Abstract
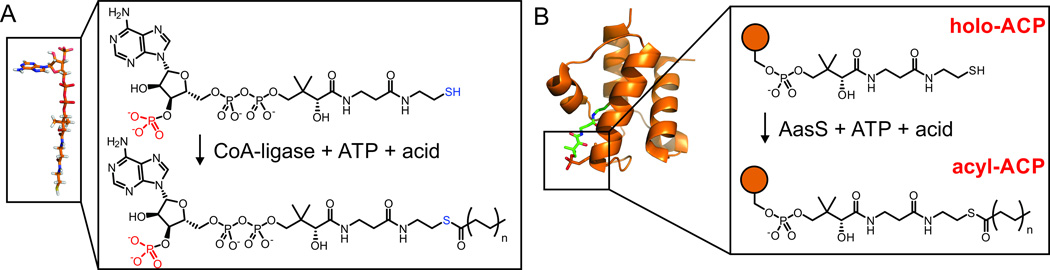
The acyl carrier protein (ACP) requires post-translational modification with a 4’-phosphopantetheine arm for activity, and this thiol-terminated modification carries cargo between enzymes in ACP-dependent metabolic pathways. We show that acyl-acyl carrier protein synthetases (AasSs) from different organisms are able to load even, odd and unnatural fatty acids onto E. coli ACP in vitro. Vibrio harveyi AasS not only shows promiscuity for the acid substrate but also is active upon various alternate carrier proteins. AasS activity also extends to functional activation in living organisms. We show that exogenously supplied carboxylic acids are loaded onto ACP and extended by the E. coli fatty acid synthase, including unnatural fatty acid analogs. These analogs are further integrated into cellular lipids. In vitro characterization of four different adenylate-forming enzymes allowed us to disambiguate CoA-ligases and AasSs, and further in vivo studies show the potential for functional application in other organisms.
Introduction
The acyl carrier protein (ACP) is a small protein that is responsible for carrying cargo from active site to active site in polyketide and fatty acid synthases. A conserved serine residue of apo-ACP becomes post-translationally modified with 4’-phosphopantetheine to form holo-ACP, (Crosby and Crump, 2012) on which nascent polyketides or fatty acids are covalently bound via a thioester linkage. For in vitro structural studies of these pathways, facile methods to install natural and unnatural cargo are required. Various techniques exist to load pantetheine or coenzyme A (CoA) probes onto carrier proteins, but all require organic synthesis. For example, activated (CoA) esters can be used to acylate the free thiol of the 4’-phoshopantetheine arm of holo-ACP, (Hitchman, et al., 1998) or pantetheinamides can be attached to apo-ACP via a “one-pot” chemoenzymatic methodology. (Worthington and Burkart, 2006) However, both methods are not efficient tools in a cellular context, since they are hampered by uptake (e.g. CoAesters do not cross cell membranes (George, et al., 2004)) or the need for expression of helper enzymes. We show here that the enzyme acyl-acyl carrier protein synthetase (AasS) can be an important tool for both in vitro and in vivo production of acyl-ACPs with natural and unnatural cargo.
Acyl-acyl carrier protein synthetase (AasS) is a member of the adenylate-forming enzymes, (Jiang, et al., 2006) a ubiquitous enzyme group characterized into three major classes: non-ribosomal peptide synthase (NRPS) adenylation domains, acyl- or aryl-CoA synthetases and oxidoreductases in class I; aminoacyl-tRNA synthetases in class II; and NRPS-independent siderophore synthetases in class III. (Schmelz and Naismith, 2009) Within class I, AasS enzymes act upon ACPs instead of coenzyme A, installing fatty acids onto the 4‘-phosphopantetheine arm of holo-ACP (Fig. 1) via hydrolysis of ATP. (Jiang, et al., 2006; Zornetzer, et al., 2006) AasS was first discovered in Escherichia coli by Ray and Cronan in 1976. (Ray and Cronan, 1976) While efficient at loading fatty acid chain lengths of up to C18 onto holo-ACP, (Flaman, et al., 2001; Rock and Garwin, 1979) the protein was unstable in vitro and difficult to purify. (Kuo and Ohlrogge, 1984; Shanklin, 2000) A second AasS sub-type was later discovered in bioluminescent bacterium Vibrio harveyi, (Byers and Holmes, 1990) the plant Arabidopsis thaliana (Koo, et al., 2005; Tjellström, et al., 2013) and cyanobacteria. (Kaczmarzyk, 2008; Kaczmarzyk and Fulda, 2010; von Berlepsch, et al., 2012) A homolog within this group from V. harveyi B392 was expressed heterologously and shown to act upon several fatty acid chain lengths both odd and even, with specificity for medium-chain fatty acids. (Jiang, et al., 2006) The enzyme was also shown to act on lipoic acid, (Jordan and Cronan, 1997) tolerate ester groups within the acyl chain, and acts on 3-OH fatty acids. (Bi, et al., 2013; Bi, et al., 2014) The V. harveyi AasS is not active on α- and ω-dicarboxylic acids and is poorly active on unsaturated fatty acids (Lin, et al., 2010) However, our understanding of the selectivity for most AasSs in terms of both ACP (Jiang, et al., 2010) and acid substrates (Jiang, et al., 2006) remains incomplete. Thus far, no studies have shown if the promiscuous AasS from V. harveyi (VhAasS) can load non-fatty acids onto carrier proteins..
Figure 1.
Activity of CoA-ligase and acyl-acyl carrier protein synthetase. A) CoA-ligases act on coenzyme A whereas B) acyl-acyl carrier protein synthetases (AasSs) act on holo-acyl carrier proteins.
Results and Discussion
To address this issue, we began by computationally screening a range of molecules by docking them into the active site of AasS using the programs Autodock/Vina and Autogrow. (Durrant, et al., 2009) Surprisingly, there is sufficient space in the active site to harbor molecules more bulky than saturated fatty acids (Table S1 and Fig. S1A-D). To confirm this observation in vitro, we tested a range of carboxylic acids for loading by VhAasS onto purified holo-Escherichia coli FAS ACP (EcACP) and monitored activity by conformationally sensitive UREA-PAGE gel and mass spectrometry. First, we loaded several even and odd fatty acids (Fig. S2A-B). Next, we assayed a panel of acids with a variety of pKas, steric bulk or other functionality (Fig. S2C-D). VhAasS appears to be highly promiscuous to the identity of acid substrates, efficiently loading azide, alkyne, phenyl, halogen-modified fatty acids (e.g. 6-bromohexanoic acid and 4-pentynoic acid) as well as 5-benzoylvaleric acid.
We also explored loading of fatty acid analogs with bulky termini, such as Nbd or dansyl fluorophore moieties, but these were not efficiently loaded onto EcACP by VhAasS (see SI). To further explore this phenomenon, we assayed for loading of 5-phenylvaleric acid and 8-phenyloctanoic acid, both of which were efficiently loaded onto EcACP by VhAasS. We subsequently nitrated both of these acids in the para position, and these too were loaded onto EcACP (data not shown). As a possible explanation for this selectivity, we hypothesized that the lipophilicity of the acid tail influences AasS catalysis, but an analysis of logP values of the unnatural acids used showed no apparent trend. The AasS does not load any carboxylic acid substrates with α-substitutions including pyrrole-2-carboxylic acid, coumarin-3-carboxylic acid or even the functionalized fatty acid 2-bromohexadecanoic acid (Fig. S2C-D). Rational for this observation might be due to a narrow opening to the substrate tunnel, but since modeling cannot give insight into this an X-ray crystal structure of VhAasS is needed. Thus far, size and shape, in combination with modifications at the α-position seem to govern substrate selectivity.
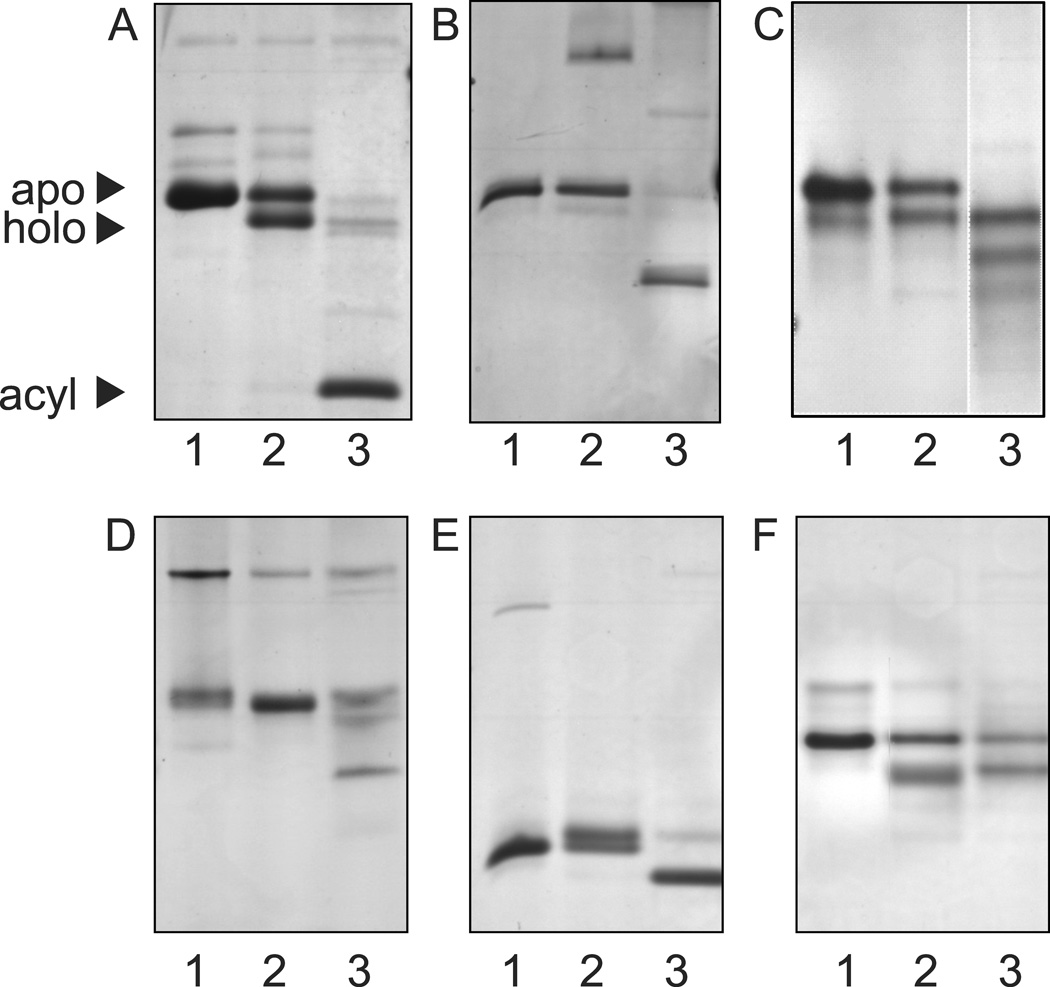
To be of general use as a tool for carrier protein modification (Fig. 1), AasS must not only be promiscuous with regard to the carboxylic acid substrate but also to the carrier protein. Previously it was shown that E. coli and Aquifex aeolicus fatty acid ACPs are substrates of VhAasS, while B. subtilis, L. lactis and the mitochondrial B. taurus ACPs are not, suggesting a quite narrow carrier protein substrate specificity for VhAasS. (Jiang, et al., 2006) To evaluate this in more detail, we widened the substrate pool by choosing three type II fatty acid synthase ACPs, two ACPs from type II polyketide synthases, and one peptidyl carrier protein (PCP) (see sequence identity in Fig. S2E). Each of these were heterologously expressed in E. coli and converted to holo- form in vitro with Sfp for use as substrates with decanoic acid (Fig. 2). Following overnight incubation, formation of the holo- or acyl-form of each carrier protein was monitored by conformationally sensitive UREA PAGE, (Post-Beittenmiller, et al., 1991) as seen in Figure 2. E. coli FAS AcpP, Mycobacterium tuberculosis type II FAS AcpM and Plasmodium falciparum FAS ACP all efficiently load decanoic acid onto the holo carrier protein. Streptomyces coelicolor ACP from the actinorhodin PKS and Syagrus glaucescens ACP from the tetracenomycin C type II PKS were both acylated. Pseudomonas fluorescencs PltL from the pyoluteorin NRPS was not acylated. This finding was not surprising, as NRPSs utilize the peptidyl carrier protein (PCP) rather than the ACP seen in FAS and PKSs.
Figure 2.
VhAasS acyl carrier protein substrate specificity. VhAass loading of decanoic acid onto different acyl carrier proteins monitored by 20% UREA-PAGE. In each panel; lane 1 is apo-ACP, lane 2 is holo-ACP formed by the reaction of apo-ACP, MgCl2, ATP, Sfp, CoA and DTT at 37 °C for 1 hour, and lane 3 is acyl-ACP formed by the reaction of holo ACP with decanoic acid and VhAasS at 37°C for 18 hours. Each panel shows these reactions on an ACP from a different organism; A Escherichia coli FAS AcpP (AcpP) B Mycobacterium tuberculosis type II FAS AcpM (AcpM) C Plasmodium falciparum FAS ACP (PfACP) D Streptomyces coelicolor ACP from the actinorhodin PKS (actACP) E Syagrus glaucescens ACP from the tetracenomycin C type II PKS (tcmACP) F Pseudomonas fluorescens PltL from the pyoluteorin NRPS (PltL).
The ability to load a range of ACPs with unnatural substrates without synthetic effort is a benefit that could be used for rapid activity assays in a variety of FAS and PKS studies. An even larger road block in studying primary and secondary metabolite pathways, however, is the lack of an efficient route to label carrier proteins in vivo and a methodology by which to monitor their pathway products. E. coli, which encodes its own AasS (NP_417313.1), has not been shown to extend and incorporate exogenously supplied fatty acids. E. coli AasS is dual-functional and membrane bound, and thus presumably unable to act in conjunction with cytosolic ACP and FAS. (Ray and Cronan, 1976) Since VhAasS can load a large variety of carrier proteins and unnatural carboxylic acids onto EcACP, we predicted that this activity could also be leveraged in vivo to incorporate exogenously supplied fatty acids into the FAS pathway. The permissiveness of the fatty acid synthase for a non-standard acyl-ACP is not without precedent. In a hallmark paper by Cronan, it was shown that in biotin synthesis FAS extends malonyl-CoA methyl ester through two iterative cycles. (Lin, et al., 2010) Also the mammalian FAS has been shown to extend nonstandard substrates in vitro by using phenyl-acetyl-CoA as starter unit. (Smith and Stern, 1983)
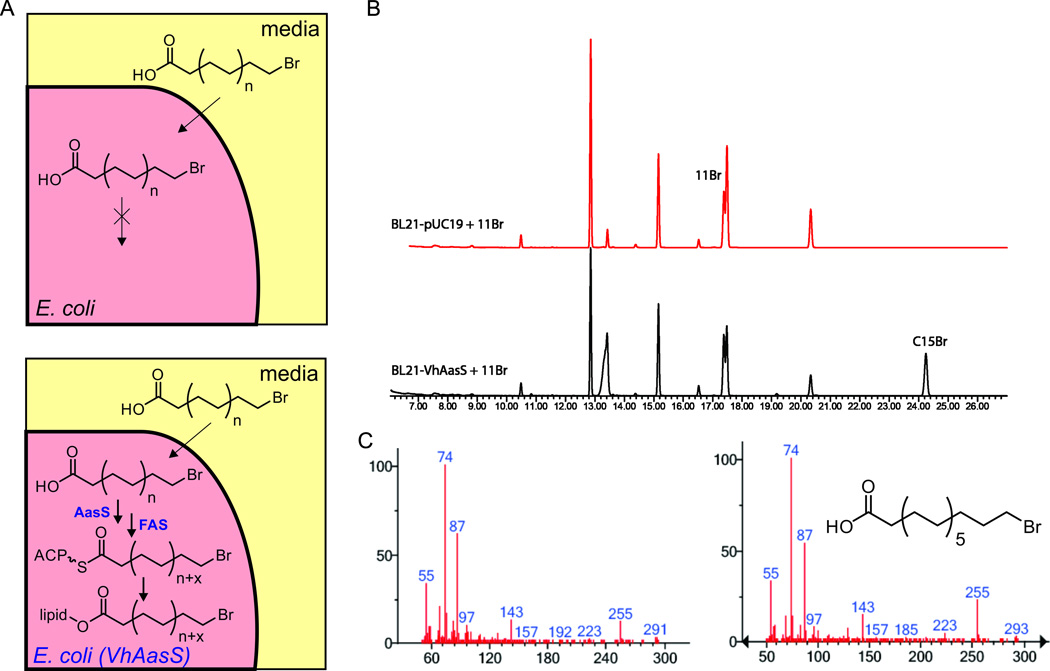
When overexpressed in E. coli BL21, (Jiang, et al., 2006) VhAasS enabled supplemented odd- and even-chain fatty acids to be taken up, extended, and incorporated into bacterial lipids. (Jiang, et al., 2010) We also supplemented functionalized fatty acids, including 8-bromooctanoic acid, and observed chain elongation only in the strain overexpressing VhAasS; whereas the control strain was unable to utilize this fatty acid (Fig. S3A-B and Table S2). Separating lipids by TLC, followed by fatty acid methyl ester (FAME) conversion and GCMS analysis, (Skipski, et al., 1964) provided an unambiguous demonstration that 8-bromooctanoic acid was elongated to 12-bromododecanoic acid and further processed into lipids (Fig. S3C-E and Table S3). 11-Bromoundecanoic acid was also incorporated and selectively elongated to 15-bromopentadecanoic acid (Fig. 3) as proven by comparison with a synthetic standard (SI). However, the vinylogous 2-octenoic acid did not appear to be elongated (Fig. S3A-B and Table S2), a finding that is in line with our in vitro data (Fig. S2C-D) indicating that AasSs are not tolerant to functionality at the α-position. The double transformant E. coli BL21 strain expressing both VhAasS along with overexpression of the E. coli thioesterase TesA shows very similar FAME profiles as that with only VhAasS, but the total fatty acid amount is increased by 3-fold (Fig. S3F). This ability of overexpressed TesA to increase fatty acid yield has been previously demonstrated for biofuel applications. (Steen, et al., 2010)
Figure 3.
In vivo activity of AasS. A) Schematic representation of feeding unnatural acids to E. coli. Top: wildtype E. coli K12 cannot utilize exogenously supplied acids. Bottom: E. coli expressing VhAasS loads supplied acids onto ACP. These acids can be extended (to a discrete chain length) by the endogenous fatty acid synthase and acyltransferases transfer unnatural chains to cellular lipids. B) GCMS chromatograms of FAME analysis of E. coli fed with 11-bromoundecanoic acid. In black the strain expressing VhAasS and in red a control strain. C) EI-MS of the peak at 24.2 minutes, identified by synthetic standard as 15-bromopentadecanoic acid.
Besides in vitro and in vivo activity of VhAasS on several unnatural carboxylic acid and carrier protein substrates, we wondered whether AasSs from different organisms show different activity or selectivity, with the hope of finding orthogonal enzymes (as has previously been shown for 4’-phosphopantetheinyl transferases (Zhou, et al., 2007). However, annotation of AasSs proved challenging due to high homology with other adenylate-forming enzymes. For example, while both E. coli and V. harveyi enzymes have been well described, the differentiation between AasSs and long-chain CoA ligases remains unclear due to vague nomenclature and the sheer number of adenylate-forming enzymes. AasSs have also been annotated as fatty acyl AMP ligases, fatty acyl CoA ligases and FadDhomologous enzymes. It has previously been suggested that acyl-ACP synthetases can be distinguished from CoA ligases by a 20 amino acid insertion between β1 and β2 of the C-subdomain (or N-terminus), distant from the active site (see Fig. S4A, region 344–364 of TTHA0604). (Arora, et al., 2009; Goyal, et al., 2012) However, Liu et al. showed recently that although FadD10 from M. tuberculosis lacks this insertion, decanoic acid is efficiently transferred in vitro to a carrier protein. (Liu, et al., 2013) This suggests that what formally defines AasS activity remains unclear. Kaczmarzyk and Fulda demonstrated that purified cyanobacterial AasPCC7942 and Slr1609 act as AasSs and do not accept CoA (Kaczmarzyk and Fulda, 2010) utilizing a radioactive assay for acyl-ACP synthetase activity. (Rock and Cronan, 1981) Despite the utility of this assay, the method separates radiolabeled products from reactants using paper chromatography. We believe that this indirect technique may not accurately distinguish CoA and ACP acceptor substrates leading to incorrect characterization. Therefore, we set out to disambiguate the activity of AasS from acyl-CoA synthetases (or CoA ligases) through in silico and in vitro studies.
In order to evaluate differences between demonstrated acyl-CoA ligases and AasSs, we constructed homology structural models of AasSs (Fig. S1E-H). Only two AasS could be modeled successfully: VhAasS based on 1ULT, a long chain acyl CoA ligase from bacterium Thermus thermophilus HB8 named TTHA0604; and cyanobacterial Ana7108 based on 3IVR, a putative long chain fatty acid CoA ligase from bacterium Rhodopseudomonas palustris CGA009. Since these modeling results were poor, we docked Vibrio harveyi ACP onto a series of X-ray crystal structures of CoA ligases, and only TTHA0604 showed convergent docking (Fig. S1E-H). Cronan and co-workers already noticed the striking homology between TTHA0604 and AasS from V. harveyi. (Jiang, et al., 2006) Therefore, we questioned whether TTHA0604 is truly a CoA ligase or a misannotated AasS. To answer this question, we first prepared a phylogenetic analysis with large sets of putative AasSs (Fig. S4B-C). To our surprise very distinct clades of these adenylate-forming enzymes formed upon construction of Neighbor Joining trees: E. coli bifunctional AasS, TTHA0604, VhAasS, and the cyanobacterial/plant AasSs form separate branches in which TTHA0604 and VhAasS are very closely related. From this analysis, we predicted that two new clades could contain novel AasS or acyl-CoA ligases.
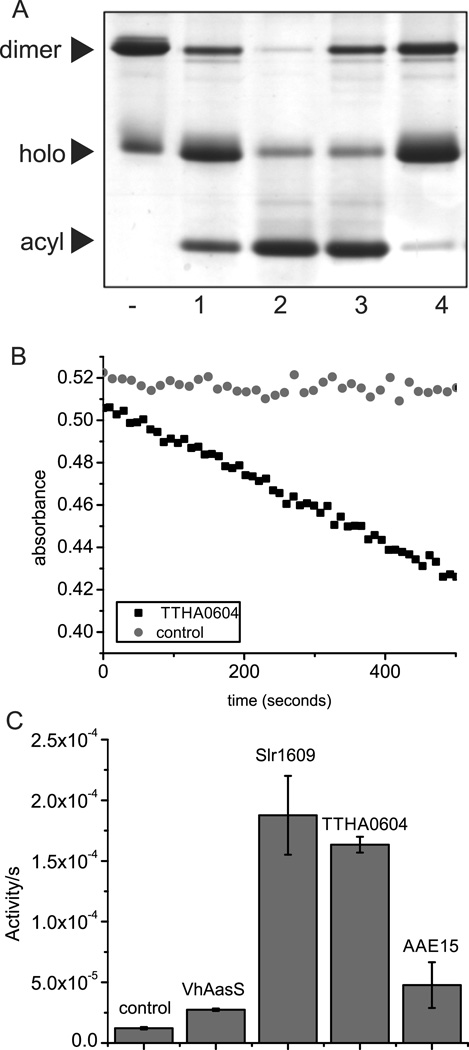
Next, we expressed VhAasS, TTHA0604 from Thermus thermophilus, AAE15 from Arabidopsis thaliana and Slr1609 from Synechocystis sp PCC 6803. These four enzymes were subsequently tested for CoA ligase and AasS activity. Acyl CoA ligase activity was assayed using an enzyme-coupled spectrophotometric assay for ATP consumption (Fig. 4B/C) (Hisanaga, et al., 2004), an assay for CoA consumption (Fig. S4D-E) (Bi, et al., 2014), and by HPLC-MS (Fig. S4F-I). Here, both TTHA0604 and Slr1609 show acyl-CoA ligase activity, as previously shown for TTHA0604. No CoA-ligase activity had previously been observed for Slr1609. In contrast, both AAE15 and VhAasS show a small amount of CoA ligase activity over background, each with a specific activity 10-fold lower than TTHA0604 and Slr1609. We then assayed these enzymes for AasS activity with E. coli ACP (EcACP) as an acceptor. Conformationally sensitive UREA PAGE was used to evaluate ACP loading with decanoic acid (Fig. 4A). VhAasS showed increased loading over Slr1609, TTHA0604, and AAE15, from which the latter two are significantly slower in catalyzing this process. Using these two assays, we functionally disambiguate CoAligases and AasSs with evidence for substrate selectivity for CoA or carrier proteins. Whereas the enzyme from V. harveyi shows excellent AasS activity but poor CoA ligase activity, T. thermophilus TTHA0604 demonstrates excellent CoA ligase but a poor AasS turnover. Interestingly, Synechocystis Slr1609 shows high activity with both CoA and ACP as substrate, whereas A. thaliana AAE15 is a poor enzyme in both cases. This difference in activity is not only reflected in vitro but also when these four enzymes were overexpressed in E. coli and their fatty acid profiles analyzed upon feeding with 8-phenyloctanoic acid (Fig S3G): only VhAasS and Slr1609 showed extended 10-phenyldecanoic acid products. This would suggest that protein-protein interactions play a role in AasS activity, and only enzymes forming the necessary interactions can be productive catalysts. Alternatively, the PPTase Sfp shows very little specificity for its carrier protein substrate, (Beld, et al., 2014) and interrogating AasSs in a similar fashion is part of ongoing work. A sequence alignment of ACPs showcases the large sequence space ACPs occupy (Fig. S2E), possibly factoring in the ability of AasSs to load them. We show here that the combination of CoA-ligase and AasS activity assays can be used to find enzymes with activity, like Slr1609, which could be used for acyl carrier protein in vitro and in vivo studies.
Figure 4.
Enzymatic activity assays of AasSs and CoA ligases. A) AasS activity of confirmed and proposed AasSs. An enzyme’s ability to load decanoic acid onto holo-EcAcpP in the presence of MgCl2, ATP and DTT overnight at 37 °C was monitored by 20% UREA-PAGE. The dash represents holo-EcAcpP and lanes 1–4 represent the enzymatic reaction with four different enzymes; Lane 1- TTHA0604 from Thermus thermophilus, Lane 2- VhAasS from Vibrio Harveyi, Lane 3- Slr1609 from Synechocystis PCC 6803, Lane 4- AAE15 from Arabidopsis thaliana.. B) Coenzyme A – decanoic acid ligase activity assay. Acyl-CoA biosynthesis is coupled via myokinase, pyruvate kinase and lactate dehydrogenase to NADH consumption, monitored at 340 nm. C) The change in absorbance over time showcasing that all four enzymes have CoA ligase activity over background. The control contains everything except CoA. Error bars represent standard deviation.
Since many cyanobacteria do harbor AasSs of the same subfamily as the bacterial V. harveyi AasS, and we showed that Slr1609 has both CoA-ligase and AasS activity (Fig. 4), we hypothesized that the wildtype Synechocystis sp PCC6803 would be able to load carboxylic acids onto its endogenous ACP, in vivo. However, many factors could be significantly different in this organism, including uptake, catabolism, protein-protein interactions between AasS and ACP and further processing by the fatty acid synthase. To our surprise, feeding 8-bromooctanoic acid led to the consumption of the acid, as shown by GCMS analyses of lyophilized media and cells (Fig. S3H-I and Table S4) with no observable bromine-containing products, suggesting dehalogenation of the fed acid. Supplementing the media with 11-bromoundecanoic acid resulted in the presence of the brominated acid in the cell, and chain extension to 15-bromopentadecanoic acid (Fig. S3H-I and Table S4). Comparing E. coli BL21 overexpressing VhAasS and the wildtype cyanobacterium shows a much lower yield of chain-extended product in the latter case, presumably due to the presence of less enzyme. However, this experiment shows that our methodology extends to different organisms, and provides valuable tools to study the flexibility of fatty acid and lipid metabolism in an important photosynthetic organism.
In our quest for a facile method to label carrier proteins and lipids in vivo, we expand the utility of AasS as a biotechnological tool in vitro by showcasing the wide range of acids that can be loaded onto carrier proteins. We envision the use of this enzyme as a general tool to quickly install and screen a wide variety of probes on carrier proteins and lipids, with no synthesis required, in order to study metabolic pathways and to prepare conjugates for structure elucidation. To our surprise, AasS activity with unnatural acids extends in vivo, where exogenously supplied acids can be efficiently introduced into living organisms. Not only are these acids transported into the cell and loaded onto the carrier protein, they are also extended by the fatty acid synthase and incorporated in lipids. Tracking lipids by in vivo AasS-catalyzed alkyne labeling, extension, transfer and subsequent click-reaction, belongs to one of the many uses we envision. (Milne, et al., 2010) Furthermore, there are very few published technologies with the ability to metabolically incorporate molecules with structural preservation. Many unique fatty acid products do not have efficient synthetic access, and these methods could be used as a tool for synthetic biology of extended hydrocarbon species. In this way, the AasS enzyme can be leveraged to gain access to metabolic pathways of organisms that have to date proved inaccessible.
Methods
In silico methods: sequence alignment, phylogeny and protein modeling/docking
Detailed in silico methods are described in the SI.
Cloning
The AasS/CoA-ligase from Synechocystis PCC 6803 is called Slr1609 and was cloned directly out of cell pellet and the gene was ligated into pET22. TTHA0604 from Thermus thermophilus was obtained from RIKEN in a pET11a vector and the gene was ligated into pET29. AAE15 (At4g14070) from Arabidopsis thaliana was obtained in pUNI51 and the gene was ligated into pET28a. AasS from V. harveyi (pYFJ84 (YFJ239), pET16b) was a generous gift of J.E. Cronan (Illinois).
Protein expression and purification
Sfp and Escherichia coli ACP (EcACP), Mycobacterium tuberculosis AcpM, Plasmodium falciparum apicoplastic ACP (PfACP), human fatty acid synthase ACP (hACP), actinorhodin ACP (actACP), ACP from tetracenomycin PKS synthase (P12884 ACPX_STRGA, tcmACP), vibriobactin NRPS carrier protein (VibB), pyoluteorin NRPS carrier protein (PltL), acyl-ACP synthetase from Vibrio harveyi (AasS), Thermus thermophilus long chain CoA ligase TTHA0604, Arabidopsis thaliana acyl-ACP synthetase (AAE15) and Synechocystis PCC 6803 acyl-ACP synthetase (Slr1609) were expressed in E. coli and purified according to standard protocols (NiNTA-resin followed by size-exclusion chromatography). A mixture of apo/holo- EcACP was transformed into pure holo-EcACP by incubation with Sfp, MgCl2 and CoA. Holo-EcACP was purified by size-exclusion chromatography. AcpH was used to convert apo/holo-carrier protein mixtures into pure apo samples.(Kosa, et al., 2012)
AasS-activity assay
We relied on conformationally sensitive UREA-PAGE analysis to distinguish apo-, holo-, crypto- and acyl- ACP formation (Post-Beittenmiller, et al., 1991). For a typical AasS reaction, a mixture of apo- and holocarrier protein was incubated with Sfp, MgCl2 and coenzyme A, in pH 8 100 mM phosphate buffer, for 1h at 37 °C, followed by the addition of ATP, MgCl2, VhAasS and acid. The reaction was run in glass vials overnight at 37 °C. Samples were taken, load dye added and run on a UREA-PAGE gel. After fixing, the bands were visualized using colloidal Coomassie (Candiano, et al., 2004) stain. Purified holo-EcACP and purified AasS were directly incubated with MgCl2, ATP and acid.
CoA-ligase activity assay
We adopted a coupled assay for the formation of AMP upon CoA-ligase activity (Hisanaga, et al., 2004). In a typical experiment fatty acid (1 µM), NADH (0.36 µM), CoA (0.6 µM), ATP (3 µM), MgCl2 (20 µM), phosphoenolpyruvate (1 µM), KCl (0.12 µM), myokinase (1U), pyruvate kinase (1U), lactate dehydrogenase (1U) and the synthase in 120 µl of pH 8 100 mM Tris were combined and the decrease in absorbance at 340 nm measured. Standard deviation was calculated using three datasets. We also used an assay for CoA consumption, monitoring the disappearance of the free thiol of CoA using DTNB, (Bi, et al., 2014) and the production of acyl-CoAs was shown by HPLC-MS.
Feeding of unnatural fatty acids
To investigate the in vivo activity, we fed E. coli BL21 and E. coli BL21 transformed with AasSs, various fatty acids. Cultures were grown in 5 ml LB, supplemented with the proper antibiotic, at 37 °C for 4h. IPTG and fatty acids were added to concentrations of 1 mM and 1 mM, respectively, and the cultures incubated at room temperature for 16h. Cultures were spun down and the cell pellets carefully washed three times with cold and sterile PBS buffer. The washed cell pellet was resuspended in 1 ml 1M methanolic acid and incubated at 65 °C for 30 minutes. The FAMEs were extracted using 1 ml of hexanes and the FAMEs separated by GCMS. For lipid analysis, cell pellets were extracted with chloroform using a modified Bligh & Dyer protocol. The lipids were separated by TLC using a mixture of chloroform/methanol. After copper sulfate, acid charring of a portion of the TLC plate. Silica was scratched off the TLC plates and either lipids eluted with 50/50 methanol/chloroform or FAMEs made in situ. FAMEs were extracted with hexanes and separated by GCMS.
Supplementary Material
Highlights.
Efficient loading of various acids on acyl carrier protein (ACP) using AasS
Acylation of ACPs with AasS from different pathways and organisms
Uptake, acylation and extension of unnatural acids in vivo, catalyzed by AasS
Significance.
We show here that acyl-acyl carrier protein synthetase (AasS) can be used in vitro to install various unnatural acids onto various carrier proteins from different pathways and organisms. This activity extends in vivo, where this enzyme enables engineered E. coli to use and extend unnatural acids.
Acknowledgements
We thank M. Jaremko, D. J. Lee, L. Tallorin for protein samples. We thank J. E. Cronan for the generous gift of the plasmid encoding V. harveyi AasS. We thank the Golden laboratory for Synechocystsis. sp. PCC 6803. RIKEN, TAIR and ABRC are thanked for plasmids. This work was supported by California Energy Commission CILMSF 500-10-039; DOE DE-EE0003373; NIH R01GM094924 and R01GM095970.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contribution
J.B. and K.F. designed research; J.B. and K.F. performed research together including cloning, protein expression, enzyme activity assays, computational docking, synthesis and feeding studies; J.B. and K.F. analyzed data; and J.B., K.F. and M.D.B wrote the paper.
References
- Arora P, Goyal A, Natarajan VT, Rajakumara E, Verma P, Gupta R, Yousuf M, Trivedi OA, Mohanty D, Tyagi A. Mechanistic and functional insights into fatty acid activation in Mycobacterium tuberculosis. Nat. Chem. Biol. 2009;5:166–173. doi: 10.1038/nchembio.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beld J, Sonnenschein EC, Vickery CR, Noel JP, Burkart MD. The phosphopantetheinyl transferases: catalysis of a post-translational modification crucial for life. Nat. Prod. Rep. 2014;31:61–108. doi: 10.1039/c3np70054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H, Wang H, Cronan JE. FabQ, a dual-function dehydratase/isomerase, circumvents the last step of the classical fatty acid synthesis cycle. Chem. Biol. 2013;20:1157–1167. doi: 10.1016/j.chembiol.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H, Yu Y, Dong H, Wang H, Cronan JE. Xanthomonas campestris RpfB is a fatty acyl-CoA ligase required to counteract the thioesterase activity of the RpfF diffusible signal factor (DSF) synthase. Mol. Microbiol. 2014;93:262–275. doi: 10.1111/mmi.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers DM, Holmes CG. A soluble fatty acyl-acyl carrier protein synthetase from the bioluminescent bacterium Vibrio harveyi. Biochem. Cell Biol. 1990;68:1045–1051. doi: 10.1139/o90-154. [DOI] [PubMed] [Google Scholar]
- Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25:1327–1333. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- Crosby J, Crump MP. The structural role of the carrier protein–active controller or passive carrier. Nat. Prod. Rep. 2012;29:1111–1137. doi: 10.1039/c2np20062g. [DOI] [PubMed] [Google Scholar]
- Durrant JD, Amaro RE, McCammon JA. AutoGrow: a novel algorithm for protein inhibitor design. Chemical Biology & Drug Design. 2009;73:168–178. doi: 10.1111/j.1747-0285.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaman AS, Chen JM, Van Iderstine SC, Byers DM. Site-directed mutagenesis of acyl carrier protein (ACP) reveals amino acid residues involved in ACP structure and acyl-ACP synthetase activity. J. Biol. Chem. 2001;276:35934–35939. doi: 10.1074/jbc.M101849200. [DOI] [PubMed] [Google Scholar]
- George N, Pick H, Vogel H, Johnsson N, Johnsson K. Specific labeling of cell surface proteins with chemically diverse compounds. J. Am. Chem. Soc. 2004;126:8896–8897. doi: 10.1021/ja048396s. [DOI] [PubMed] [Google Scholar]
- Goyal A, Verma P, Anandhakrishnan M, Gokhale RS, Sankaranarayanan R. Molecular basis of the functional divergence of fatty acyl-AMP ligase biosynthetic enzymes of Mycobacterium tuberculosis. J. Mol. Biol. 2012;416:221–238. doi: 10.1016/j.jmb.2011.12.031. [DOI] [PubMed] [Google Scholar]
- Hisanaga Y, Ago H, Nakagawa N, Hamada K, Ida K, Yamamoto M, Hori T, Arii Y, Sugahara M, Kuramitsu S, et al. Structural basis of the substrate-specific two-step catalysis of long chain fatty acyl-CoA synthetase dimer. J. Biol. Chem. 2004;279:31717–31726. doi: 10.1074/jbc.M400100200. [DOI] [PubMed] [Google Scholar]
- Hitchman TS, Crosby J, Byrom KJ, Cox RJ, Simpson TJ. Catalytic self-acylation of type II polyketide synthase acyl carrier proteins. Chem. Biol. 1998;5:35–47. doi: 10.1016/s1074-5521(98)90085-0. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Chan CH, Cronan JE. The soluble acyl-acyl carrier protein synthetase of Vibrio harveyi B392 is a member of the medium chain acyl-CoA synthetase family. Biochemistry. 2006;45:10008–10019. doi: 10.1021/bi060842w. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Morgan-Kiss RM, Campbell JW, Chan CH, Cronan JE. Expression of Vibrio harveyi acyl-ACP synthetase allows efficient entry of exogenous fatty acids into the Escherichia coli fatty acid and lipid A synthetic pathways. Biochemistry. 2010;49:718–726. doi: 10.1021/bi901890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan SW, Cronan JE. A new metabolic link: the acyl carrier protein of lipid synthesis donatates lipoic acid to the pyruvate dehydrogenase complex in Escherichia coli and mitochondria. J. Biol. Chem. 1997;272:17903–17906. doi: 10.1074/jbc.272.29.17903. [DOI] [PubMed] [Google Scholar]
- Kaczmarzyk D. PhD Thesis: Acyl-acyl carrier protein synthetases from bluegreen algae and plants. Niedersächsische Staats-und Universitätsbibliothek Göttingen; 2008. [Google Scholar]
- Kaczmarzyk D, Fulda M. Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling. Plant Physiol. 2010;152:1598–1610. doi: 10.1104/pp.109.148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo AJ, Fulda M, Ohlrogge JB. Identification of a plastid acyl-acyl carrier protein synthetase in Arabidopsis and its role in the activation and elongation of exogenous fatty acids. Plant J. 2005;44:620–632. doi: 10.1111/j.1365-313X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Kosa NM, Haushalter RW, Smith AR, Burkart MD. Reversible labeling of native and fusion-protein motifs. Nat. Methods. 2012;9:981–984. doi: 10.1038/nmeth.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TM, Ohlrogge JB. Acylation of plant acyl carrier proteins by acyl-acyl carrier protein synthetase from Escherichia coli Arch. Biochem. Biophys. 1984;230:110–116. doi: 10.1016/0003-9861(84)90091-2. [DOI] [PubMed] [Google Scholar]
- Lin S, Hanson RE, Cronan JE. Biotin synthesis begins by hijacking the fatty acid synthetic pathway. Nat. Chem. Biol. 2010;6:682–688. doi: 10.1038/nchembio.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Ioerger TR, Wang F, Sacchettini JC. Structures of Mycobacterium tuberculosis FadD10 protein reveal a new type of adenylate-forming enzyme. J. Biol. Chem. 2013;288:18473–18483. doi: 10.1074/jbc.M113.466912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne SB, Tallman KA, Serwa R, Rouzer CA, Armstrong MD, Marnett LJ, Lukehart CM, Porter NA, Brown HA. Capture and release of alkyne-derivatized glycerophospholipids using cobalt chemistry. Nat. Chem. Biol. 2010;6:205–207. doi: 10.1038/nchembio.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post-Beittenmiller D, Jaworski J, Ohlrogge J. In vivo pools of free and acylated acyl carrier proteins in spinach. Evidence for sites of regulation of fatty acid biosynthesis. J. Biol. Chem. 1991;266:1858–1865. [PubMed] [Google Scholar]
- Ray TK, Cronan JE. Activation of long chain fatty acids with acyl carrier protein: demonstration of a new enzyme, acyl-acyl carrier protein synthetase, in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1976;73:4374–4378. doi: 10.1073/pnas.73.12.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C, Garwin J. Preparative enzymatic synthesis and hydrophobic chromatography of acyl-acyl carrier protein. J. Biol. Chem. 1979;254:7123–7128. [PubMed] [Google Scholar]
- Rock CO, Cronan JE., Jr Acyl-acyl carrier protein synthetase from Escherichia coli. Methods Enzymol. 1981;71 Pt C:163–168. doi: 10.1016/0076-6879(81)71023-1. [DOI] [PubMed] [Google Scholar]
- Schmelz S, Naismith JH. Adenylate-forming enzymes. Curr. Opin. Struct. Biol. 2009;19:666–671. doi: 10.1016/j.sbi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanklin J. Overexpression and purification of the Escherichia coli inner membrane enzyme acyl–acyl carrier protein synthase in an active form. Protein Expression Purif. 2000;18:355–360. doi: 10.1006/prep.2000.1206. [DOI] [PubMed] [Google Scholar]
- Skipski V, Peterson R, Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem. J. 1964;90:374. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Stern A. The effect of aromatic CoA esters on fatty acid synthetase: Biosynthesis of ω-phenyl fatty acids. Arch. Biochem. Biophys. 1983;222:259–265. doi: 10.1016/0003-9861(83)90523-4. [DOI] [PubMed] [Google Scholar]
- Steen EJ, Kang Y, Bokinsky G, Hu Z, Schirmer A, McClure A, Del Cardayre SB, Keasling JD. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature. 2010;463:559–562. doi: 10.1038/nature08721. [DOI] [PubMed] [Google Scholar]
- Tjellström H, Strawsine M, Silva J, Cahoon EB, Ohlrogge JB. Disruption of plastid acyl: acyl carrier protein synthetases increases medium chain fatty acid accumulation in seeds of transgenic Arabidopsis. FEBS Lett. 2013;587:936–942. doi: 10.1016/j.febslet.2013.02.021. [DOI] [PubMed] [Google Scholar]
- von Berlepsch S, Kunz H-H, Brodesser S, Fink P, Marin K, Flügge U-I, Gierth M. The acyl-acyl carrier protein synthetase from Synechocystis sp. PCC 6803 mediates fatty acid import. Plant Physiol. 2012;159:606–617. doi: 10.1104/pp.112.195263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington AS, Burkart MD. One-pot chemo-enzymatic synthesis of reporter-modified proteins. Org. Biomol. Chem. 2006;4:44–46. doi: 10.1039/b512735a. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Cironi P, Lin AJ, Xu Y, Hrvatin S, Golan DE, Silver PA, Walsh CT, Yin J. Genetically encoded short peptide tags for orthogonal protein labeling by Sfp and AcpS phosphopantetheinyl transferases. ACS Chem. Biol. 2007;2:337–346. doi: 10.1021/cb700054k. [DOI] [PubMed] [Google Scholar]
- Zornetzer GA, White RD, Markley JL, Fox BG. Preparation of isotopically labeled spinach acyl–acyl carrier protein for NMR structural studies. Protein Expression Purif. 2006;46:446–455. doi: 10.1016/j.pep.2005.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.