Abstract
Heart failure (HF) is a major public health problem affecting more than 5 million Americans and more than 23 million patients worldwide. The epidemiology of HF is evolving. Data suggests that the incidence of HF peaked in the mid 1990s and has since declined. Survival after HF diagnosis has improved, leading to an increase in prevalence. The case mix is also changing, as a rising proportion of patients with HF have preserved ejection fraction and multimorbidity is increasingly common. After diagnosis, HF can have a profound associated morbidity. Hospitalizations in HF remain both frequent and costly, though they may be declining as a result of preventive efforts. The need for skilled nursing facility care in HF has risen. The role of palliative medicine in the care of patients with advanced HF is evolving as we learn how to best care for this population with a large symptom burden.
Keywords: heart failure, epidemiology, incidence, prevalence, mortality, readmission
INTRODUCTION
An estimated 5.1 million adults are currently living with heart failure (HF) in the United States (U.S.), a clinical syndrome with a high associated morbidity and mortality[1]. The magnitude of this public health problem is reflected by the large economic burden imposed; the total cost of care for patients with HF is $31 billion and estimated to increase to $70 billion by 2030.[2] An appreciation of the factors that impact secular trends in HF is important to understanding the epidemic and anticipating future population needs. Strategies to prevent HF will reduce the incidence, while strategies to treat patients with established HF will reduce mortality, resulting in an increased prevalence of HF. As the prevalence of HF increases, our ability to care for the growing population of patients with HF becomes more complex, and issues such as readmissions and long-term care become of mounting importance. This review will focus on recent evidence regarding secular trends in the epidemiology and outcomes of HF.
EPIDEMIOLOGY OF HEART FAILURE
Definition of Heart Failure
An understanding of the variability in the definition used for HF is needed to interpret the reported results of epidemiologic studies. The American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) guidelines define HF by stage (A to D)[3], where only stage C and D patients have had active symptoms of HF. Stage A patients include the large proportion of the U.S. adult population who have one or more risk factors for HF, such as hypertension and diabetes, whereas stage B patients have cardiac structural abnormalities but have never had clinical symptoms of HF. Most epidemiologic studies examining the prevalence of HF are restricting their definition to include only those patients with stage C (current or past symptoms of HF) of D (refractory, advanced symptoms) HF.
As HF is a clinical syndrome rather than a disease, it requires a clinical evaluation incorporating both elements of the clinical history and signs uncovered during physical examination and testing for diagnosis. Several criteria have been proposed to diagnose HF, including the Framingham criteria [4], the Gothenburg criteria [5], the Boston criteria [6], and the European Society of Cardiology (ESC) Criteria [7]. Each relies on data that can be obtained from self-report, medical record documentation, and physical examination verifying that symptoms and signs of HF are present. In addition, each of the criteria also requires incorporation of data from testing such as chest radiograph (Framingham [4], Boston [6]), electrocardiogram (Gothenburg [5]), and cardiac imaging (ESC [7]).
The Framingham criteria require the presence of two major or one major and two minor criteria to diagnose HF.[4] Examples of major and minor criteria include paroxysmal nocturnal dyspnea, orthopnea, neck vein distension, rales, cardiomegaly, jugular venous pressure elevation, ankle edema, dyspnea on exertion, and pleural effusion. The Boston criteria categorize HF into definite, possible, or unlikely based on a score calculated by summarizing components of the history (such as dyspnea on exertion and orthopnea), physical examination (such as jugular venous distension) and chest radiography (such as pleural effusions and pulmonary edema). [6] Both the Boston and Framingham criteria have 100% sensitivity for the diagnosis of HF when compared with a cardiologist categorization. The Gothenburg criteria combines cardiac and pulmonary signs and symptoms of HF with use of HF medications (diuretics, digoxin) to define an HF stage, including 0 (HF absent), 1 (only cardiac symptoms present), 2 (cardiac symptoms plus either pulmonary symptoms or medication use), 3 (cardiac and pulmonary symptoms and medication use) to 4 (death due to HF).[5] Finally, the ESC criteria require objective evidence of cardiac dysfunction in addition to symptoms of HF, such that cardiac imaging with echocardiography or another modality is required.[7]
Di Bari et al compared the 4 sets of criteria in an elderly Italian population.[8] HF was diagnosed in 11.9%, 10.7%, 20.8%, and 9.0% of participants using the Framingham, Boston, Gothenburg, and ESC criteria, respectively. The Boston criteria best predicted adverse cardiovascular events, including cardiovascular death and HF-related hospitalization, and are therefore recommended for use in older adults.
Incidence and Prevalence
In the United States, an estimated 5.1 million Americans are living with HF, with 550,000 new cases diagnosed each year.[1] A summary of studies that have examined the incidence and prevalence of HF are shown in Table 1. The prevalence and incidence of HF vary widely depending on the study population and HF diagnostic criteria used. While studies frequently use validated diagnostic criteria such as Framingham, Boston, and others previously reviewed, some rely on self-report or billing codes for the diagnosis of HF, the accuracy of which are unclear. Furthermore, many studies require a hospitalization event for detection and diagnosis, thereby missing outpatient cases. As HF can often be managed in an ambulatory setting, these studies may underestimate the incidence and prevalence of the diagnosis. In addition, shifts in coding of hospital discharge diagnoses to maximize reimbursement[9] may impact temporal trends observed.
Table 1.
Studies Reporting on the Incidence and Prevalence of Heart Failure
| Population Source | Years | First Author | Diagnostic Criteria | Incidence | Prevalence | Mortality |
|---|---|---|---|---|---|---|
| North American Studies | ||||||
| NHANES-I | 1971–1975 | Schocken[88] | Self-report and clinical score | --- | 1–2% self-report 2% clinical score |
10 year: 43% self-report, 38% clinical score |
| Medicare beneficiaries (≥65) | 1986 1993 |
Croft[12] | Initial hospitalization with HF | 1986: White: 22.4/1000 p*y Black: 22.4/1000 p*y 1993: White: 24.6/1000 p*y Black: 26.1/1000 p*y |
--- | In hospital 1986 White: 13%, Black: 11% 1993 White: 10%, Black: 9% |
| Atherosclerosis Risk in Communities Study | 1987–2002 | Loehr[17] | Initial hospitalization for HF or HF death | Black women 8.1/1000 p*y Black men: 9.1/1000 p*y White women 3.4/1000 p*y White men 6.0/1000 p*y |
--- | 1-year 22% (similar by race) |
| Cardiovascular Health Study | 1989–1995 | Gottdiener[42] | Physician diagnosis and HF treatment | Women: 14.6/1000 p*y Men: 26.2/1000 p*y |
--- | --- |
| Henry Ford Health System | 1989–1999 | McCollough[15] | Framingham | 1989: Women 3.7/1000p*y Men 4.0/1000 p*y 1999: Women 4.2/1000p*y Men 3.7/1000 p*y |
1989: 4% 1999: 14% |
1-year 17% for incident cases |
| Framingham Heart Study | 1950–1999 | Levy[19] | Framingham | 1990–99: Women 3.3/1000p*y Men 5.6/1000 p*y |
--- | 5-year age-adjusted 1950–69: Men 70% Women 57% 1990–99: Men 59% Women 45% |
| Olmsted County | 1979–2000 | Roger[20] | Framingham | Women: 2.9/1000 p*y Men: 3.8/1000 p*y |
--- | 5 year age adjusted 1979–84: 57% 1996–2000: 52% |
| Worcester, Massachusetts | 2000 | Goldberg[10] | Framingham Hospitalized only | Women: 2.5/1000 p*y Men: 1.9/1000 p*y |
--- | In-hospital 5.1% |
| Kaiser Permanente Northwest Region Health Plan | 1970–74 & 1990–94 | Barker[46] | Framingham | Women: 8.6/1000 p*y Men: 11.7/1000 p*y Women: 11.8/1000 p*y Men: 12.7/1000 p*y |
--- | 5-year age-adjusted 1970–74: Men 83% Women 61% 1990–94: Men 69% Women 65% |
| Multi-Ethnic Study of Atherosclerosis | 2000–2005 | Bahrami[16] | Physician diagnosis + medical treatment for HF | Black: 4.6/1000 p*y Hispanic: 3.5/1000 p*y White: 2.4/1000 p*y Chinese: 1.0/1000 p*y |
--- | --- |
| Medicare beneficiaries (≥65yo) | 1994–2003 | Curtis[13] | 1 inpatient or 3 outpatient billing diagnoses of HF | 1994: 32.2/1000 p*y 2003: 29.1/1000 p*y |
1994: 9% 2003: 12% |
5 year risk-adjusted 1994: Men 68% Women 62% 2003: Men 65% Women 60% |
| Ontario, Canada | 1997–2008 | Yeung[23] | Inpatient and outpatient billing diagnoses of HF | 1997: 4.5/1000 p*y 2007: 3.1/1000 p*y |
--- | 1 year risk-adjusted 1997: 27% 2007: 25% |
| CARDIA study (enrolled at 18–30yo) | 1985–2006 | Bibbins-Domingo[14] | Hospitalization for HF + medical treatment | Black women: 1.1% in 20 years follow-up Black men: 0.9% |
--- | Death from HF by age 50 in 3 black men (4.5% of deaths) and 2 black women (7.7% deaths) |
| European Studies | ||||||
| West London | 1995–1996 | Cowie[89,90] | ESC | Women: 1.2/1000 p*y Men: 1.4/1000 p*y |
--- | 1-year unadjusted 62% |
| Scotland | 1990–1996 | Stewart[21] | Initial hospitalization for HF (primary code) | 1996 Women: 1.9/1000 p*y Men: 2.2/1000 p*y |
--- | 1-year, sex-adjusted 1990: Men 37%, Women 40% 1996: Men 37%, Women 36% |
| 4 practices in England (≥45) | 1995–1999 | Davies[91] | ESC | --- | Women 1.7% Men 3.0% |
--- |
| 3 practices Denmark (≥40) | 1993–1995 | Nielsen[92] | Boston | --- | 6.4% | --- |
| Rotterdam Study (Netherlands, ≥55) | 1989–2000 | Bleumink[11] | ESC | Women: 12.5/1000 p*y Men: 17.6/1000 p*y |
1999: 7% | 1 year 37% 5 years 65% |
| Sweden | 1987–2006 | Barasa[18] | Initial HF hospitalization | Age 55–84 1987–91: 7.2/1000 p*y 2002–06: 6.0/1000 p*y |
--- | 1 year age 55–84 1987–91: 39% 2002–06: 27% |
| Eastern Finland | 1986–88 | Remes[93] | Boston | Women: 1.0/1000 p*y Men: 4.0/1000 p*y |
--- | --- |
| Other Locations | ||||||
| Western Australia | 1990–2005 | Teng[22] | Initial hospitalization for HF (primary code) | Women: 0.9/1000 p*y Men: 1.3/1000 p*y |
--- | 1 year unadjusted 1990–93: 31% 2002–05: 23% |
Similarly, the population under examination can have major implications on reported findings. It is well established that the risk of HF increases with advancing age, with an incidence of 0.3 per 1000 in those <55 years old up to 18 per 1000 for those ≥85 years[10], with estimates as high as 47 per 1000 in nonagenarians. [11] Therefore, studies limited to older populations, such as those focused on Medicare beneficiaries[12,13] tend to have higher incidence rates, while those in young populations[14] may have very low incidence rates. The incidence of HF also varies by race and sex. Several U.S. studies, including those in Medicare beneficiaries[12], those in the Henry Ford Health System[15], and in participants of the Multi-Ethnic Study of Atherosclerosis (MESA)[16], Atherosclerosis Risk in Communities (ARIC)[17], and the Coronary Artery Risk Development in Young Adults (CARDIA)[14] studies have reported a higher incidence of HF in blacks compared with whites. Only MESA specifically examined incidence rates in other races and reported the highest incidence in blacks, followed by Hispanics, whites, and Chinese individuals[16]. Most, but not all[10,14], studies have found a higher incidence of HF in men compared with women.
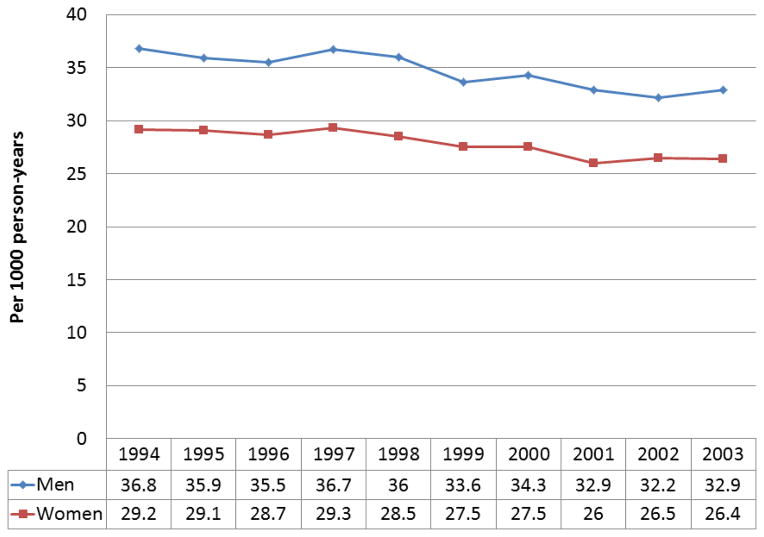
Secular trends in the incidence of HF have been examined in many studies.[12,13,15,18–23] Data reported from several studies suggest there may have been a decline in the incidence of HF since the mid-1990s. Croft reported an increase in the incidence of HF among Medicare beneficiaries in 1993 compared with 1986[12], though this may have been influenced by changes in billing patterns during the time period. Subsequently, Curtis reported a decline in the incidence of HF among Medicare beneficiaries from 1994 to 2003 (Figure).[13] Similar declines after the mid-1990s in Western Australia, Scotland, Sweden, and Ontario Canada were reported.[18,21–23] Two well-characterized population-based studies in the U.S, the Framingham Heart Study and Olmsted County study saw no changes in incidence through 2000 in men[19,20], though a 31–40% decline in women from 1990–99 was seen in Framingham[19]. More contemporary data evaluating trends in the incidence of HF that have occurred in the last decade are needed.
Figure 1. Incidence of Heart Failure in Medicare Beneficiaries, 1994 to 2003.
The incidence of heart failure declined from 32 per 1000 person-years in 1994 to 29 per 1000 person-years in 2003 (p<0.01). The incidence was higher in men than women but declined in both sexes over time. Data from [13].
The prevalence of HF varies from 1–14% based on available data from the U.S. and Europe (Table 1). The prevalence has increased over time due to improved survival after diagnosis of HF and aging of the population. Estimation of the lifetime risk for the development of HF is important for population health planning and risk communication. In the Framingham Heart Study, a primarily white U.S. population, the lifetime risk of HF ranged from 20–33%.[24] A recent effort combining data from the Cardiovascular Health Study cohort and the Chicago Heart Association Detection Project in Industry estimated the lifetime risk of developing HF from age 45 through 95 years. The risk was similar for black and white women (ranging from 24–46%) and lower for black (20–29%) compared to white (30–42%) men.[25] This divergence in overall risk of HF, which tends to be highest in black men, and a lower lifetime risk of HF in black men appeared to be due to higher competing risks for non-cardiovascular death among black men, due to causes such as homicide and renal failure. The Netherlands’ Rotterdam Study reported a lifetime risk of HF of 33% for men and 29% for women from the age of 55, which is overall similar to the U.S. findings.[11]
Impact of Ejection Fraction
HF can occur in patients with preserved and reduced left ventricular ejection fraction (EF). While HF patients with preserved (HFpEF) and reduced (HFrEF) EF have a high associated mortality and share similar clinical symptoms of HF[26–29], in many ways they are different. They tend to occur in different patient populations[26,28,30], respond differently to therapies[27,31–34], and display different patterns of ventricular and cellular remodeling[35].
Different thresholds to define preserved EF in HF have been proposed, primarily ranging from >40–55%. Large U.S. national HF registries have used ≥40% as the cutpoint[31,36], while the Olmsted County[26,28,29,37] studies have defined preserved EF as ≥50%, which is in accordance with the ACCF/AHA guidelines.[3] While estimates have varied according to the study population and EF cutpoint used, approximately half of all patients with HF have HFpEF.[38] Table 2 summarizes epidemiologic studies reporting on the prevalence and clinical characteristics of patients with HFpEF. In general, they are more likely to be older, female, have comorbidities such as hypertension and atrial fibrillation, and less likely to have clinically-evident ischemic heart disease compared with their HFrEF counterparts.
Table 2.
Selected Studies Reporting on the Prevalence and Characteristics of Patients with HFpEF
| Population Source | Years of Study | First Author | Definition of HFpEF | Prevalence of HFpEF | Demographic Characteristics Associated with HFpEF |
|---|---|---|---|---|---|
| Olmsted County | 1987–2001 | Owan[28] | EF≥50% | 47% (2167/4596) | Older age, female, higher BMI, lower hemoglobin, hypertension, atrial fibrillation |
| Olmsted County | 2003–2005 | Bursi[26] | EF≥50% | 55% (308/556) | Older age, female, no prior myocardial infarction |
| Framingham Heart Study | 1981–2004 | Lee[94] | EF>45% | 41% (220/534) | Hypertension, female sex, atrial fibrillation, no prior myocardial infarction, lack of LBBB |
| Framingham Heart Study | 1981–2008 | Ho[95] | EF>45% | 43% (196/457) | Older age, higher BMI, smoking, atrial fibrillation |
| Ontario, Canada | 1999–2001 | Bhatia[30] | EF>50% | 31% (880/2802) | Older age, female, hypertension, atrial fibrillation |
| Strong Heart Study | 1993–1995 | Devereaux[96] | EF≥55% | 53% (50/95) | Older age, female, less ischemic heart disease |
| Cardiovascular Health Study | 1989–1993 | Gottdiener[97] | EF≥55% | 22.3% (60/269) | Older age, female, hypertension, less ischemic heart disease, lower serum creatinine |
| OPTIMIZE registry | 2003–2004 | Fonarow | EF≥40% | 51% (21149/41267) | Older age, female, white, less ischemic heart disease |
| ADHERE registry | 2001–2004 | Yancy[98] | EF≥40% | 50% (26322/52187) | Older age, female, hypertension, no prior myocardial infarction |
| Community hospital registry | 1995 & 1997 | Philbin[99] | EF>50% | 24% (312/1291) | Older age, female, higher body weight, valvular etiology for HF |
| PREVEND study | 1997–2010 | Brouwers[100] | EF≥50% | *34% (125/374) | Female, non-smoker, lower serum creatinine |
| Cardiovascular Research Network | 2005–2008 | Gurwitz[101] | EF≥50% | *52% (6210/11994) | Older age, female, hypertension, white, non-cardiac comorbidities |
| Denmark registry | 1993–1996 | Gustaffson[102] | Based on WMI | 40% (2218/5491) | Older age, female, less ischemic heart disease |
| UK-HEART study | 1993–1995 | MacCarthy[103] | EF≥50% | 31% (163/522) | Lower serum creatinine |
| Euro HF Survey | 2000–2001 | Lenzen[104] | EF≥40% | 46% (3148/6806) | Older age, female, hypertension, atrial fibrillation, less ischemic heart disease |
BMI= body mass index, HFpEF= heart failure with preserved ejection fraction, EF= ejection fraction, WMI= wall motion index
Represents the proportion of newly diagnosed HF cases that had preserved EF in the study period
There are very few data informing us on secular trends in the incidence and prevalence of HFpEF. To the best of our knowledge, no study has specifically examined trends in the incidence of HFpEF, and these data are needed. Among patients hospitalized with HF in Olmsted County, Minnesota, the proportion with HFpEF increased from 38% to 54% from 1987 to 2001.[28] Given the aging of the population and increase in the comorbidity burden of patients with HF, one could hypothesize a similar trend in the incidence of HFpEF over the same time period.
Etiology of Heart Failure
Several population-based epidemiologic studies have examined the contribution of risk factors to the development of HF.[39–45] Several common factors that predispose to HF in the population have been identified, most notably hypertension (present in 44–91% of cases at incident diagnosis)[10,22,40,43,46], diabetes (18–23%)[10,22,23,40,46], coronary artery disease (29–63%)[10,13,23,40,46], obesity (25%)[40], and a history of smoking (51%).[40] It should be recognized that multiple risk factors may co-exist and interact with each other in an individual patient. In Olmsted County, the risk of developing HF was highest for patients with coronary heart disease and diabetes.[40] However, both the prevalence of a risk factor and its associated risk for the outcome are needed to determine the population impact of a risk factor on a disease (i.e. population attributable risk). Coronary disease and hypertension had the highest population attributable risks for HF, with each responsible for 20% of cases.[40] In the ARIC cohort, lack of optimal control of five factors including blood pressure, cholesterol, diabetes, smoking and body mass was estimated to account for 88.8% of incident HF events.[41] Subsequently, the population impact that a modest reduction in the prevalence of modifiable risk factors in the population, including smoking, diabetes, hyperlipidemia, hypertension, and obesity were estimated.[39] They reported that a 5% decrement in the prevalence of diabetes in the U.S., for example, may prevent 30,000 incident HF cases annually. Thus, even small reductions in the prevalence of risk factors as a result of preventative health efforts may translate into large improvements in our ability to prevent the onset of HF in the population. However, while data from the National Health Examination and Nutrition Survey (NHANES) demonstrated that the prevalence of risk factors including hypertension, hyperlipidemia, and smoking have declined, the prevalence of obesity and diabetes have risen.[47,48] These data suggest the importance of obesity and diabetes in the genesis of HF may rise, and underscore the importance of targeted preventive efforts to address these two emerging epidemics.
The burden of risk factors in patients with established HF has increased over time[49,50], and the majority of patients with HF exhibit multimorbidity[51]. In patients with incident HF, the number of risk factors per person increased by 30% from 1979–2002[49]. Furthermore, the number of patients with HF with five or more chronic conditions increased from 42.1% in 1988–94 to 58.0% in 2003–08[51], and the prevalence of multimorbidity is higher in patients with HFpEF.[52] Thus, multimorbidity, which is highly prevalent in older adults[53], and increasing in prevalence in patients with HF, deserves further examination. Multimorbidity has strong implications in the clinical management of patients with HF, where both the comorbidity burden and the interaction of specific comorbidities can affect the metabolism of medications, determine eligibility for advanced heart failure therapies, and have implications on prognosis.
SECULAR TRENDS IN OUTCOMES AFTER HEART FAILURE DIAGNOSIS
Mortality
Numerous studies have consistently shown that mortality from HF has steadily declined in recent decades[12,13,18,19,21–23,46,54,55], largely reflecting the introduction of medications, such as angiotensin converting enzyme inhibitors and beta blockers, which improve survival in patients with reduced EF. Secular trends in mortality from the time of initial diagnosis of HF are summarized in Table 1. However, despite these improvements, HF remains associated with poor outcomes. After initial diagnosis of HF, the estimated survival is 72–75% at 1 year[18,19,23] and 35–52% at 5 years.[18–20] Most studies have suggested that women have better survival than men after diagnosis, adjusting for age. In Framingham, estimated 5-year mortality was 59% in men and 45% in women from 1990–99.[19] Similar improvements in survival over time and sex differences were reported in Olmsted County[20], elderly Medicare beneficiaries[13], and the Kaiser Permanente system[46] through the 1990s. More recent data from Medicare beneficiaries has suggested that mortality may have reached a plateau from 2001–2005.[56]
In-hospital mortality has also improved. A report using a large national dataset of U.S. hospital discharges found that in-hospital mortality declined by 27% from 4.5% in 2001 to 3.3% in 2009, though no improvements were seen in younger individuals.[57] In Medicare beneficiaries hospitalized with HF, in-hospital mortality declined from 8.5% in 1993 to 4.3% in 2006.[58] However, this was balanced, in part, by an increase in early post-discharge mortality (from discharge to 30-days post-discharge), such that the total 30-day mortality rate only declined by 2.1% during the same period. This may reflect a movement toward discharging patients earlier as reimbursement from U.S. government payers does not increase with longer length of stay.
There are very few studies examining the cause of death in patients with HF. In Olmsted County, 43% of deaths were due to non-cardiovascular causes, and the proportion was higher in patients with preserved EF.[50] Over time, a shift in the distribution of deaths occurred, with a decrease in the proportion of cardiovascular deaths from 74% from 1979–84 to 51% from 1997–2002. Concomitant increases in patient age and comorbidity burden were observed over the study period, which were felt to impact the shift toward non-cardiovascular causes of death observed. In contrast, trial populations including highly selected patients with reduced EF have shown a much lower proportion of non-cardiovascular deaths.[59,60]
Readmissions
Heart failure is the leading cause of hospitalization among Medicare beneficiaries in the U.S. Patients hospitalized with HF have the highest 30-day readmission rate (~25%) of any diagnosis[61], over half of patients are readmitted within one year, and multiple readmissions are common.[49,62] In total, there are more than 1 million hospitalizations for HF each year in the U.S.[63]. Annual total direct medical costs for patients with HF are $21 billion and expected to increase to $53 billion by 2030[2], and hospitalizations account for up to three-quarters of those costs.[64] Thus, hospitalizations in patients with HF are a major public health problem, and have been a focus of the debate on healthcare reform. One of the provisions in the Affordable Care Act established the Hospital Readmissions Reduction Program[65], which required the Center for Medicare and Medicaid Services (CMS) to begin financial penalizing hospitals with higher-than-expected 30-day readmission rates for HF, pneumonia, and acute myocardial infarction. Beginning in fiscal year 2013, two-thirds of hospitals who were identified as “underperformers” faced a financial penalty of up to 1% of their total Medicare base payments, which was increased to a maximum 2% penalty in 2014.
While patients hospitalized with HF are at high risk for readmission, the majority of hospitalizations in patients with HF are due to reasons other than HF. In Medicare beneficiaries, only 37% of readmissions within 30 days of a HF hospitalizations are for HF[61], and total cardiovascular causes only account for about half of 30-day readmissions.[66] Over the lifetime after HF diagnosis, the average patient is hospitalized about once a year, and most (62%) hospitalizations are for non-cardiovascular causes.[49] Common non-cardiovascular reasons for (re)admission include respiratory tract infections and other pulmonary disorders, renal disorders, and fractures,[49,61] likely reflecting the comorbidity burden in the population. While patients with HFpEF are more likely to experience non-cardiovascular hospitalizations than those with HFrEF,[67] the overall risk of hospitalization is similar in both groups.[30,49]
Several studies examining trends in hospitalizations for HF have demonstrated a peak in rates in the 1990s, followed by declines thereafter. These reflect reports from the U.S.[54,57], Canada[68], Sweden[69], the Netherlands[70], New Zealand[71], and Scotland[72]. Using data from a national database of hospital discharges in the U.S., the HF hospitalization rate declined by a relative 26.9% from 2001 to 2009.[57] However, the declines were limited to older individuals, a pattern which was also seen in an Australian study.[22] While HF hospitalization rates appear to have declined since 2000, 30-day readmission rates after a HF hospitalization were stable in Medicare beneficiaries from 2004–2006.[73] However, recent data from CMS suggests that there has been a reduction in the 30-day all-cause hospital readmission rate in 2012 to 18.4% compared with 19% from 2007–11.[74] These trends were not specific to patients discharged following a HF hospitalization, and further analyses are needed to determine whether efforts aimed at reducing readmissions in patients with HF have been successful nationally.
Use of Long-term Care Facilities
As the population of patients with HF has become older, use of long-term care facilities has risen. From 2000–2004, 13.4% of those hospitalized for HF were discharged to skilled nursing facilities (SNF), compared with only 6.8% from 1980–84.[75] Among Medicare beneficiaries in the Get With the Guidelines program, 24.1% were discharged to a SNF after a HF hospitalization, though this varied widely by region, with highest use in the Northeastern U.S.[76] Patients discharged to a SNF after HF hospitalizations are at particularly high risk for adverse outcomes, with over half dying within one year.[76] While they may also be at slightly higher risk for readmission than those who are discharged home[76], variability in national rates of SNF use explain very little of the variation in 30-day readmission rates.[77]
Impact of Advanced Heart Failure Therapies
The introduction and advancement in mechanical circulatory support technology has revolutionized our ability to care for selected patients with advanced HF. Orthotopic heart transplantation has been an option for several decades, but the availability of suitable organ donors has limited the number of heart transplants to approximately 2200 per year in North America, which has been stated to be “epidemiologically trivial”[78] when considering the burden of HF in the population. However, left ventricular assist devices (LVAD) can now be used as both a bridge to transplantation (until a suitable organ becomes available) or as destination therapy (LVAD remains in situ until death). LVADs implanted as destination therapy improve survival and quality of life for patients with advanced HF who otherwise would be ineligible for heart transplantation due to advanced age and comorbidity.[79] Unfortunately, their use is currently limited to the subset of patients that have very reduced EF (<25%), advanced symptoms despite optimal medical therapy, no other life-limiting illnesses, and are interested in device support. While the exact number of potentially eligible patients is unknown, estimates have ranged from 25,000 to 250,000, which represent only a small fraction of the more than 5 million Americans living with HF. Therefore, their use likely has minimal impact on secular population trends in outcomes of HF at this time. However, as technology continues to advance and evolve, smaller, less invasive devices may become options for a broader range of individuals.
Trends in End-of-Life Care
As previously noted, HF is a syndrome with a high associated mortality, and roughly 5% of patients have end-stage disease that is refractory to medical therapy. The majority of these patients are ineligible for advanced HF therapies such as LVAD or heart transplant due to age, comorbidities, EF or personal preference. HF can have a profound impact on an individual’s quality of life, and the symptom burden for patients with HF is as high as those with advanced cancer.[80] Palliative care focuses on relieving and preventing suffering for these patients, and palliative medicine specialists can be instrumental in helping patients to define goals of care and providing emotional support for the patient, family and caregivers. While palliative care may be appropriate for patients with HF at any stage of the disease, hospice is a specific medical benefit provided by Medicare and many other insurers to provide comfort-focused care in patients who only have months to live. While use of hospice services in patients with HF at the end-of-life has increased since 2000[81–83], rates of hospice enrollment in patients with HF are still less than half those of patients with cancer.[81] Recent data from Medicare[82] and Olmsted County[84] suggest that approximately 40% of patients with HF enroll in hospice prior to death. While there are no national trends on use of palliative medicine services in patients with HF, the proportion of hospitals with palliative care programs grew to 62% in 2009, which is an increase of 134% compared with 2000.[85] The importance of palliative care in the treatment of patients with advanced HF has been recognized by several agencies including the such as the AHA[86] and ESC[87], and will likely continue to grow and evolve over time. As the clinical trajectory of HF can be unpredictable in individuals, there is a recognized need to proactively and iteratively discuss end-of-life wishes with patients with HF and to help them develop a plan for end-of-life care that aligns with their values, goals, and preferences.[86]
Conclusions
The HF population is changing. The incidence of HF varies across studies, but overall suggests that the incidence has decreased since the mid-1990s. The etiology and risk factors for HF are evolving, with improvements in the population burden of some factors such as hypertension, and an upsurge in obesity and diabetes, all of which will impact future trends in HF incidence. Improvement in survival after diagnosis has led to an increase in the prevalence of HF, and the age and comorbidity burden have resulted in an increasing proportion of patients with HFpEF. Hospitalizations in patients with HF remain frequent and costly, but may be declining. Delivering high-quality, patient-centered care for the growing population of patients with HF who are often elderly with multimorbidity continues to represent a formidable challenge.
Acknowledgments
Dr. Shannon M. Dunlay is supported by an NIH Career Development Award (K23 HL116643).
Footnotes
The authors have no disclosures or potential conflicts of interest
Conflict of Interest
Shannon M. Dunlay and Véronique L. Roger declare that they have no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
• Of Importance
•• Of Major Importance
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2•.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. This paper estimates the future costs and epidemiology of heart failure in the United States, which is useful for understanding the epidemic of heart failure and anticipating future population needs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–52. doi: 10.1161/CIR.0b013e31829e8807. These are the most recent U.S. guidelines for the care of patients with heart failure and include several important changes in management compared with earlier versions. [DOI] [PubMed] [Google Scholar]
- 4.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. New Engl J Med. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, et al. Cardiac and pulmonary causes of dyspnoea--validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8:1007–14. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 6.Carlson KJ, Lee DC, Goroll AH, Leahy M, Johnson RA. An analysis of physicians’ reasons for prescribing long-term digitalis therapy in outpatients. J Chron Dis. 1985;38:733–9. doi: 10.1016/0021-9681(85)90115-8. [DOI] [PubMed] [Google Scholar]
- 7.Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–40. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 8.Di Bari M, Pozzi C, Cavallini MC, Innocenti F, Baldereschi G, De Alfieri W, et al. The diagnosis of heart failure in the community. Comparative validation of four sets of criteria in unselected older adults: the ICARe Dicomano Study. J Am Coll Cardiol. 2004;44:1601–8. doi: 10.1016/j.jacc.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Psaty BM, Boineau R, Kuller LH, Luepker RV. The potential costs of upcoding for heart failure in the United States. Am J Cardiol. 1999;84:108–9. A9. doi: 10.1016/s0002-9149(99)00205-2. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg RJ, Spencer FA, Farmer C, Meyer TE, Pezzella S. Incidence and hospital death rates associated with heart failure: a community-wide perspective. Am J Med. 2005;118:728–34. doi: 10.1016/j.amjmed.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25:1614–9. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 12.Croft JB, Giles WH, Pollard RA, Casper ML, Anda RF, Livengood JR. National trends in the initial hospitalization for heart failure. J Am Geriatr Soc. 1997;45:270–5. doi: 10.1111/j.1532-5415.1997.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 13.Curtis LH, Whellan DJ, Hammill BG, Hernandez AF, Anstrom KJ, Shea AM, et al. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med. 2008;168:418–24. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 14.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, et al. Racial differences in incident heart failure among young adults. New Engl J Med. 2009;360:1179–90. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39:60–9. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 16.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–45. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–22. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 18.Barasa A, Schaufelberger M, Lappas G, Swedberg K, Dellborg M, Rosengren A. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur Heart J. 2014;35:25–32. doi: 10.1093/eurheartj/eht278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, et al. Long-term trends in the incidence of and survival with heart failure. New Engl J Med. 2002;347:1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 20.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 21.Stewart S, MacIntyre K, MacLeod MM, Bailey AE, Capewell S, McMurray JJ. Trends in hospitalization for heart failure in Scotland, 1990–1996. An epidemic that has reached its peak? Eur Heart J. 2001;22:209–17. doi: 10.1053/euhj.2000.2291. [DOI] [PubMed] [Google Scholar]
- 22.Teng TH, Finn J, Hobbs M, Hung J. Heart failure: incidence, case fatality, and hospitalization rates in Western Australia between 1990 and 2005. Circ Heart Fail. 2010;3:236–43. doi: 10.1161/CIRCHEARTFAILURE.109.879239. [DOI] [PubMed] [Google Scholar]
- 23.Yeung DF, Boom NK, Guo H, Lee DS, Schultz SE, Tu JV. Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007. Canadian Med Asso J. 2012;184:E765–73. doi: 10.1503/cmaj.111958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 25•.Huffman MD, Berry JD, Ning H, Dyer AR, Garside DB, Cai X, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol. 2013;61:1510–7. doi: 10.1016/j.jacc.2013.01.022. This is the first paper to estimate lifetime risk of heart failure in both black and white men and women and highlights important racial differences in risk in men. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 27.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–45. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 28.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. New Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 29.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. New Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 31.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–77. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 32.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–50. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 33.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 34.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 35.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–13. doi: 10.1161/CIRCULATIONAHA.110.954388. discussion 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West R, Liang L, Fonarow GC, Kociol R, Mills RM, O’Connor CM, et al. Characterization of heart failure patients with preserved ejection fraction: a comparison between ADHERE-US registry and ADHERE-International registry. Eur J Heart Fail. 2011;13:945–52. doi: 10.1093/eurjhf/hfr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5:720–6. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Avery CL, Loehr LR, Baggett C, Chang PP, Kucharska-Newton AM, Matsushita K, et al. The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2012;60:1640–6. doi: 10.1016/j.jacc.2012.07.022. This analysis including nearly 15,000 patients participating it the ARIC study demonstrates that even a modest reduction in the prevalence of risk factors for heart failure could translate into a large decrease in the annual incidence of heart failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunlay SM, Weston SA, Jacobsen SJ, Roger VL. Risk factors for heart failure: a population-based case-control study. Am J Med. 2009;122:1023–8. doi: 10.1016/j.amjmed.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–7. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–37. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 43.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–62. [PubMed] [Google Scholar]
- 44.Loehr LR, Rosamond WD, Poole C, McNeill AM, Chang PP, Deswal A, et al. The potentially modifiable burden of incident heart failure due to obesity: the atherosclerosis risk in communities study. Am J Epidemiol. 2010;172:781–9. doi: 10.1093/aje/kwq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 46.Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970–1974 and 1990–1994. Circulation. 2006;113:799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- 47.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 48.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–74. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 49.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–7. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med. 2011;124:136–43. doi: 10.1016/j.amjmed.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saczynski JS, Go AS, Magid DJ, Smith DH, McManus DD, Allen L, et al. Patterns of comorbidity in older adults with heart failure: the Cardiovascular Research Network PRESERVE study. J Am Geriatr Soc. 2013;61:26–33. doi: 10.1111/jgs.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10:142–51. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306:1669–78. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutcliffe S, Phillips C, Watson D, Davidson C. Trends in heart failure mortality in England and Wales since 1950. Eur J Intern Med. 2007;18:576–80. doi: 10.1016/j.ejim.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 56.Curtis LH, Greiner MA, Hammill BG, Kramer JM, Whellan DJ, Schulman KA, et al. Early and long-term outcomes of heart failure in elderly persons, 2001–2005. Arch Intern Med. 2008;168:2481–8. doi: 10.1001/archinte.168.22.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Chen J, Dharmarajan K, Wang Y, Krumholz HM. National trends in heart failure hospital stay rates, 2001 to 2009. J Am Coll Cardiol. 2013;61:1078–88. doi: 10.1016/j.jacc.2012.11.057. Highlights the fact that while in-hospital mortality and length of stay have improved for some groups of patients hospitalized with heart failure, gains have not been seen in younger patients and blacks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bueno H, Ross JS, Wang Y, Chen J, Vidan MT, Normand SL, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303:2141–7. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Connor CM, Miller AB, Blair JE, Konstam MA, Wedge P, Bahit MC, et al. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: results from Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) program. Am Heart J. 2010;159:841–849. e1. doi: 10.1016/j.ahj.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 60.Remme WJ, Cleland JG, Erhardt L, Spark P, Torp-Pedersen C, Metra M, et al. Effect of carvedilol and metoprolol on the mode of death in patients with heart failure. Eur J Heart Fail. 2007;9:1128–35. doi: 10.1016/j.ejheart.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 61.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. New Engl J Med. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 62.Chun S, Tu JV, Wijeysundera HC, Austin PC, Wang X, Levy D, et al. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail. 2012;5:414–21. doi: 10.1161/CIRCHEARTFAILURE.111.964791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61:1259–67. doi: 10.1016/j.jacc.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunlay SM, Shah ND, Shi Q, Morlan B, VanHouten H, Long KH, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4:68–75. doi: 10.1161/CIRCOUTCOMES.110.957225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hospital Readmissions Reduction Program. Affordable Care Act., 2012.
- 66.Setoguchi S, Stevenson LW. Hospitalizations in patients with heart failure: who and why. J Am Coll Cardiol. 2009;54:1703–5. doi: 10.1016/j.jacc.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ezekowitz JA, Kaul P, Bakal JA, Quan H, McAlister FA. Trends in heart failure care: has the incident diagnosis of heart failure shifted from the hospital to the emergency department and outpatient clinics? Eur J Heart Fail. 2011;13:142–7. doi: 10.1093/eurjhf/hfq185. [DOI] [PubMed] [Google Scholar]
- 69.Schaufelberger M, Swedberg K, Koster M, Rosen M, Rosengren A. Decreasing one-year mortality and hospitalization rates for heart failure in Sweden; Data from the Swedish Hospital Discharge Registry 1988 to 2000. Eur Heart J. 2004;25:300–7. doi: 10.1016/j.ehj.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 70.Mosterd A, Reitsma JB, Grobbee DE. Angiotensin converting enzyme inhibition and hospitalisation rates for heart failure in the Netherlands, 1980 to 1999: the end of an epidemic? Heart. 2002;87:75–6. doi: 10.1136/heart.87.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wasywich CA, Gamble GD, Whalley GA, Doughty RN. Understanding changing patterns of survival and hospitalization for heart failure over two decades in New Zealand: utility of ‘days alive and out of hospital’ from epidemiological data. Eur J Heart Fail. 2010;12:462–8. doi: 10.1093/eurjhf/hfq027. [DOI] [PubMed] [Google Scholar]
- 72.Jhund PS, Macintyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5. 1 million people. Circulation. 2009;119:515–23. doi: 10.1161/CIRCULATIONAHA.108.812172. [DOI] [PubMed] [Google Scholar]
- 73.Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, Keenan PS, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gerhardt G, Yemane A, Hickman P, Olschlaegger A, Rollins E, Brennan N. Data Show Reduction in Medicare Hospital Readmission Rates in 2012. Medicare and Medicaid Research Review. 2013;3 doi: 10.5600/mmrr.003.02.b01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S. 1979 to 2004. J Am Coll Cardiol. 2008;52:428–34. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 76.Allen LA, Hernandez AF, Peterson ED, Curtis LH, Dai D, Masoudi FA, et al. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail. 2011;4:293–300. doi: 10.1161/CIRCHEARTFAILURE.110.959171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen J, Ross JS, Carlson MD, Lin Z, Normand SL, Bernheim SM, et al. Skilled nursing facility referral and hospital readmission rates after heart failure or myocardial infarction. Am J Med. 2012;125:100, e1–9. doi: 10.1016/j.amjmed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stewart GC, Stevenson LW. Keeping left ventricular assist device acceleration on track. Circulation. 2011;123:1559–68. doi: 10.1161/CIRCULATIONAHA.110.982512. discussion 1568. [DOI] [PubMed] [Google Scholar]
- 79.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. New Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 80.Bekelman DB, Rumsfeld JS, Havranek EP, Yamashita TE, Hutt E, Gottlieb SH, et al. Symptom burden, depression, and spiritual well-being: a comparison of heart failure and advanced cancer patients. J Gen Intern Med. 2009;24:592–8. doi: 10.1007/s11606-009-0931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Setoguchi S, Glynn RJ, Stedman M, Flavell CM, Levin R, Stevenson LW. Hospice, opiates, and acute care service use among the elderly before death from heart failure or cancer. Am Heart J. 2010;160:139–44. doi: 10.1016/j.ahj.2010.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Unroe KT, Greiner MA, Hernandez AF, Whellan DJ, Kaul P, Schulman KA, et al. Resource use in the last 6 months of life among medicare beneficiaries with heart failure, 2000–2007. Arch Intern Med. 2011;171:196–203. doi: 10.1001/archinternmed.2010.371. [DOI] [PubMed] [Google Scholar]
- 83.Unroe KT, Greiner MA, Johnson KS, Curtis LH, Setoguchi S. Racial differences in hospice use and patterns of care after enrollment in hospice among Medicare beneficiaries with heart failure. Am Heart J. 2012;163:987–993. e3. doi: 10.1016/j.ahj.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 84.Dunlay SM, Swetz KM, Redfield MM, Mueller PS, Roger VL. Resuscitation preferences in community patients with heart failure. Circ Cardiovasc Qual Outcomes. 2014;7:353–9. doi: 10.1161/CIRCOUTCOMES.113.000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meier DE. Increased access to palliative care and hospice services: opportunities to improve value in health care. The Milbank quarterly. 2011;89:343–80. doi: 10.1111/j.1468-0009.2011.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86•.Allen LA, Stevenson LW, Grady KL, Goldstein NE, Matlock DD, Arnold RM, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125:1928–52. doi: 10.1161/CIR.0b013e31824f2173. This scientific statement from the American Heart Association discusses the importance of shared decision making in patients with advanced heart failure and provides detailed guidance for clinicians on how and when to discuss goals of care and end-of-life preferences with patients with advanced heart failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jaarsma T, Beattie JM, Ryder M, Rutten FH, McDonagh T, Mohacsi P, et al. Palliative care in heart failure: a position statement from the palliative care workshop of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009;11:433–43. doi: 10.1093/eurjhf/hfp041. [DOI] [PubMed] [Google Scholar]
- 88.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–6. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 89.Cowie MR, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, Suresh V, et al. Incidence and aetiology of heart failure; a population-based study. Eur Heart J. 1999;20:421–8. doi: 10.1053/euhj.1998.1280. [DOI] [PubMed] [Google Scholar]
- 90.Cowie MR, Wood DA, Coats AJ, Thompson SG, Suresh V, Poole-Wilson PA, et al. Survival of patients with a new diagnosis of heart failure: a population based study. Heart. 2000;83:505–10. doi: 10.1136/heart.83.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, et al. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet. 2001;358:439–44. doi: 10.1016/s0140-6736(01)05620-3. [DOI] [PubMed] [Google Scholar]
- 92.Nielsen OW, Hilden J, Larsen CT, Hansen JF. Cross sectional study estimating prevalence of heart failure and left ventricular systolic dysfunction in community patients at risk. Heart. 2001;86:172–8. doi: 10.1136/heart.86.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Remes J, Reunanen A, Aromaa A, Pyorala K. Incidence of heart failure in eastern Finland: a population-based surveillance study. Eur Heart J. 1992;13:588–93. doi: 10.1093/oxfordjournals.eurheartj.a060220. [DOI] [PubMed] [Google Scholar]
- 94.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–7. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–86. doi: 10.1161/CIRCHEARTFAILURE.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Devereux RB, Roman MJ, Liu JE, Welty TK, Lee ET, Rodeheffer R, et al. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. Am J Cardiol. 2000;86:1090–6. doi: 10.1016/s0002-9149(00)01165-6. [DOI] [PubMed] [Google Scholar]
- 97.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–9. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 98.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 99.Philbin EF, Rocco TA, Jr, Lindenmuth NW, Ulrich K, Jenkins PL. Systolic versus diastolic heart failure in community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med. 2000;109:605–13. doi: 10.1016/s0002-9343(00)00601-x. [DOI] [PubMed] [Google Scholar]
- 100.Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–31. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 101.Gurwitz JH, Magid DJ, Smith DH, Goldberg RJ, McManus DD, Allen LA, et al. Contemporary prevalence and correlates of incident heart failure with preserved ejection fraction. Am J Med. 2013;126:393–400. doi: 10.1016/j.amjmed.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gustafsson F, Torp-Pedersen C, Brendorp B, Seibaek M, Burchardt H, Kober L. Long-term survival in patients hospitalized with congestive heart failure: relation to preserved and reduced left ventricular systolic function. Eur Heart J. 2003;24:863–70. doi: 10.1016/s0195-668x(02)00845-x. [DOI] [PubMed] [Google Scholar]
- 103.MacCarthy PA, Kearney MT, Nolan J, Lee AJ, Prescott RJ, Shah AM, et al. Prognosis in heart failure with preserved left ventricular systolic function: prospective cohort study. BMJ. 2003;327:78–9. doi: 10.1136/bmj.327.7406.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lenzen MJ, Scholte op Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, et al. Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. Eur Heart J. 2004;25:1214–20. doi: 10.1016/j.ehj.2004.06.006. [DOI] [PubMed] [Google Scholar]