mass analyzer. Anal Chem. 2011;83:5469–5475. doi: 10.1021/ac2007976. [DOI] [PubMed] [Google Scholar]
- 13.Lietz CB, Richards AL, Ren Y, Trimpin S. Inlet ionization: protein analyses from the solid state without the use of a voltage or a laser producing up to 67 charges on the 66 kDa BSA protein. Rapid Commun Mass Spectrom. 2011;25:3453–3456. doi: 10.1002/rcm.5233. [DOI] [PubMed] [Google Scholar]
- 14.Robichaud G, Garrard KP, Barry JA, Muddiman DC. MSiReader: an open-source interface to view and analyze high resolving power MS imaging files on Matlab platform. J Am Soc Mass Spectrom. 2013;24:718–721. doi: 10.1007/s13361-013-0607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.