Abstract
The intrinsic coagulation activity of silica nanoparticles strongly depends on their surface curvature. Nanoparticles with higher surface curvature do not denature blood coagulation factor XII on its surface, providing a coagulation ‘silent’ surface, while nanoparticles with lower surface curvature shows denaturation and concomitant coagulation.
Introduction
Blood compatibility of synthetic nanomaterials is an essential prerequisite for their use in drug delivery,1, 2 gene therapy,3-5 and bioimaging.6,7 Previous studies have demonstrated that upon systemic administration, nanoparticles (NPs) can trigger series of biochemical reactions with major blood components,8,9 leading to blood coagulation10,11 and vascular thrombosis.12 This activation of the coagulation pathway can further result in multiple organ failure and death.13
Upon contact with blood, NPs adsorb a wide variety of plasma proteins onto the surface that are closely associated with blood coagulation process.14-16 In particular, blood coagulation factor XII (FXII), a key zymogen of coagulation cascade, can initiate intrinsic coagulation pathway upon contact with foreign materials.17,18 Moreover, the denaturation of FXII can further increase the intrinsic coagulation process.19 Previous reports have demonstrated that the denaturation of adsorbed proteins on NP surfaces strongly depends on the curvature of NPs.20,21 However, the effect NP surface curvature on FXII and consequent intrinsic coagulation activity has not been to our knowledge investigated. Herein, we report the effect of nano-scale curvature on plasma coagulation activity using silica NPs of different sizes. In our findings, the coagulation activity of silica NPs decreases with the decreasing NP size (higher curvature). This curvature-dependent coagulation activity was further confirmed by the interaction of NPs with FXII; a lower degree of denaturation/unfolding of FXII was observed for NPs with higher curvature, leading to little to no plasma coagulation activity (Scheme 1). These results suggest that the interaction between FXII and NPs can be controlled by simply tuning the curvature of NPs and provide a means to either activate or inactivate intrinsic coagulation, independent of NP chemical composition.
Results and discussions
Commercially available silica NPs were used to investigate the size dependence of coagulation activity (see supporting information Table I and Figure S1). The in vitro intrinsic coagulation activity was measured by monitoring the amount of generated thrombin in human plasma by a modified activated partial thromboplastin time method (APTT).22 Since intrinsic coagulation activity correlates with the effective surface area of pro-coagulants,23 we investigated NP coagulation activity at four different surface areas (Figure 1). Flat glass was chosen as a positive control because of its strong pro-coagulant activity24 and as a representative of SiO2 surface with least surface curvature. Silica NPs of different sizes were incubated with human plasma and the amount of thrombin generation was determined by measuring the optical density (O.D.) of the chromogenic thrombin substrate S-2238 over time (405 nm). At a surface area of 0.4 cm2 in 180 μL plasma solution, none of the silica NPs showed intrinsic coagulation (Figure 1a); coagulation was only observed with the flat glass (surface area ~0.4 cm2) (positive control). For higher surface areas, the activation of intrinsic coagulation system was more prominent; larger NPs (diameters from 12 nm to 85 nm) showed substantial coagulation activity over time (Figure 1b-c). In contrast, NPs with smaller diameters (4 nm and 7 nm) did not show coagulation except at a very high surface area of 10 cm2 (Figure 1d), demonstrating NP curvature is a critical determinant of intrinsic coagulation activity.
Figure 1.
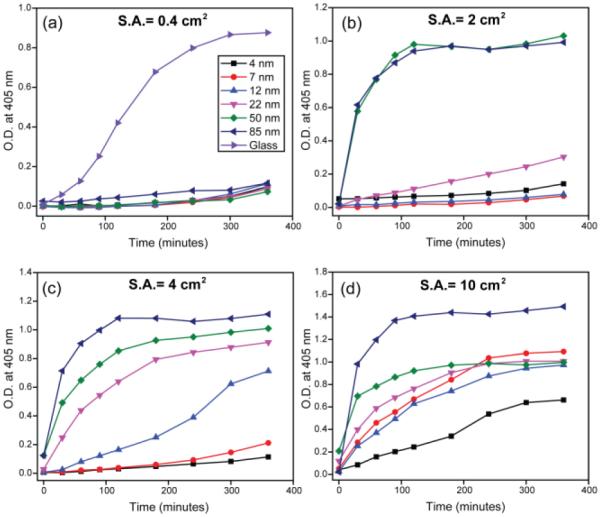
Size dependent intrinsic coagulation activity of silica NPs over time with surface area (S.A.) (a) 0.4 cm2, (b) 2 cm2, (c) 4 cm2, and (d) 10 cm2. Results are the averages of at least twelve replicates. O.D. denotes optical density.
To establish a clear relation between NP surface curvature and intrinsic coagulation activity, we further performed experiments at a constant surface area and at a fixed time (3 hr). As anticipated, the intrinsic coagulation activity of silica NPs increased with increasing NP size (Figure 2a and Figure S2), demonstrating clear surface curvature-dependent coagulation activity. We further investigated the dose-dependent coagulation activity of silica NPs of different sizes. As observed in Figure 2b, each NP showed a threshold concentration for the intrinsic coagulation activity, indicating that critical quantities of pro-coagulants are required to start intrinsic blood coagulation pathway. The threshold concentration shifts to higher values for smaller NPs, suggesting the smaller NPs generated less thrombin at a constant concentration and incubation time. In other words, NPs of lower curvature are more coagulation active compared to higher curvature ones at same concentration. It also mirrors our results in Figure 1a where flat glass (lowest curvature) is most active than all NPs. These results clearly demonstrate that at a given concentration, larger NPs are most active in initiating the intrinsic coagulation system, and that activity is reduced with increasing NP curvature.
Figure 2.
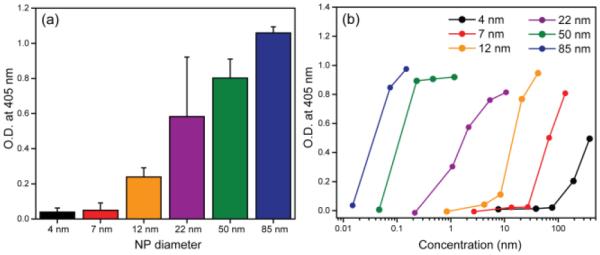
(a) Intrinsic coagulation activity after 3 hr incubation in the presence of silica NPs. Surface area of NPs were 4 cm2 in 180 μL of test solution. Error bars represent standard deviation of more than 12 replicates. (b) Effect of NP concentration on intrinsic coagulation activities on nanoparticle concentrations after 90 min incubation. Results are the averages of at least twelve replicates. O.D. denotes optical density.
Previous studies have demonstrated that denatured FXII on material surface is 500 times more susceptible to proteolytic activation than its native form.25 Given the fact that proteins absorbed on large NPs lose their native structure and function due to denaturation,26 we hypothesized that the intrinsic blood coagulation activity (vide supra) is associated with the adsorption and denaturation of FXII on NP surface. To test the possible denaturation of FXII on silica NP surface, we measured the circular dichroism (CD) spectra of FXII in the presence of NPs with three different sizes at a constant surface area of 0.1 m2/ml (Figure 3a). As anticipated, no change in the CD spectrum of FXII was observed in the presence of 4 nm silica NPs, further demonstrating the inert nature of NPs with high surface curvature. However, a red shift of the minimum at ~217 nm was observed for larger NPs, with concomitant structural changes at higher wavelengths, indicating denaturation/structural changes of FXII in presence of NPs with lower curvature.
Figure 3.
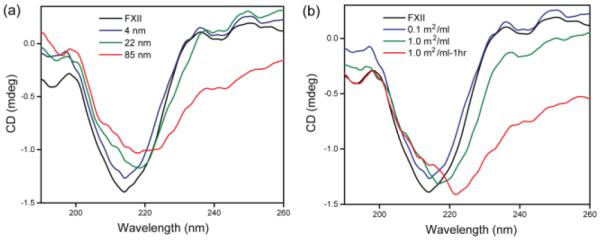
(a) Effect of NP surface curvature on the denaturation of FXII at room temperature. Surface areas of all silica NPs were 0.1 m2/ml. (b) CD spectra of FXII and FXII with 4 nm silica NPs with increasing surface area and incubation time at room temperature.
Smaller NPs also demonstrated intrinsic coagulation activity when at high surface areas (Figure 1d). To test the effect of surface area of smaller NPs on the denaturation of FXII, CD spectra were taken in the presence of 4 nm silica NPs at two different surface areas. FXII with 0.1 m2/ml of 4 nm silica NPs demonstrated no significant alteration of CD spectra of FXII (vide supra). However, when surface area of 4 nm silica NPs was increased to 1 m2/ml, a red shift was observed at the negative minimum (Figure 3b). Moreover, the CD spectrum change was enhanced with longer incubation time (1h) for this NP, demonstrating that denaturation of FXII is facilitated by larger surface area of procoagulant as well as prolonged incubation time. These trends of FXII denaturation are similar with intrinsic coagulation activation indicated in Figure 1, attesting to our hypothesis that structural change of FXII on NP surfaces leads to intrinsic coagulation activity. Taken together, adsorption of FXII on NPs with high curvature leads to no denaturation, restricting proteolytic cleavage of FXII. In contrast, FXII adsorbs on low curvature NPs with significant denaturation, thereby becomes susceptible to proteolytic cleavage by other enzyme such as kallikrein, leading to intrinsic coagulation.
Conclusions
In summary, we demonstrated that in vitro intrinsic coagulation activity of nanomaterials is highly correlated with the NP size; NPs with high surface curvature (small size) were coagulation inert while NPs with low curvature (large size) showed coagulation at a given surface area. This coagulation behavior was shown to depend on the structural loss of FXII upon NP contact; greater degree of FXII denaturation on the surface of larger NPs caused higher coagulation activity. These studies indicate that particles with high surface curvature provide an essentially coagulation-inert surface, an important issue for enhancing blood compatibility for nanomedicine. Conversely, particles with lower curvature provide a potential tool for inducing coagulation, an important issue for treatmnt of traumatic injury.
Experimental Section
Materials
The chromogenic substrate S-2238 was purchased from Chromogenix (Milano, Italy). Normal control human plasma “Plasma Control N” was obtained from Siemens Healthcare (Malvern, PA). Phosphatidyl-L-serine was purchased from MP Biomedicals. 4 nm silica nanoparticles dispersion was obtained from Alfa Aesar (Ward Hill, MA). Silica nanoparticle dispersion of 7 nm (Ludox SM), 12 nm (Ludox HS), and 22 nm (Ludox TM) were purchased from Sigma-Aldrich. 50 nm (NexSil 85-40) and 85 nm (NexSil 125-40) silica nanoparticle dispersions were purchased from Nyacol Nano Technologies, Inc. (Ashland, MA). MPC polymer (poly(2-methacryloyloxyethyl phosphorylcholine) (Lipidure-CM5206) and MPC polymer coated 96 well plate “Lipidure-Coat A-F96” was purchased from NOF Corporation (Tokyo, Japan). FXII was purchased from Haematologic Technologies Inc. (Essex Junction, VA) and used as received.
Intrinsic coagulation activity
The test solution consisted of 10 ml of 0.1 M Tris. HCl (pH = 7.4), 0.6 ml of 5 N NaCl aq., 0.4 ml of 0.5 M CaCl2 aq., 0.5 ml of 1 mM phosphatidyl-L-serine aq., 0.4 ml of 5 mM S-2238 aq., and 0.5 ml of human plasma. The silica nanoparticle dispersion was added to the above solution and the total volume was adjusted to 18 ml using Milli-Q water. 180 μl of test solution was loaded into MPC polymer coated 96 well plate and incubated at 37°C. Thrombin generation, determined by conversion of S-2238, was measured by the absorbance at 405 nm in a mircoplate reader (Spectramax M2, Molecular Devices).
CD Measurement
The CD spectra of FXII in presence of silica nanoparticles were measured using J-720 spectropolarimeter (Jasco) using a quartz cuvette with a 1 mm path length. 320 μl FXII solution (3.6 mg/ml) was diluted with 1.4 ml of 0.1 M Tris. HCl (pH = 7.4). Silica nanoparticle dispersion in Tris. HCl was added, and then the total concentration of FXII was adjusted to 4.5 μM using 0.1 M Tris.HCl. CD measurement was done from 190 to 250 nm at a rate of 100 nm/min.
Supplementary Material
Scheme 1.

Effect of NP surface curvature on intrinsic coagulation. NPs with higher surface curvature (smaller size) do not denature FXII on their surface and thereby are coagulation inert, however, NPs with smaller curvature denatures FXII, initiating coagulation cascade.
Acknowledgements
This research was supported in part by a grant from the National Institutes of Health (EB014277).
Footnotes
Electronic Supplementary Information (ESI) available: Physical properties and scanning electron micrographs (SEM) of silica NPs, intrinsic coagulation activity after 3 hr. See DOI: 10.1039/b000000x/
References
- 1.Duncan B, Kim C, Rotello VM. J. Control. Release. 2010;148:122. doi: 10.1016/j.jconrel.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis ME, Chen Z, Shin DM. Nat. Rev. Drug Discov. 2008;7:771. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 3.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Nature. 2010;464:1067. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanasty R, Dorkin JR, Vegas A, Anderson D. Nat. Mater. 2013;12:967. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 5.Ding Y, Jiang Z, Saha K, Kim CS, Kim ST, Landis RF, Rotello VM. Mol Ther. 2014;22:1075. doi: 10.1038/mt.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. ACS Nano. 2008;2:889. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasnovik DE, Nahrendorf M, Weissleder R. Basic Res. Cardiol. 2008;103:122. doi: 10.1007/s00395-008-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi J, Reipa V, Hitchins VM, Goering PL, Malinauskas RA. Toxicol. Sci. 2011;123:133. doi: 10.1093/toxsci/kfr149. [DOI] [PubMed] [Google Scholar]
- 9.Saha K, Moyano DF, Rotello VM. Mater. Horiz. 2014;1:102. doi: 10.1039/C3MH00075C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nabeshi H, Yoshikawa T, Matsuyama K, Nakazato Y, Arimori A, Isobe M, Tochigi S, Kondoh S, Hirai T, Akase T, Yamashita T, Yamashita K, Yoshida T, Nagano K, Abe Y, Yoshioka Y, Kamada H, Imazawa T, Itoh N, Kondoh M, Yagi K, Mayumi T, Tsunoda S, Tsutsumi Y. Nanotechnology. 2012;23:045101. doi: 10.1088/0957-4484/23/4/045101. [DOI] [PubMed] [Google Scholar]
- 11.Jones CF, Campbell RA, Brooks AE, Assemi S, Tadjiki S, Thiagarajan G, Mulcock C, Weyrich AS, Brooks BD, Ghandehari H, Grainger DW. ACS Nano. 2012;6:9900. doi: 10.1021/nn303472r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, Radomski MW. Br. J. Phamacol. 2005;146:882. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levi M, Keller TT, van Group E, ten Cate Cardiovasc. Res. 2003;60:26. doi: 10.1016/s0008-6363(02)00857-x. H. [DOI] [PubMed] [Google Scholar]
- 14.Deng ZJ, Liang MT, Monteiro M, Toth I, Minchin RF. Nat. Nanotechnol. 2011;6:39. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- 15.Sacchetti C, Motamedchaboki K, Magrini A, Palmieri G, Mattei M, Bernardini S, Bottini N, Bottini M. ACS Nano. 2013;7:1974. doi: 10.1021/nn400409h. [DOI] [PubMed] [Google Scholar]
- 16.Ilinskaya AN, Dobrovolskaia MA. Nanomedicine. 2013;8:969. doi: 10.2217/nnm.13.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renne T, Schmaier AH, Nickel KF, Blomback M, Maas C. Blood. 2012;120:4296. doi: 10.1182/blood-2012-07-292094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stavrou E, Schmaier AH. Thromb. Res. 2010;125:210. doi: 10.1016/j.thromres.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuel M, Pixley RA, Villanueva MA, Colman RW, Villanueva GB. J. Biol. Chem. 1992;267:19691. [PubMed] [Google Scholar]
- 20.Vertegel AA, Siegel RW, Dordick JS. Langmuir. 2004;20:6800. doi: 10.1021/la0497200. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Adv. Drug. Delivery Rev. 2009;61:428. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aronson DL, Chang P, Kessler CM. Circulation. 1992;85:1706. doi: 10.1161/01.cir.85.5.1706. [DOI] [PubMed] [Google Scholar]
- 23.Volger EA, Graper JC, Harper GR, Sugg HW, Lander LM, Brittain WJ. J. Biomed. Mater. Res. 1995;29:1005. doi: 10.1002/jbm.820290813. [DOI] [PubMed] [Google Scholar]
- 24.Zhuo R, Miller R, Bussard KM, Siedlecki CA, Volger VA. Biomaterials. 2005;26:2965. doi: 10.1016/j.biomaterials.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Griffin JH. Proc. Natl. Acad. Sci. U.S.A. 1978;75:1998. doi: 10.1073/pnas.75.4.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shang W, Nuffer JH, Dordick JS, Siegel RW. Nano Lett. 2007;7:1991. doi: 10.1021/nl070777r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


