Abstract
Rationale
Neuropsychological testing is widespread in adult cocaine abusers, but lacking in teens. Animal models may provide insight into age-related neuropsychological consequences of cocaine exposure.
Objectives
Determine whether developmental plasticity protects or hinders behavioral flexibility after cocaine exposure in adolescent vs. adult rats.
Methods
Using a yoked-triad design, one rat controlled cocaine delivery and the other two passively received cocaine or saline. Rats controlling cocaine delivery (1.0 mg/kg) self-administered for 18 sessions (starting P37 or P77), followed by 18 drug-free days. Rats next were tested in a strategy set shifting task, lasting 11–13 sessions.
Results
Cocaine self-administration did not differ between age groups. During initial set formation, adolescent-onset groups required more trials to reach criterion and made more errors than adult-onset groups. During the set shift phase, rats with adult-onset cocaine self-administration experience had higher proportions of correct trials and fewer perseverative + regressive errors than age-matched yoked-controls or rats with adolescent-onset cocaine self-administration experience. During reversal learning, rats with adult-onset cocaine experience (self-administered or passive) required fewer trials to reach criterion and the self-administering rats made fewer perseverative + regressive errors than yoked-saline rats. Rats receiving adolescent-onset yoked-cocaine had more trial omissions and longer lever press reaction times than age-matched rats self-administering cocaine or receiving yoked-saline.
Conclusions
Prior cocaine self-administration may impair memory to reduce proactive interference during set shifting and reversal learning in adult-onset but not adolescent-onset rats (developmental plasticity protective). Passive cocaine may disrupt aspects of executive function in adolescent-onset but not adult-onset rats (developmental plasticity hinders).
Keywords: Adolescent, Adult, Cocaine, Self-administration, Strategy set shifting task
Introduction
It is well established that functioning of the prefrontal cortex (PFC) is disrupted in adult cocaine abusers (Bolla et al. 2004; Volkow et al. 1992). Compared to healthy controls, abstinent cocaine abusers have reduced neural activity in orbitofrontal cortex (OFC), anterior cingulate (ACC) and dorsolateral prefrontal cortex (DLPFC). Gray matter densities in OFC and ACC also are lower in abstinent cocaine abusers (Franklin et al. 2002; Matochik et al. 2003; Moreno-Lopez et al. 2012; Tanabe et al. 2009). Consistent with these structural differences, abstinent cocaine abusers exhibit deficits in decision-making, a process associated with neural activity within OFC, DLPFC and medial PFC (Bechara et al. 2001; Bolla et al. 2003). Cocaine abusers additionally show greater working memory deficits during abstinence than healthy controls, an effect that is associated with reduced activation of the ACC (Hester and Garavan 2004; van der Plas et al. 2009). Concerning attentional networks, cocaine abusers have problems with sustained attention during abstinence, with effect sizes between 0.40 and 1.10 across multiple studies (Jovanovski et al. 2005). During the Wisconsin Card Sorting Test, which measures behavioral flexibility, abstinent cocaine abusers are likely to make more perseverative errors and complete fewer categories than healthy controls, which are outcomes indicative of executive and attentional impairment (Madoz-Gúrpide et al. 2011; Woicik et al. 2011).
Whereas a considerable amount of neuropsychological testing has been conducted in adult cocaine abusers to assess executive function, comparable testing in adolescent subjects is lacking. According to the 2013 Monitoring the Future Study, past month use of cocaine or crack has declined slightly or remained stable over the past four years amongst 8th – 12th graders. Importantly, prevalence of cocaine use among college students (period of transition from adolescence to young adulthood) is on an upward trajectory (e.g., Kasperski et al. 2011; Williams et al. 2006), suggesting there is a cohort of teens who initiate and continue to use cocaine throughout adolescence despite potential negative consequences to neuropsychological function. As these consequences are largely understudied, preclinical animal models that directly compare adult-onset and adolescent-onset cocaine exposure may provide insight into age-related neuropsychological consequences.
Previous research in adolescent and adult rats, either trained to self-administer 1 mg/kg cocaine or passively receiving cocaine or saline in a yoked manner (triad design), has shown differential effects of cocaine exposure on neurocognitive function measured during adulthood after an 18-day drug-free period. Using a task that measures amygdala-related stimulus-reward learning (Kantak et al. 2001), deficits were observed after adult-onset cocaine exposure (self-administered or passively received), but not after adolescent-onset cocaine exposure (Kerstetter and Kantak 2007). Employing a task that measures OFC-related non-spatial working memory (Di Pietro et al. 2004), a similarly designed study in adult and adolescent triads showed that rats from both drug-onset ages had deficits (Harvey et al. 2009). Notably, working memory deficits were exclusive to rats of both ages self-administering cocaine and were more pronounced in rats with adolescent-onset than adult-onset cocaine exposure.
The current study used the same triad design as described above to examine performance of rats in an automated strategy set-shifting task similar in concept to the Wisconsin Card Sorting Test (Floresco et al. 2008). Behavioral flexibility is required for reaching criterion levels of learning during set shift and reversal-learning phases that follow an initial discrimination-learning phase (Birrell and Brown 2000; Boulougouris et al. 2007; Floresco et al. 2008; Ghods-Sharifi et al. 2008; Ragozzino et al. 1999; Stefani et al. 2003). Because rats must shift attention from one stimulus dimension to another across phases to earn reward, proactive interference in the form of perseverative and regressive errors can emerge to slow learning if memory of the preceding discrimination strategy is retrieved while engaging in the new discrimination (Floresco et al. 2008). Faster acquisition of the new discrimination during the set shift or reversal-learning phase could reflect either facilitated learning (e.g., Cain et al. 2011) or a failure to store or retrieve memory of the preceding discrimination strategy (e.g., Chess et al. 2011). Assessment of multiple measures of performance can help disambiguate these alternative interpretations. Furthermore, the triad design for cocaine delivery helps disambiguate the contribution of contingent vs. noncontingent presentations of environmental events to changes in cognitive function.
Materials and methods
Subjects
Adult (n=24; arrival on postnatal day 65, [P65]) and adolescent (n=30; arrival on P25) non-littermate male rats of the Wistar/Cr strain (Charles River Laboratories, Wilmington, MA, USA) were housed individually in plastic cages (24 × 22 ×20 cm) within a temperature- (21–23°C) and light-(08:00 hours on; 20:00 hours off) controlled vivarium. During self-administration sessions, rats were allowed free access to food and water except for 2 days prior to and 2 days following initiation of self-administration sessions, when food was restricted to approximately 16 g per day. Food also was restricted during the 18-day drug-free period and the 13–15 sessions of the strategy set shifting task. During this second period of food restriction, approximately 16 g of food per day were provided, which maintained rats at no less than 85–90% of a growth-adjusted free-feeding body weight. Rats had unlimited access to water in their home cages between experimental sessions. All experimental procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals as well as specific national laws. The Boston University Institutional Animal Care and Use Committee approved this study.
Apparatus
Chambers (Model ENV-008CT, Med Associates, St. Albans, VT, USA) used to conduct self-administration and strategy set shifting sessions were outfitted with two retractable levers, a white stimulus light mounted above each lever, a food receptacle, a pellet dispenser, a house light, and an infusion pump with a delivery leash/liquid swivel/counterbalanced arm assembly. A sound-attenuating cubicle, with an exhaust fan for ventilation, enclosed each chamber. A computer was programmed in Medstate Notation and connected to an interface (Med Associates) to control experimental events.
Drugs
Cocaine hydrochloride (gift from NIDA, Bethesda, MD, USA) was dissolved in 0.9% sterile saline containing 3 IU heparin per mL, to a final cocaine concentration of 2.68 mg/mL. Throughout self-administration sessions, a unit dose of 1.0 mg/kg was delivered intravenously by infusing the 2.68 mg/mL solution at a rate of 1.8 mL/min. In order to attain a dose of 1.0 mg/kg, the infusion duration was adjusted for each animal’s daily body weight (1.2 s/100 g). Yoked control rats were passively exposed to either the saline/heparin solution (yoked-saline) or to the cocaine solution (yoked-cocaine), also delivered at a rate of 1.2 s/100 g body weight.
Lever Shaping and Surgery
Four days following arrival, food was removed from the home cages in the morning, and that same evening animals were autoshaped overnight in the experimental chambers to respond on either lever under a fixed-ratio 1 (FR1) schedule of food reinforcement (45 mg chocolate-flavored precision pellets; Bio-Serv, Frenchtown, NJ, USA). Water was available in the experimental chambers for this phase of the study. Following autoshaping, food was available ad libitum in the home cages, and catheter surgery took place 2 to 3 days later. Jugular vein catheters were implanted in both adult and adolescent rats using back mounts as the site of catheter attachment. Post-surgical care and catheter maintenance procedures were as previously described (Harvey et al. 2009). All rats were given 5 to 6 days to recover from surgery prior to initiating self-administration sessions.
Self-Administration Procedures
Self-administration was conducted during the light phase for 18 sessions, with five sessions per week (Monday through Friday). The first self-administration session was conducted on P37 in adolescent rats and on P77 in adult rats. Each session was 2 h in duration, with levers inserted into the chamber at the start of sessions and retracted at the end of sessions. Prior to initiating self-administration sessions, adult and adolescent rats each were divided into triads (n=8 adult triads and n=10 adolescent triads). In each triad, one rat controlled cocaine delivery and the other two rats passively received either cocaine or saline. Rats self-administering cocaine (1.0 mg/kg) were initially trained under an FR1 reinforcement schedule with a 20-s timeout (TO) period after each infusion. To create a drug-associated light cue that was distinct from the light cue used in the strategy set shifting task (see below), the house light flashed (2 sec on/2 sec off) during the infusion and the 20-s TO period for each member of the triad. Use of a visual cue with the same parameters for both procedures is a potentially confounding variable because after acquiring salience in the self-administration procedure, the same visual cue could influence the rate of new learning during the strategy set shifting task. The active lever was counterbalanced to the left or right across subjects self-administering cocaine. There were no scheduled consequences for responses made on the inactive lever. Response requirements were gradually increased to a terminal FR5 20-s TO reinforcement schedule during which rats self-administering cocaine pressed the active lever five times to receive a cocaine infusion. Adult rats reached the FR5 response requirement after an average of 6.5±0.2 sessions and adolescent rats after an average of 7.8±0.5 sessions. Rats remained at this terminal schedule for the remainder of the 18 sessions. Responses made on either the active (light-paired) or inactive (non light-paired) lever by the yoked rats passively receiving cocaine or saline were recorded but had no scheduled consequences. Adolescent rats completed the 18th self-administration session on P60 and adult rats on P100. Following these sessions, rats underwent an 18-day drug-free period in their home cages.
Strategy Set Shifting Task Procedures
Following the 18-day drug-free period (P61–P78 in adolescent-onset rats or P101–P118 in adult-onset rats), animals were tested in the operant version of the strategy set-shifting task (Floresco et al. 2008; Harvey et al. 2013). The task was divided into four phases: habituation, initial set formation, set shift and reversal learning. The habituation phase was conducted Monday-Friday over eight sessions (completed by P87 or P127), and was used to train rats to lever press for food reward within 10 sec of lever insertion into the chamber and to establish a lever position bias. Stimulus lights above the levers were not illuminated during the habituation phase. During the three experimental phases of the strategy set shifting task, sessions were conducted Monday–Friday, and an individual session lasted no more than 300 trials. If a rat did not reach criterion within 300 trials, sessions were continued on subsequent days until criterion was reached. For the initial set, each trial was initiated with a 20 sec TO period in a darkened chamber. A randomly selected stimulus light was then illuminated, starting 3 sec before the house light was turned on and the levers inserted into the chamber. Within 10 sec of lever insertion, rats were required to press the lever that had the stimulus light illuminated above it, regardless of the lever position bias, to earn a food pellet (visual cue discrimination). Levers were retracted after a lever was pressed (correct or incorrect) or if 10 sec elapsed without a lever press being made (trial omission). Following a correct lever press and pellet delivery (45 mg chocolate-flavored precision pellets; Bio-Serv, Frenchtown, NJ, USA), the stimulus light and house light remained illuminated for 4 sec. After an incorrect lever press or an omitted trial, the stimulus light and house light were extinguished immediately. Daily sessions continued until the criterion was reached (eight consecutive correct lever choices, Floresco et al. 2008). The initial set formation phase was completed after 1–3 sessions (adolescent-onset groups) or after 1–2 sessions (adult-onset groups); most rats completed the initial set formation phase in 1 session. The next day, rats underwent a shift to a discrimination that required the rat to press the lever that was opposite its lever position bias, regardless of which stimulus light was illuminated to earn a food pellet (egocentric spatial response discrimination). The daily sessions continued until the criterion was reached (ten consecutive correct choices, Floresco et al. 2008). All rats completed the set shift phase in 1 session. The following day, reversal learning was examined that required the rat to press the lever that was the same as its lever position bias, regardless of which stimulus light was illuminated to earn a food pellet (reversal of egocentric spatial response discrimination). The daily sessions continued until the criterion was reached (ten consecutive correct choices, Floresco et al. 2008). The reversal phase was completed in 1 session except in one rat (adult-onset) that completed this phase in 2 sessions. The same trial contingencies as outlined for the initial set formation phase were in effect for the set shift and reversal-learning phases.
Data Analyses
The self-administration data were analyzed to determine if behavior during sessions was similar or different for adolescent and adult rats. Prior to analysis, responses on active (light-paired) and inactive (non light-paired) levers were averaged in individual subjects across sessions conducted under the FR5 contingency (approximately 10–11 sessions). Response data were analyzed by a three-factor (drug-onset age × treatment condition × lever) ANOVA, with repeated measures for the lever factor. Infusion data were analyzed over all 18 sessions by a two-factor (drug-onset age × session number) ANOVA, with repeated measures on the session number factor. The measures evaluated for each phase of the strategy set shifting task were 1) trials competed to reach criterion, 2) number of omitted trials, 3) average lever press reaction time, 4) proportion of correct trials to total trials completed, and 5) number of total errors. In addition, proportions of error subtypes to total errors made (see below) were compared during the set shift and reversal-learning phases. The first five dependent measures were analyzed by a two-factor (drug-onset age × treatment condition) ANOVA and the error subtype measure was analyzed by a three-factor (drug-onset age × treatment condition × error subtype) ANOVA, with repeated measures for the error subtype factor. Tukey tests were used for all post-hoc testing, where appropriate. Prior to ANOVA analysis, the proportions of correct trials and proportions of each error subtype were arcsine transformed (arcsine [square root x+1]), as data ranged between 0 and 1.
Subtypes of errors made during the set shift phase were examined in blocks of eight trials and consisted of perseverative, regressive and never-reinforced errors (Floresco et al. 2008; 2009). Perseverative and regressive errors were recorded when a rat pressed a lever with the stimulus light illuminated above it on trials that required the rat to press the opposite lever. Errors were perseverative when a rat pressed the incorrect lever on six or more trials per block of eight trials. Once a rat made five or fewer incorrect choices in a block of eight trials for the first time, the incorrect lever choices in subsequent blocks were then scored as regressive errors. Never-reinforced errors were recorded when a rat pressed the incorrect lever on trials for which the correct lever had the stimulus light illuminated above it (i.e. a choice that was not reinforced during either the initial set or set shift phase). Perseverative errors are an index of how well the previously acquired strategy is suppressed, regressive errors are an index of how well the new strategy is maintained, and never-reinforced errors are an index of how well the new strategy is acquired (Floresco et al. 2008). Errors during the reversal-learning phase were examined in blocks of sixteen trials and consisted of perseverative and regressive errors (Floresco et al. 2009). Once a rat made ten or fewer incorrect choices in a block of sixteen trials for the first time, the incorrect lever choices in subsequent blocks were then scored as regressive errors. As never reinforced errors are not possible to commit during the reversal-learning phase, total errors consisted of perseverative + regressive errors. An additional two-factor ANOVA examined drug-onset age and treatment condition differences in the number of perseverative + regressive errors to assess the contribution of proactive interference during the set shift phase.
During the course of training, two adolescent rats actively self-administering cocaine lost catheter function. The yoked-saline and yoked-cocaine rats paired to these two rats were subsequently assigned to other rats that controlled drug delivery. For adolescent-onset groups, this resulted in n=8 rats actively self-administering cocaine and n=10 rats passively receiving either cocaine or saline. For adult-onset groups, one rat from the passive cocaine group lost catheter function, resulting in n=8 rats either actively self-administering cocaine or passively receiving saline and n=7 rats passively receiving cocaine.
Results
Self-Administration Behavior
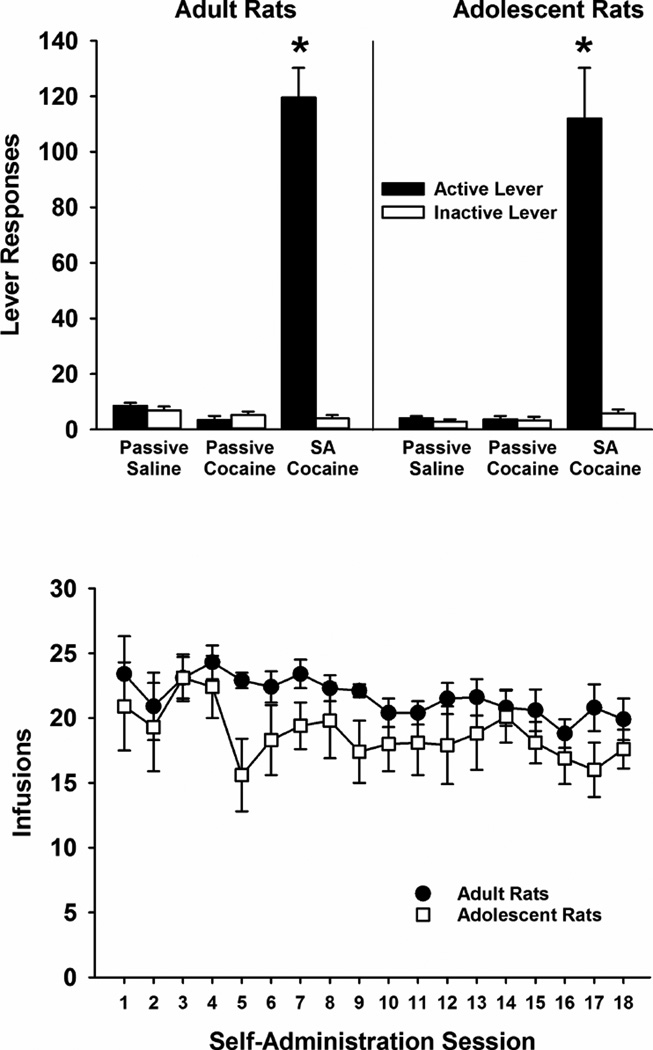
Cocaine self-administration behavior did not differ significantly between adult and adolescent rats. A three-factor ANOVA of lever responses revealed significant main effects of treatment condition (F [2,45]=114, p≤0.001) and lever (F [1,45]=138, p≤0.001), and a significant treatment condition × lever interaction (F [2,45]=129, p≤0.001). Post-hoc testing demonstrated that differences were due to a greater number of active than inactive lever responses being emitted by rats self-administering cocaine under the FR5 contingency (p≤0.001). The number of active and inactive lever responses was not significantly different in rats passively receiving either yoked-cocaine or yoked-saline. The drug-onset age × lever interaction, the drug-onset age × treatment condition interaction, and the drug-onset age × treatment condition × lever interaction were not significant, indicating that responding was similar across age groups at each lever and at each treatment condition (Fig. 1, top panel). Additionally, the number of infusions earned by rats self-administering cocaine (Fig. 1, bottom panel) did not differ significantly between age groups overall (F [1, 14]=2.3, p>0.05) or between age groups across each of the 18 self-administration sessions (F [17, 238]=0.6, p>0.05). Adult rats earned an overall average of 22±1 infusions per session and adolescent rats earned an overall average of 19±2 infusions per session. Infusions did, however, significantly differ overall across sessions (F [17, 238]=1.9, p≤0.02). Post-hoc testing indicated that there were more infusions during session 4 than session 16 (p≤0.05). There were no other betweensession differences in number of infusions.
Fig. 1.
Mean ± SEM number of active and inactive lever presses (upper panel) and infusions (lower panel) in adult and adolescent rats self-administering cocaine (SA cocaine) or receiving yoked-saline (passive saline) or yoked-cocaine (passive cocaine). * p ≤ 0.05 compared to all other conditions at each age.
Strategy Set Shifting Performance
Initial Set Formation Phase
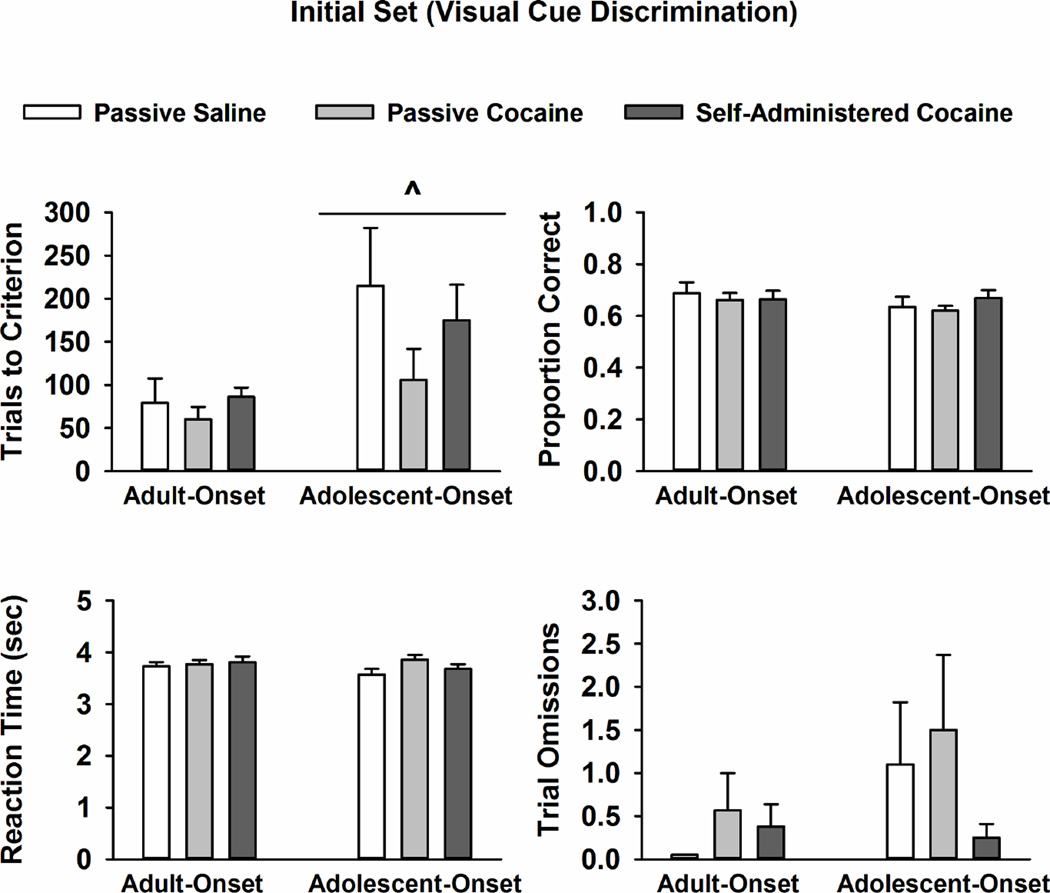
Data obtained during the initial set formation phase are displayed in Fig. 2. Analysis of trials completed to reach criterion showed a significant main effect of drug-onset age (F [1, 45] = 6.7, p ≤ 0.01), with adolescent-onset groups requiring more trials to reach criterion than adult-onset groups. Within each age group, trials to criterion did not differ by treatment condition. Drug-onset age and/or treatment condition did not significantly impact the proportion of correct trials, lever press reaction time, or number of trial omissions during the initial set phase. Analysis of total number of errors (Table 1) revealed a significant main effect of drug-onset age, with adolescent-onset groups making significantly more total errors than adult-onset groups during this initial phase (F [1, 45] = 6.1, p ≤ 0.02).
Fig. 2.
Mean ± SEM trials completed to reach criterion, proportion correct trials, lever press reaction time (sec), and trial omissions during the initial set formation phase in rats with adult-onset and adolescent-onset self-administration experience or passive saline or passvie cocaine exposure. ^ p ≤ 0.05 compared to adult-onset rats overall.
Table 1.
Total number of errors (mean ± S.E.M.) during each phase of the strategy set shifting task in rats with adult-onset and adolescent-onset cocaine or saline exposure.
| Cocaine-Onset Age | Treatment Condition | Initial Set | Set Shift | Reversal Learning |
|---|---|---|---|---|
| Adult | Yoked-Saline | 28.5 ± 11.9 | 23.9 ± 3.4 | 52.0 ± 8.8 |
| Yoked-Cocaine | 21.0 ± 5.7 | 22.7 ± 5.7 | 32.6 ± 4.3 c | |
| Self-Administered Cocaine | 31.4 ± 7.1 | 11.5 ± 2.9 b | 29.0 ± 4.1 c | |
| Adolescent | Yoked-Saline | 91.6 ± 31.6 a | 18.1 ± 3.9 | 40.0 ± 5.3 |
| Yoked-Cocaine | 43.5 ± 16.3 a | 17.6 ± 1.4 | 44.6 ± 5.3 | |
| Self-Administered Cocaine | 64.9 ± 18.9 a | 22.9 ± 5.3 | 31.5 ± 3.5 |
p ≤ 0.02 compared to adult-onset treatment conditions (main effect of age);
p ≤ 0.05 compared to age-matched yoked-saline and yoked-cocaine treatment conditions;
p ≤ 0.05 compared to the age-matched yoked-saline treatment condition.
Set Shift Phase
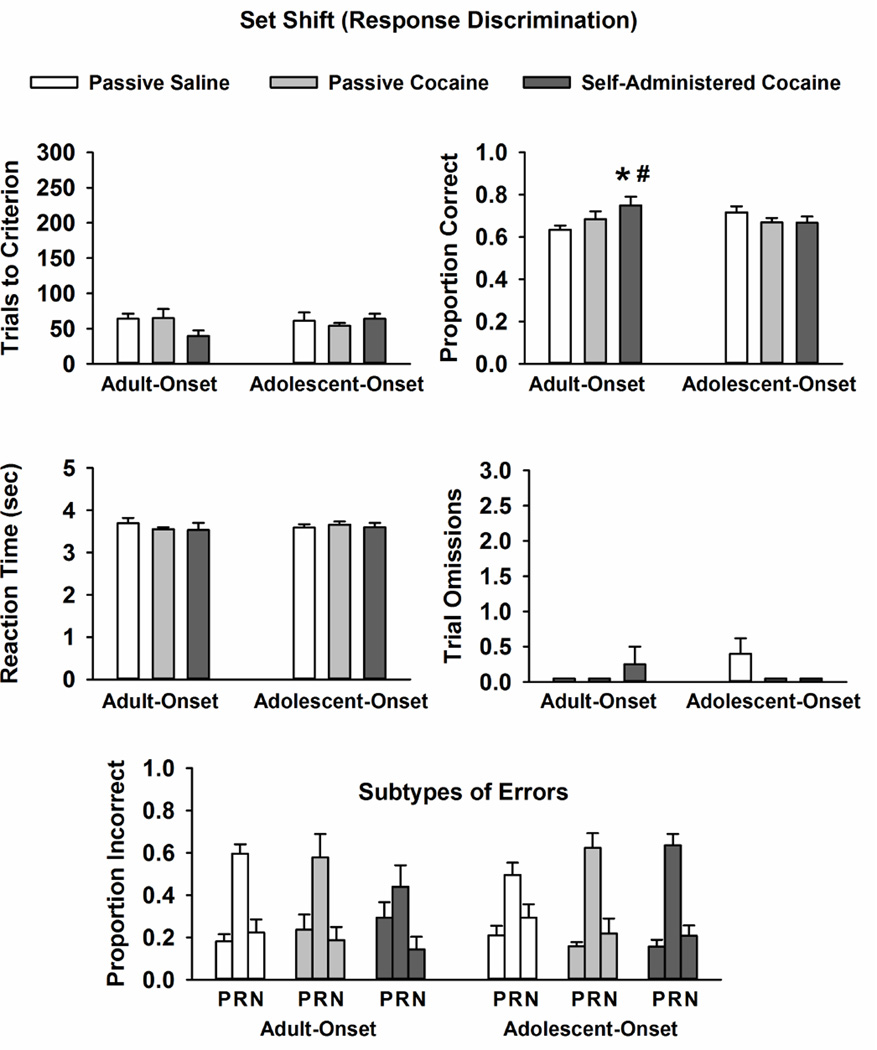
Data obtained during the set shift phase are displayed in Fig. 3. Analysis of trials to reach criterion, lever press reaction time, and trial omissions revealed no differences due to drug-onset age and/or treatment condition. For proportion of correct trials, there was a significant drug-onset age × treatment condition interaction (F [2, 45] = 3.5, p ≤ 0.04). Post-hoc testing demonstrated that within adult-onset groups, rats actively self-administering cocaine had a greater proportion of correct trials during the set shift phase than age-matched rats passively receiving yoked-saline (p ≤ 0.03). Within adolescent-onset groups, treatment condition did not impact the proportion of correct trials. Moreover, rats with adult-onset cocaine self-administration experience had a greater proportion of correct trials than rats with adolescent-onset cocaine self-administration experience (p ≤ 0.04). Error analysis during the set shift phase revealed a significant main effect of error subtype (F [2, 90] = 39, p ≤ 0.001) that did not differ by drug-onset age and/or treatment condition. Post-hoc testing demonstrated that there was a greater proportion of regressive than perseverative or never reinforced errors across all conditions (p ≤ 0.001). Analysis of total number of errors (Table 1) revealed a drug-onset age × treatment condition interaction (F [2, 45] = 3.1, p ≤ 0.05). Rats with adult-onset cocaine self-administration experience made significantly fewer total errors than age-matched rats passively receiving yoked-saline or yoked-cocaine during the set shift phase (p ≤ 0.05). Analysis of perseverative + regressive errors also revealed a drug-onset age × treatment condition interaction (F [2, 45] = 5.3, p ≤ 0.01). Rats with adult-onset cocaine self-administration experience made significantly fewer perseverative + regressive errors (9.6±2.8) than age-matched rats passively receiving yoked-saline (17.9±2.5; p ≤ 0.01) or yoked-cocaine (19.4±5.2; p ≤ 0.05) and compared to rats with adolescent-onset cocaine self-administration experience (16.8 ± 2.5; p ≤ 0.03).
Fig. 3.
Mean ± SEM trials completed to reach criterion, proportion correct trials, lever press reaction time (sec), trial omissions, and error subtypes (P: perseverative; R: regressive; N: never-reinforced) during the set shift phase in rats with adult-onset and adolescent-onset self-administration experience or passive saline or passvie cocaine exposure. * p ≤ 0.05 compared to age-matched rats passively receiving yoked-saline; # p ≤ 0.05 compared to same treatment condition in the other age group.
Reversal-Learning Phase
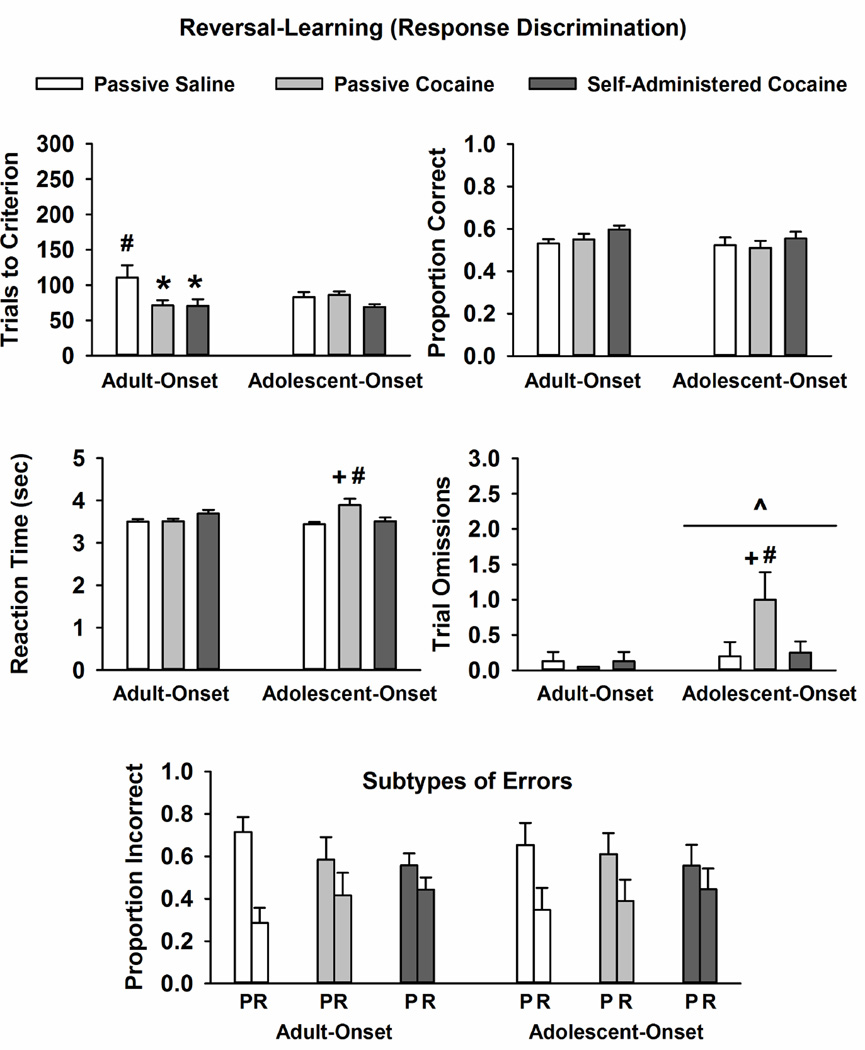
Data obtained during the reversal-learning phase are displayed in Fig. 4. Analysis of trials completed to reach criterion revealed a significant main effect of treatment condition (F [2, 45] = 4.4, p ≤ 0.02) and a trend for a drug-onset age × treatment condition interaction (F [2, 45] = 2.7, p ≤ 0.07). Within adult-onset groups, rats actively self-administering and passively receiving yoked-cocaine required fewer trials to reach criterion during the reversal-learning phase than rats passively receiving yoked-saline (p ≤ 0.01 and 0.02, respectively). Moreover, rats with adult-onset yoked-saline exposure required more trials to reach criterion than rats with adolescent-onset yoked-saline exposure (p ≤ 0.02). Within adolescent-onset groups, treatment condition did not impact the number of trials to reach criterion. Drug-onset age and/or treatment condition did not significantly impact the proportion of correct trials.
Fig. 4.
Mean ± SEM trials completed to reach criterion, proportion correct trials, lever press reaction time (sec), trial omissions, and error subtypes (P: perseverative; R: regressive) during the reversal-learning phase in rats with adult-onset and adolescent-onset self-administration experience or passive saline or passive cocaine exposure. # p ≤ 0.05 compared to same treatment condition in the other age group; * p ≤ 0.05 compared to the age-matched rats passively receiving yoked-saline; + p ≤ 0.05 compared to age-matched rats self-administering cocaine and passively receiving yoked-saline; ^ p ≤ 0.05 compared to adult-onset rats overall.
For lever press reaction time, there was a significant drug-onset age × treatment condition interaction (F [2, 45] = 4.4, p ≤ 0.02), and post-hoc testing demonstrated that rats passively receiving yoked-cocaine during adolescence had longer reaction times than age-matched rats actively self-administering cocaine or passively receiving yoked-saline (p ≤ 0.02 and 0.002, respectively). Moreover, rats with adolescent-onset yoked-cocaine exposure had longer lever press reaction times than rats with adult-onset yoked-cocaine exposure (p ≤ 0.01). Within adult-onset groups, lever press reaction time was not impacted by treatment condition.
Analysis of trial omissions revealed a significant main effect of drug-onset age (F [1, 45] = 4.3, p ≤ 0.04), with adolescent-onset groups omitting more trials, overall, than adult-onset groups. There was also a trend for a drug-onset age × treatment condition interaction for trial omissions (F [2, 45] = 2.4, p ≤ 0.09). Within adolescent-onset groups, the number of trial omissions was greater in rats passively receiving yoked-cocaine than in rats actively self-administering cocaine or passively receiving yoked-saline (p ≤ 0.05 and 0.03, respectively). Moreover, rats with adolescent-onset yoked-cocaine exposure had more trial omissions than rats with adult-onset yoked-cocaine exposure (p ≤ 0.01).
Lastly, error analysis during the reversal-learning phase revealed a significant main effect of error subtype (F [1, 45] = 10.6, p ≤ 0.002) that did not differ by drug-onset age and/or treatment condition. There was a greater proportion of perseverative than regressive errors across all conditions (p ≤ 0.002). Analysis of number of total errors (perseverative + regressive, Table 1) revealed a significant main effect of treatment condition (F [2, 45] = 4.2, p ≤ 0.02) and a trend for a drug-onset age × treatment condition interaction (F [2, 45] = 2.5, p ≤ 0.09). Post-hoc testing demonstrated that rats with adult-onset cocaine self-administration experience and passive cocaine exposure made significantly fewer perseverative + regressive errors than age-matched rats passively receiving yoked-saline (p ≤ 0.05). There were no significant differences in the number of perseverative + regressive errors in adolescent-onset groups.
Discussion
Initial Set Formation: Influence of Age
Adult and adolescent rats consumed similar amounts of cocaine and engaged in comparable rates of contingent lever pressing. Though it is likely that the brief periods of cocaine withdrawal that were imposed on weekends produced neurochemical changes in the brain (e.g., Conrad et al. 2010; Gabriele et al. 2012; Wydra et al. 2013), these changes and the weekend withdrawal did not appear to impact cocaine self-administration behavior, particularly as a function of age. Thus, any dissociable age-related or treatment condition-related effects during the three phases of the set shifting task can not be attributed to different amounts or patterns of cocaine consumed by adults vs. adolescents.
In the present study, rats with adolescent-onset cocaine or saline exposure (now young adults) required more trials to reach criterion than the rats with adult-onset cocaine or saline exposure (now more mature adults) during the initial visual cue discrimination. The total errors made reflected the same age difference. In other studies, adolescent rats also were relatively slow in learning the initial visual cue discrimination in the strategy set shifting task (Harvey et al. 2013). In a set shifting task for which rats must dig in a scented-medium cup for a hidden reward, adolescent rats required more trials than young adult rats to reach criterion during all phases of the task (Newman and McGaughy 2011). Research has suggested that formation of an attentional set requires intact functioning of the PFC in general (Crofts et al. 2001) and of the orbital subregion in particular (Chase et al. 2012). In humans, the PFC is the last to mature, with gray matter loss (maturation) steadily increasing throughout adolescence into young adulthood (Gogtay et al. 2004; Sowell et al. 2001) and continuing to increase from young adulthood until middle age (Aine et al. 2006; Sowell et al. 2003). In rats, the ability to behave flexibly requires dopamine D1 receptors in mPFC and OFC (Floresco 2013; Winter et al. 2009). Notably, D1 receptors within PFC do not reach full adult levels until P100 (Andersen et al. 2000). Thus, the slower initial discrimination learning by adolescent vs. young adult rats (Newman and McGaughy 2011) and by young adult vs. more mature adult rats (present findings) supports the idea that rodent PFC function matures along a continuum until PFC development is complete (Spear 2000). The age-related difference only in initial discrimination learning may relate to the difficulty rats had overall in learning the visual cue discrimination. The response discrimination and its reversal were relatively easier to learn by rats, and thus perhaps less susceptible to age-related differences in PFC maturation. Though age at testing impacted the speed of learning the initial visual cue discrimination, prior cocaine exposure had no influence during this phase of testing. Consequently, an important question, addressed below, is whether developmental plasticity protects or hinders PFC-related behavioral flexibility during the set shifting and reversal-learning phases after long-term cocaine exposure.
Interaction of Age and Cocaine Exposure on Set Shfting and Reversal Learning
Following self-administration training, age and cocaine-related changes in performance during the set shift and reversal-learning phases were found. Notably, the task measures were obtained after a prolonged drug-free period; thus, differences were not related to a direct influence of cocaine but rather to a consequence of prior repeated exposure (either active or passive exposure, depending on the measure). The training dose of cocaine used (1 mg/kg) is a dose that produces a clinically relevant (2 µM) peak plasma level in rats (Booze et al. 1997); in human addicts, peak plasma levels reach 1–3 µM after typical doses of cocaine (Paly et al. 1982). The 1 mg/kg training dose produced a daily i.v. intake of cocaine of ~20 mg/kg (or ~10 mg/kg/hr), which falls near the range of hourly cocaine intakes (12–20 mg/kg) reported after escalation in extended access models (e.g., Allen et al. 2007; Carroll et al. 2010; Knackstedt et al. 2007; Liu et al. 2005). As abstinent cocaine abusers with prefrontal deficits are reported to use ~10–20 mg/kg/day (e.g., Bolla et al. 2003; Madoz-Gúrpide et al. 2011), the present study effectively serves as a model of set shifting performance of individuals who abused cocaine during adolescence or adulthood.
Set Shift Phase
During the set shift phase, rats with adult-onset cocaine self-administration experience completed a significantly higher proportion of correct trials compared to age-matched yoked-saline controls and to rats with adolescent-onset cocaine self-administration experience, despite all groups having an equivalent number of trials to criterion. A higher proportion of correct trials in this phase can emerge from a variety of influences. First, this may signify weakened memory (storage or retrieval) of the reinforced strategy acquired in the initial phase. Indeed, a general feature of rat behavior during the set shift phase was comission of a significantly higher proportion of regressive errors than perseverative or never reinforced errors. Other investigators who have used this task have reported either more perseverative than regressive errors (Floresco et al 2008; Thai et al 2013), more regressive than perseverative errors (Hankosky et al 2013), or the same number of regressive and perseverative errors (Harvey et al 2013) during the set shift phase. Regressive errors and perseverative errors arise from proactive interference, with the former reflecting a natural tendency of rats to revert to the original reinforced strategy that was learned during the initial discrimination phase after being reinforced at least 25% of the time during the set shift phase (Floresco et al. 2008). Weakened memory of the original learned strategy would produce less proactive interference and result in a greater proportion of correct trials when contingencies change. In line with this, rats with adult-onset cocaine self-administration experience committed fewer perseverative + regressive errors than age-matched rats receiving yoked-saline or yoked-cocaine during the set shift phase, suggesting less proactive interference by the preceeding phase in the self-administering group. Human laboratory testing shows a different profile from the rats during a set shift in that abstinent cocaine abusers commit more total errors, perseverative errors and non-perseverative errors, and have a smaller percentage of correct choices, than healthy controls (Hanlon et al. 2011; Madoz-Gurpide et al. 2011; Woicik et al. 2009; Woicik et al. 2011). These measures are indicative of behavioral flexibility deficits, which were not reproduced during the set shift phase in rats exposed to cocaine self-administration or passive cocaine delivery.
The lack of behavioral flexibility deficits during the set shift phase in cocaine abstinent rats of both ages may relate to the level of task difficulty. In published work conducted subsequent to the present study, we reported that the performance of adolescent Spontaneously Hypertensive Rats (a model for attention deficit/hyperactivity disorder) during the set shift and reversal-learning phases was more deteriorated and learning was more detered compared to controls when the task was made more taxing by imposing a 15-sec delay between a correct lever press and food pellet delivery (Harvey et al. 2013). When a 0-sec delay was used between a correct lever press and food pellet delivery (current design), Spontaneously Hypertensive Rats exhibited faster learning during the set shift and reversal-learning phases compared to controls. It is posssible that if a 15-sec delay were used in the present study, rats with adult-onset and adolescent-onset cocaine self-administration experience would have shown behavioral flexibility deficts during the set shift phase.
Factors other than weakened memory of the original strategy by prior cocaine self-administration experience may underlie the higher proportion of correct trials and smaller number of total and perseverative + regressive errors during the set shift phase in the adult-onset rats. It should be noted, however, that abstinent cocaine abusers do exhibit memory deficits in several other types of neuropsychological tests (e.g., Fox et al. 2009; O’Malley et al. 1992; van Gorp et al. 1999; Woicik et al. 2009), and metabolic activity is reduced in circuits related to learning and memory after withdrawal from self-administered cocaine in adult rats (Calipari et al. 2013). Of particular interest are findings showing that abstinent cocaine abusers performed better than healthy controls in simple motor learning tasks (Gillen et al. 1998; van Gorp et al. 1999). Such an effect might explain the higher proportion of correct trials in this study because we used an operant-based learning task involving a simple response discrimination (always press left lever or always press right lever, depending on original lever position bias) for the set shift phase.
Adolescent-onset cocaine self-administration experience or its passive delivery did not influence behavior during the set shift phase. These results are consistent with a past study showing that following injections of progressively increasing doses of cocaine (5–15 mg/kg over 12 days) and 10 or 24 drug-free days, trials to reach criterion during the set shift phase (digging in a scented-medium cup for a hidden reward) did not differ from vehicle controls (Black et al. 2006). In this respect, rats with adolescent-onset cocaine self-administration experience reacted differently than rats with adult-onset cocaine self-administration experience during the set shift phase (no increase in proportion of correct trials, no decrease in perseverative + regressive errors, and no decrease in total errors in younger rats). This age-related difference in rats may relate to studies in people showing that, as individuals mature, there is increasing recruitment of cortical regions during attentional switching (Casey et al. 2004). Attentional switching is gated almost entirely by subcortical (striatal) mechanisms in younger individuals. Though not the same process as set shifting, attentional switching is an executive function requiring behavioral flexibility and is critically dependent on PFC (Rossi et al. 2009). In the current study, as PFC function may not be fully mature in the young adult rats (Andersen et al. 2000), this might preclude the rats with adolescent-onset cocaine self-administration experience from being susceptible to cocaine-induced reductions in proactive interference and/or to motor learning improvement during the set shift phase.
Reversal-Learning Phase
During the reversal-learning phase, all groups had a greater proportion of perseverative than regressive errors, suggesting behavior was less flexible during this phase compared to the preceeding set shift phase in general. Unknown is whether this is an order effect or due to the type of behavioral flexibility test. In past studies, more regressive than perseverative errors (Thai et al 2013; Dalton et al 2011), more perseverative than regressive errors (Haluk and Floresco 2009), or the same number of perseverative and regressive errors (Harvey et al 2013) have been reported during the reversal-learning phase of this task in rats. In human laboratory studies of probabilistic reversal learning based on a visual discrimination, cocaine and stimulant-dependent individuals either made a similar number of perseverative errors and a greater number of spontaneous (regressive) errors than healthy controls (Ersche et al. 2011), or a greater number of total errors and perseverative errors than healthy controls (Ersche et al. 2008). Despite this natural tendancy toward response perseveration during the reversal-learning phase, rats with adult-onset cocaine self-administration experience required fewer trials to reach criterion and made fewer perseverative + regressive errors compared to age-matched yoked-saline, but not yoked-cocaine, controls. Thus, increased speed of reversal learning during abstinence in both adult-onset cocaine groups may reflect weakened memory (less proactive interference) of the previous discrimination strategy or improved simple motor learning (Gillen et al. 1998; van Gorp et al. 1999), as rats were required to always choose a particular lever (same as the original lever position bias) to receive food reward. However, past research showed that reversal learning is disrupted in abstinent cocaine abusers and in adult rats with prior cocaine self-administration experience or passive cocaine exposure (Stalnaker et al. 2009). The tasks used in those studies appear to be more complex (go/no-go odor discrimination task, probabilistic reversal-learning task) than the reversal-learning task (reversal of the response discrimination) used here. As with the set shift phase, it is possible that behavioral flexibility deficits during the reversal-learning phase would have been obtained following adult-onset cocaine exposure if demands of the task were more taxing (Harvey et al. 2013).
In contrast to adult-onset rats, rats with adolescent-onset passively yoked-cocaine exposure showed performance deficits during the reversal-learning phase. The observed deficits were not of the type related to speed or accuracy of reversal learning, but were related to these rats having longer lever press reaction times (decision-making impairment) and more trial omissions (attentional/motivational impairment). These latter measures in human subjects reflect executive function impairment (Drechsler et al. 2010; Hervey et al. 2006; Reeve and Schandler 2001; Seidman et al. 1997), suggesting that passive cocaine exposure in adolescent rats disrupts some aspects of executive function, but not behavioral flexibility, during abstinence. It is interesting that rats with adolescent-onset cocaine self-administration experience did not exhibit the same deficits, suggesting the consequences of contingent and non-contingent cocaine delivery on PFC function can be different. In our past work, we found that non-spatial working memory was disrupted in rats with adolescent-onset cocaine self-administration experience, but not in age-matched rats passively receiving yoked-cocaine (Harvey et al. 2009). Considered together, these findings indicate that compared to age-matched rats passively receiving yoked-cocaine, rats with adolescent-onset cocaine self-administration experience are relatively worse at retaining information over a short delay (working memory), which is consistent with their being relatively better at abandoning previously learned responses when the contingencies change (reversal learning). Previous research examining catechol-O-methyltransferase polymorphism supports the view that decreased network stability underlying working memory can be associated with increased flexibility and switching in cognitive tasks, and vice versa (Bilder et al. 2004). As non-spatial working memory and reversal learning performances require the intact functioning of the OFC (Di Pietro et al. 2004; Ghods-Sharifi et al. 2008), it is likely that active and passive cocaine exposure in a brain that is not fully mature may cause long-term OFC dysfunction, albeit the nature of the dysfunction is expressed differently. In structural plasticity studies, spine density in rat OFC is reduced by experimenter-administered cocaine and unchanged by self-administered cocaine (Ferrario et al. 2005; Kolb et al. 2004). On the other hand, the transcription factor deltaFosB is increased in rat OFC to a greater extent after self-administered cocaine than passively yoked-cocaine (Winstanley et al. 2007). These and other factors may underlie differences between active and passive cocaine exposure in adolescent rats on OFC-related neurocognitive functioning in adulthood after cocaine is withdrawn.
Conclusions
This study set out to address the question of whether developmental plasticity protects or hinders PFC-related behavioral flexibility after long-term withdrawal from cocaine exposure in adolescent compared to adult rats. Future studies that use the more difficult version of the strategy set shifting task may be required to establish concurrent validity with human cocaine abusers regarding behavioral flexibility deficits, in order to show predictive validity with respect to the consequences of cocaine use on behavioral flexibility in adolescents. Though deficits in behavioral flexibility were not revealed under the experimental conditions used in this study, the results showed that cocaine self-administration experience affected the young adult (adolescent-onset) and more mature adult (adult-onset) rats differently during the set shift and reversal learning phases, and that the manner of cocaine delivery (self-administered vs. passively yoked) affected the young adult (adolescent-onset) rats differently during the reversal learning phase. Consequently, these kinds of relative differences have heuristic value for addressing the question of whether developmental plasticity protects or hinders other aspects of the behavioral effects of cocaine.
Impairment in some aspects of executive function was observed during the reversal-learning phase in rats passively receiving yoked-cocaine during adolescence. Passively yoked-cocaine can be considered a form of imposed subordination, as rats have no control over cocaine delivery. Though speculative, this finding suggests that when cocaine use is coerced via peer-pressure during adolescence, some aspects of executive function may be hindered in adulthood. Some support for this idea includes research showing that imposed subordination in young mice produces impaired exploratory behaviors and general learning disability compared to mice with dominant status (Colas-Zelin et al. 2012) and research showing that an animal’s social rank (dominant or subordinate) influences behavioral responses to a variety of psychoactive drugs (Czoty and Nader 2013; Nesher et al. 2013). Human laboratory tests show that one’s relative perceived rank in a social hierarchy (lower or higher) influences cortical activation patterns differently (Zink et al. 2008) and that the perception of social hierarchy cues in monkeys modulates adaptive behavior, including the direction of visual attention (Deaner et al. 2005) that may be mediated in part by the OFC (Marsh et al. 2009). It remains to be determined whether coerced drug use via peer-pressure signifies subordination in adolescents.
Rats with adolescent-onset cocaine self-administration experience did not show any behavioral deficits, but they failed to show cocaine-induced reductions in perseverative + regressive errors, increases in the proportion of correct trials, and decreases in trials to criterion during the set shift and/or reversal-learning phases, as exhibited by rats with adult-onset cocaine self-administration experience. Thus, the immaturity of the PFC during adolescence may protect against cocaine-induced memory impairment and/or cocaine-induced improvement in simple motor learning during adulthood when cocaine is used intentionally during adolescence.
Acknowledgements
This research was supported by NSF grant SMA 0835976 to the CELEST Science of Learning Center (B. Shinn-Cunningham, PI). Experiments comply with the current laws of the country in which they were performed.
Footnotes
The authors declare no conflicts of interest.
References
- Aine CJ, Woodruff CC, Knoefel JE, Adair JC, Hudson D, Qualls C, Bockholt J, Best E, Kovacevic S, Cobb W, Padilla D, Hart B, Stephen JM. Aging: compensation or maturation? Neuroimage. 2006;32:1891–1904. doi: 10.1016/j.neuroimage.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RM, Uban KA, Atwood EM, Albeck DS, Yamamoto DJ. Continuous intracerebroventricular infusion of the competitive NMDA receptor antagonist, LY235959, facilitates escalation of cocaine self-administration and increases break point for cocaine in Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;88:82–88. doi: 10.1016/j.pbb.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black YD, Maclaren FR, Naydenov AV, Carlezon WA, Jr, Baxter MG, Konradi C. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. J Neurosci. 2006;26:9656–9665. doi: 10.1523/JNEUROSCI.2391-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booze RM, Lehner AF, Wallace DR, Welch MA, Mactutus CF. Dose-response cocaine pharmacokinetics and metabolite profile following intravenous administration and arterial sampling in unanesthetized, freely moving male rats. Neurotoxicol Teratol. 1997;19:7–15. doi: 10.1016/s0892-0362(96)00180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Cain RE, Wasserman MC, Waterhouse BD, McGaughy JA. Atomoxetine facilitates attentional set shifting in adolescent rats. Dev Cogn Neurosci. 2011;1:552–559. doi: 10.1016/j.dcn.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Beveridge TJ, Jones SR, Porrino LJ. Withdrawal from extended-access cocaine self-administration results in dysregulated functional activity and altered locomotor activity in rats. Eur J Neurosci. 2013 doi: 10.1111/ejn.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Gao Y, Brimijoin S, Anker JJ. Effects of cocaine hydrolase on cocaine self-administration under a PR schedule and during extended access (escalation) in rats. Psychopharmacology (Berl) 2010;213:817–829. doi: 10.1007/s00213-010-2040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Davidson MC, Hara Y, Thomas KM, Martinez A, Galvan A, Halperin JM, Rodriguez-Aranda CE, Tottenham N. Early development of subcortical regions involved in non-cued attention switching. Dev Sci. 2004;7:534–542. doi: 10.1111/j.1467-7687.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- Chase EA, Tait DS, Brown VJ. Lesions of the orbital prefrontal cortex impair the formation of attentional set in rats. Eur J Neurosci. 2012;36:2368–2375. doi: 10.1111/j.1460-9568.2012.08141.x. [DOI] [PubMed] [Google Scholar]
- Chess AC, Raymond BE, Gardner-Morse IG, Stefani MR, Green JT. Set shifting in a rodent model of attention-deficit/hyperactivity disorder. Behav Neurosci. 2011;125:372–382. doi: 10.1037/a0023571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas-Zelin D, Light KR, Kolata S, Wass C, Denman-Brice A, Rios C, Szalk K, Matzel LD. The imposition of, but not the propensity for, social subordination impairs exploratory behaviors and general cognitive abilities. Behav Brain Res. 2012;232:294–305. doi: 10.1016/j.bbr.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Ford K, Marinelli M, Wolf ME. Dopamine receptor expression and distribution dynamically change in the rat nucleus accumbens after withdrawal from cocaine self-administration. Neuroscience. 2010;169:182–194. doi: 10.1016/j.neuroscience.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, Roberts AC. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Nader MA. Effects of dopamine D2/D3 receptor ligands on food-cocaine choice in socially housed male cynomolgus monkeys. J Pharmacol Exp Ther. 2013;344:329–338. doi: 10.1124/jpet.112.201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GL, Ma LM, Phillips AG, Floresco SB. Blockade of NMDA GluN2B receptors selectively impairs behavioral flexibility but not initial discrimination learning. Psychopharmacology (Berl) 2011;216:525–535. doi: 10.1007/s00213-011-2246-z. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr Biol. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Complementary tasks to measure working memory in distinct prefrontal cortex subregions in rats. Behav Neurosci. 2004;118:1042–1051. doi: 10.1037/0735-7044.118.5.1042. [DOI] [PubMed] [Google Scholar]
- Drechsler R, Rizzo P, Steinhausen HC. The impact of instruction and response cost on the modulation of response-style in children with ADHD. Behav Brain Funct. 2010;6:31. doi: 10.1186/1744-9081-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Abbott S, Craig KJ, Muller U, Suckling J, Ooi C, Shabbir SS, Clark L, Sahakian BJ, Fineberg NA, Merlo-Pich EV, Robbins TW, Bullmore ET. Response perseveration in stimulant dependence is associated with striatal dysfunction and can be ameliorated by a D(2/3) receptor agonist. Biol Psychiatry. 2011;70:754–762. doi: 10.1016/j.biopsych.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an "inverted-U" toward a family of functions. Front Neurosci. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Fox HC, Jackson ED, Sinha R. Elevated cortisol and learning and memory deficits in cocaine dependent individuals: relationship to relapse outcomes. Psychoneuroendocrinology. 2009;34:1198–1207. doi: 10.1016/j.psyneuen.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O'Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Gabriele A, Pacchioni AM, See RE. Dopamine and glutamate release in the dorsolateral caudate putamen following withdrawal from cocaine self-administration in rats. Pharmacol Biochem Behav. 2012;103:373–379. doi: 10.1016/j.pbb.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem. 2008;89:567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Gillen RW, Kranzler HR, Bauer LO, Burleson JA, Samarel D, Morrison DJ. Neuropsychologic findings in cocaine-dependent outpatients. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:1061–1076. doi: 10.1016/s0278-5846(98)00057-8. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Hankosky ER, Kofsky NM, Gulley JM. Age of exposure-dependent effects of amphetamine on behavioral flexibility. Behav Brain Res. 2013;252:117–125. doi: 10.1016/j.bbr.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl) 2011;218:681–692. doi: 10.1007/s00213-011-2360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RC, Dembro KA, Rajagopalan K, Mutebi MM, Kantak KM. Effects of self-administered cocaine in adolescent and adult male rats on orbitofrontal cortex-related neurocognitive functioning. Psychopharmacology (Berl) 2009;206:61–71. doi: 10.1007/s00213-009-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RC, Jordan CJ, Tassin DH, Moody KR, Dwoskin LP, Kantak KM. Performance on a strategy set shifting task during adolescence in a genetic model of attention deficit/hyperactivity disorder: methylphenidate vs. atomoxetine treatments. Behav Brain Res. 2013;244:38–47. doi: 10.1016/j.bbr.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF, Tonev S, Eugene Arnold L, Keith Conners C, Hinshaw SP, Swanson JM, Hechtman L. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006;12:125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Green-Jordan K, Valencia E, Kremin T, Eichenbaum HB. Cognitive task performance after lidocaine-induced inactivation of different sites within the basolateral amygdala and dorsal striatum. Behav Neurosci. 2001;115:589–601. doi: 10.1037//0735-7044.115.3.589. [DOI] [PubMed] [Google Scholar]
- Kasperski SJ, Vincent KB, Caldeira KM, Garnier-Dykstra LM, O'Grady KE, Arria AM. College students' use of cocaine: results from a longitudinal study. Addict Behav. 2011;36:408–411. doi: 10.1016/j.addbeh.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Kantak KM. Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacology (Berl) 2007;194:403–411. doi: 10.1007/s00213-007-0852-6. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Kolb B, Pellis S, Robinson TE. Plasticity and functions of the orbital frontal cortex. Brain Cogn. 2004;55:104–115. doi: 10.1016/S0278-2626(03)00278-1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Effects of extended-access self-administration and deprivation on breakpoints maintained by cocaine in rats. Psychopharmacology (Berl) 2005;179:644–651. doi: 10.1007/s00213-004-2089-y. [DOI] [PubMed] [Google Scholar]
- Madoz-Gurpide A, Blasco-Fontecilla H, Baca-Garcia E, Ochoa-Mangado E. Executive dysfunction in chronic cocaine users: an exploratory study. Drug Alcohol Depend. 2011;117:55–58. doi: 10.1016/j.drugalcdep.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Blair KS, Jones MM, Soliman N, Blair RJ. Dominance and submission: the ventrolateral prefrontal cortex and responses to status cues. J Cogn Neurosci. 2009;21:713–724. doi: 10.1162/jocn.2009.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez L, Stamatakis EA, Fernandez-Serrano MJ, Gomez-Rio M, Rodriguez-Fernandez A, Perez-Garcia M, Verdejo-Garcia A. Neural correlates of the severity of cocaine, heroin, alcohol, MDMA and cannabis use in polysubstance abusers: a resting-PET brain metabolism study. PLoS One. 2012;7:e39830. doi: 10.1371/journal.pone.0039830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Dev Psychobiol. 2011;53:391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley S, Adamse M, Heaton RK, Gawin FH. Neuropsychological impairment in chronic cocaine abusers. Am J Drug Alcohol Abuse. 1992;18:131–144. doi: 10.3109/00952999208992826. [DOI] [PubMed] [Google Scholar]
- Paly D, Jatlow P, Van Dyke C, Jeri FR, Byck R. Plasma cocaine concentrations during cocaine paste smoking. Life Sci. 1982;30:731–738. doi: 10.1016/0024-3205(82)90606-3. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve WV, Schandler SL. Frontal lobe functioning in adolescents with attention deficit hyperactivity disorder. Adolescence. 2001;36:749–765. [PubMed] [Google Scholar]
- Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Exp Brain Res. 2009;192:489–497. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Faraone SV, Weber W, Mennin D, Jones J. A pilot study of neuropsychological function in girls with ADHD. J Am Acad Child Adolesc Psychiatry. 1997;36:366–373. doi: 10.1097/00004583-199703000-00015. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology. 2009;56(Suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai CA, Zhang Y, Howland JG. Effects of acute restraint stress on set-shifting and reversal learning in male rats. Cogn Affect Behav Neurosci. 2013;13:164–173. doi: 10.3758/s13415-012-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol. 2009;31:706–719. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorp WG, Wilkins JN, Hinkin CH, Moore LH, Hull J, Horner MD, Plotkin D. Declarative and procedural memory functioning in abstinent cocaine abusers. Arch Gen Psychiatry. 1999;56:85–89. doi: 10.1001/archpsyc.56.1.85. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Williams J, Pacula RL, Chaloupka FJ, Wechsler H. College students' use of cocaine. Subst Use Misuse. 2006;41:489–509. doi: 10.1080/10826080500521755. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DE, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter S, Dieckmann M, Schwabe K. Dopamine in the prefrontal cortex regulates rats behavioral flexibility to changing reward value. Behav Brain Res. 2009;198:206–213. doi: 10.1016/j.bbr.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang GJ, Volkow ND, Goldstein RZ. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34:1112–1122. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woicik PA, Urban C, Alia-Klein N, Henry A, Maloney T, Telang F, Wang GJ, Volkow ND, Goldstein RZ. A pattern of perseveration in cocaine addiction may reveal neurocognitive processes implicit in the Wisconsin Card Sorting Test. Neuropsychologia. 2011;49:1660–1669. doi: 10.1016/j.neuropsychologia.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydra K, Golembiowska K, Zaniewska M, Kaminska K, Ferraro L, Fuxe K, Filip M. Accumbal and pallidal dopamine, glutamate and GABA overflow during cocaine self-administration and its extinction in rats. Addict Biol. 2013;18:307–324. doi: 10.1111/adb.12031. [DOI] [PubMed] [Google Scholar]
- Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58:273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]