Abstract
An intensive, 9-session Motivational Interviewing (IMI) intervention was assessed using a randomized clinical trial of 217 methamphetamine (MA) dependent persons. Intensive motivational interviewing (IMI) was compared with a standard single standard session of MI (SMI) combined with eight nutrition education sessions. Interventions were delivered weekly over two months. All study participants also received standard outpatient group treatment three times per week. Both study conditions showed significant decreases in MA use and ASI drug scores, but there were no significant differences between the two conditions. However, reductions in ASI psychiatric severity scores and days of psychiatric problems during the past 30 days were found for clients in the IMI condition but not SMI. SMI may be equally beneficial to IMI in reducing MA use and problem severity, but IMI may help alleviate co-occurring psychiatric problems that are unaffected by shorter MI interventions. Additional studies are needed to assess the problems, populations, and contexts for which IMI is effective.
Keywords: Methamphetamine, Motivational Interviewing, Randomized Clinical Trial, Psychotherapy, Treatment Dose
1. Introduction
Motivational Interviewing (MI) has achieved wide support for effectiveness in the addiction research literature. Although meta-analyses have shown MI to be effective for alcohol as well as illicit drug disorders1,2,3, the findings are strongest and most consistent for the treatment of alcohol problems. Studies of MI for illicit drug addiction have differed from studies for alcohol in several respects. First, nearly all of the drug studies have examined MI as preparation for more intensive treatment.1 MI for alcohol problems has been shown to be effective as a stand-alone treatment for alcohol problems4–6 and as a preparation for more intensive treatment.7,8 Second, the outcomes of MI for illicit drug problems are mixed. While a number of reviews and meta-analyses have concluded MI is effective as a preparation for more intensive drug treatment,1,9,2 a number of studies have reported non-significant findings relative to a variety of comparison conditions.10,11,12,13,14 As noted by Carroll and colleagues,15 most of these studies reporting non-significant findings used relatively large samples and rigorous, well-controlled study designs.
Although MI has been used to treat substance use disorders among a range of populations, relatively few attempts have been made to modify its content, process, or intensity to improve its impact or better address the needs of specific populations.16 For example, despite evidence of a dose effect for MI1,3 there has been limited effort toward developing more intensive models of MI. Polcin et al16 suggested that more MI sessions might be especially important for populations with higher severity of illicit drug dependence.
Intensive motivational interviewing (IMI)17 was developed as a 9-session intervention for the treatment of methamphetamine (MA) dependence. The overarching rationale for nine sessions is that it gives the client and therapist more time to address implementation of the change plan, including obstacles and barriers that emerge as the plan is enacted. It also enables the therapist and client time to address the variety of problems presented by clients with MA dependence. Although the intervention is delivered using a manual format that allows for consistency of the MI approach, the therapist is given considerable latitude in terms of problems and topics addressed in the sessions. The content of the first three sessions is taken largely from National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN) Studies of MI-based interventions.18,14
The first session focuses on identification of problems and motivation to change them. Problem discussion is facilitated through the use of worksheets that identify problems and through feedback from the therapist. Results from assessments used for research purposes (e.g., the Addiction Severity Index) are used by the therapist to provide feedback to the client about problems. The second session focuses on addressing ambivalence, the “pros and cons” of making or not making changes regarding substance use and related problems, again facilitated by the use of worksheets. The goal of session three is to use the discussions in the first two sessions to develop a change plan that included goals, plans for achieving them and ways that obstacles might be addressed. Unlike the NIDA CTN studies, IMI includes six additional sessions that focus on implementation of the change plan over time. This allows time for addressing obstacles, modifying goals, formulating new plans for achieving goals, and developing new goals once initial goals are achieved. The final session addresses termination and what if any additional services are needed.
Initial piloting of the intervention was conducted on a sample of 30 MA dependent individuals to assess acceptability, feasibility, and initial outcomes.19 IMI was used as a stand-alone intervention without any other services. Relative to MA use pre-treatment, participants during treatment reduced the number of days of MA use and provided fewer positive urine screens. Effect sizes for reductions in MA use and positive urine screens corresponded to medium effects as defined in Cohen.20 Measure of adherence and fidelity to IMI were those used by Ball et al18 and Winhusen et al.14 Results showed the therapist consistently used MI interventions with a high level of skill. For a more complete description of the intervention, piloting procedures, and adherence measures see Galloway et al.19
This paper takes the next step in the development of IMI by reporting outcomes on a randomized clinical trial of 217 MA dependent persons over a six month time period. The goal was to assess MA outcomes of individuals assigned to IMI versus a comparison condition at an intensive outpatient treatment program. Participants were interviewed at baseline and at 2-, 4-, and 6-month follow-up to assess MA use and a variety of other areas of functioning.
2. Methods
2.1 Sample
Study participants were recruited onsite at an outpatient substance abuse treatment facility in Northern California or by advertisement in local newspapers, community bulletin boards, and online postings. The majority were recruited through online postings (33%) followed by referral from a friend or study participant (23%), and through the local newspapers (18%). In order to maximize generalizability, inclusion into the study was liberal and required that participants be 18 years or older, meet 12-month DSM IV criteria for MA dependence and have the ability to read and understand English. Additionally, participants needed to provide contact information so they could be reached for follow-up interviews.
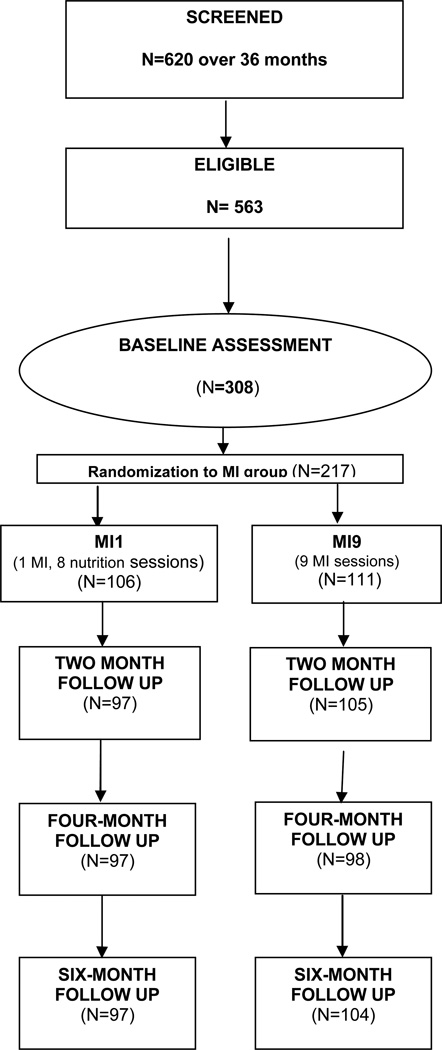
Figure 1 shows that 620 individual were screened over a 36 month period. Of those, 563 were found to meet the eligibility criteria. Typically, screenings were conducted before scheduling a baseline interview. The lag between screening and baseline interviews resulted in 308 individuals completing baseline interviews, 217 of whom were randomized into the study.
Figure 1.
Recruitment and retention
Study procedures were described by a research associate and participants who were interested in participating were asked to sign an informed consent before beginning the baseline interview. Individuals were screened by the outpatient treatment facility staff for serious medical or psychiatric problems that would exclude them from study participation and made appropriate referral to other services. Individuals with psychiatric conditions that could be managed on an outpatient basis were referred to mental health services while they participated in the study. Once assessed as meeting criteria for participation, individuals signed an informed consent for the study, completed a baseline interview and scheduled their first MI session. All study procedures were approved by the Public Health Institute institutional review board (IRB).
2.2 Procedures
Participants were assigned to a study condition based on stratified randomization procedures that ensured gender and MA severity were proportional in both conditions. MA severity was determined by past 30 day use at the baseline interview, operationalized as 10 or more days of use vs. less than 10 days of use. Participants randomized to the Intensive MI condition met with a therapist weekly over a 9-week period. Participants in the comparison condition received a single 90-minute session of standard MI (SMI)21 along with eight nutrition education sessions designed to achieve time equivalence. Topics covered in nutrition education included issues such as weight management, exercise, cholesterol, nutritional content of food, and the food pyramid. All MI sessions were audiotaped and 34% were randomly selected and rated using the Yale Adherence and Competence Scale (YACS).15,18,22 All three study therapists readily met minimum standards for competence and adherence throughout the study. For further details about the intervention manual and adherence monitoring please see Galloway et al19. Individuals in both study conditions took part in outpatient group sessions consisting of cognitive behavioral interventions that emphasized craving management. Group sessions took place 3 times a week for up to 12 weeks, eight weeks of active treatment and four weeks of aftercare.
Research interviews were conducted at baseline and 2-, 4-, and 6-month follow-up. The research study provided treatment at no cost to the participants and payment of $30 for the baseline interview, and $50 at the 2-, 4-, and 6-month interviews. Follow-up rates were excellent with over 90.0% completing interviews at each follow-up time point. Over 87% of the participants completed all follow-up interviews.
2.3 Measures
2.3.1 Baseline Assessment
Demographic information was collected at the baseline interview and included gender, age, marital status, highest educational attainment, and race/ethnicity.
DSM-IV Checklist for Drug and Alcohol Dependence was used at baseline to determine inclusion criteria of past 12-month MA dependence as well as 12-month dependence of other drugs, including alcohol. Items are based on DSM IV diagnostic criteria.23,24
2.3.2 Outcome Measures
Timeline Follow-Back (TLFB) was used to record the subject’s self-report use of MA (the primary outcome). During the first 9 weeks of the study the TLFB was administered weekly. Thereafter it was administered at 2-, 4-, and 6-month follow-up. The TLFB has been used extensively in a variety of drug and alcohol studies,25 including CTN studies of MI14,15 and has shown strong test-retest reliability as well as construct validity using collateral reports and urine samples.
Urine Screens for MA use were used to assess concordance with self-reported MA use.
Addiction Severity Index – Lite (ASI) is a standardized, structured interview that assesses past 30 days problem severity in seven areas. These seven areas include medical, employment, drug, alcohol, legal, family/social and psychiatric status. Problem severity is rated on a scale of 0.0 – 1.0 with a higher score indicative of more problem severity. The ASI–Lite version does not include the interviewer ratings of problem severity in the composite score calculations.26,27 Administration of the ASI was at baseline and 2, 4, and 6 month follow-up.
Psychiatric symptoms were assessed using composite scores on the Addiction Severity Index Psychiatric Scale. In addition, we examined the following past 30 day individual items on this scale: number of days experiencing anxiety, number of days experiencing depression and number of days experiencing any psychiatric problems.
Retention was assessed as the number of standard outpatient treatment sessions attended and as the number of MI and nutrition sessions attended.
2.4 Data Analysis
Data were analyzed using SPSS version 1828 and Stata version 13.29 ANOVA and χ2 tests of independence were used to test for differences in baseline demographic variables (age, marital status, education, and race/ethnicity), attendance at treatment groups, abstinence rates, and average ASI scores (alcohol, drug, psych severity) across time, separately for each study condition by gender.
Longitudinal analyses were carried out using random effects modeling.30 The specific model estimated for each gender separately was y(i,t) = αi + β1Ai + β2Oi + + β3Si + β4ASIPi + ΣγjTi,j + ΣθkTi,kGi + εi,t for j=1, …, 3; k=0,…,3 (0 = baseline, and 1, 2, 3 = 2, 4, and 6 month follow-ups, respectively) where A is an indicator for the whether the respondent was age 30 or over, O is the number of outpatient sessions attended, S is the number of MI session attended during treatment, ASIP is the baseline ASI psychiatric severity, G is an group indicator variable for the IMI treatment condition (with SMI the reference), and the T are indicator variables for the time of measurement. Correlation within-individual over time was incorporated into the model via the random intercept term αi = α+ ui, where the ui were assumed to have a constant correlation within individual (ρ) across time and independent across individuals. The adjusted treatment effect for the SMI condition at each of the 2, 4, and 6-month follow-up interviews (i.e., within-individual changes in outcome for each follow-up compared to baseline) was estimated by γ1, γ2, and γ3, respectively; for IMI, the corresponding estimates were defined as θ1-θ0, θ2-θ0, and θ3-θ0, respectively. The null hypotheses H0: γ1=γ2=γ2 and H0: θ1=θ2=θ2 were tested using standard accumulated linear contrasts in Stata and neither was rejected for any of the outcomes examined, indicating treatment effects (i.e., average differences from baseline) within each condition were not different across the three follow-up interviews. Therefore, the treatment effect estimates for each of the two conditions were estimated separately as the averages of the 2-, 4-, and 6-month individual treatment effects. All models were estimated using the xtmelogit (for dichotomous outcomes) and xtmixed (for continuous outcomes) in Stata.
3. Results
3.1 Sample characteristics
The total study sample comprised 217 participants (110 men; 107 women) with 106 participants (53 men, 53 women) in the SMI group and 111 participants (57 men, 54 women) in the IMI group. No differences in demographic variables were found between study groups, either overall or within gender, on baseline age, ethnicity, education, marital status, presence of children in the household, housing instability (homelessness) during the course of the study, or being in a controlled environment in the past 30 days (see Table 1). The average age of study participants ranged from 37.5 (Intensive MI men) to 39.3 (Intensive MI women). Over half of all study participants had completed at least some college education (57.2%). The majority (67.3%) were Caucasian, with the highest percentage being among women in the Intensive MI condition (74.1%).
Table 1.
Sample demographic and treatment characteristics by treatment group and gender
| Standard MI (MI1) | Intensive MI (MI9) | |||||
|---|---|---|---|---|---|---|
| Overall (n=106) |
Men (n=53) |
Women (n=53) |
Overall (n=111) |
Men (n=57) |
Women (54) |
|
| Age | ||||||
| 18–29 | 21.7 | 20.8 | 22.6 | 21.6 | 21.1 | 22.2 |
| 30–39 | 29.2 | 30.2 | 28.3 | 36.0 | 42.1 | 29.6 |
| 40–49 | 31.1 | 24.5 | 37.7 | 27.9 | 24.6 | 31.5 |
| 50 plus | 17.9 | 24.5 | 11.3 | 14.4 | 12.3 | 16.7 |
| Ethnicity | ||||||
| White | 67.0 | 60.4 | 73.6 | 67.6 | 61.4 | 74.1 |
| Black | 8.5 | 7.5 | 9.4 | 8.1 | 7.0 | 9.3 |
| Hispanic | 12.3 | 15.1 | 9.4 | 11.7 | 12.3 | 11.1 |
| Other | 12.3 | 17.0 | 7.5 | 12.6 | 19.3 | 5.6 |
| Education | ||||||
| High school or less | 40.0 | 42.3 | 37.7 | 45.5 | 48.2 | 42.6 |
| Some college or more | 60.0 | 57.7 | 62.3 | 54.5 | 51.8 | 57.4 |
| Marital Status | ||||||
| Married or lives with partner | 16.0 | 17.0 | 15.1 | 14.4 | 12.3 | 16.7 |
| Never married | 50.9 | 54.7 | 47.2 | 45.0 | 45.6 | 44.4 |
| Separated/widowed/divorced | 33.0 | 28.3 | 37.7 | 40.5 | 42.1 | 38.9 |
| Children < 18 in the home | ||||||
| Yes | 44.3 | 41.5 | 47.2 | 50.5 | 42.1 | 59.3 |
| Homeless during study | ||||||
| Yes | 34.9 | 26.4 | 43.4 | 36.0 | 33.3 | 38.9 |
| Controlled environment 30 days | ||||||
| Yes | 19.8 | 17.0 | 22.6 | 25.2 | 31.6 | 18.5 |
| # MI Sessions attended | 4.2** (.3) | 4.9 (.4) | 3.6** (.3) | 5.3 (.3) | 5.6 (.4) | 5.1 (.4) |
| # Outpatient Sessions attended | 14.7 (1.1) | 16.4 (1.7) | 13.0 (1.5) | 14.3 (1.0) | 14.9 (1.4) | 13.7 (1.3) |
| Baseline ASI Psychiatric Severity | .31*** (.02) | .26* (.03) | .35** (.03) | .40 (.02) | .36 (.03) | .44 (.02) |
Indicates a significant difference, either overall or within gender, between treatment conditions at the .05, .01, .001 level
Note:
# MI Sessions attended includes attendance at nutrition groups for participants in the comparison condition.
Attendance at outpatient therapy groups during the study did not differ between the study conditions. However, those in the Intensive MI condition and, in particular, women attended significantly more MI sessions compared to nutrition sessions in the Standard MI condition. In addition, baseline psychiatric severity was significantly higher for both men and women in the Intensive MI condition compared to the Standard MI condition.
3.2 Methamphetamine use and drug severity
Table 2 shows univarite averages at each interview for primary outcomes as well as estimates of the overall and gender-specific adjusted longitudinal treatment effects for each of the Standard (SMI) and Intensive (IMI) conditions. For both the SMI and IMI conditions, univariate average PDA estimates increase from baseline to the 2-month follow-up with little additional change between subsequent 4-and 6-month follow-ups. Additionally, no differences in average PDA estimates were found between SMI and IMI conditions for any of the interviews. Estimates of the adjusted longitudinal average treatment effects indicated significant increases in PDA for each of the SMI and IMI conditions both overall as well as for men and women separately. However, no differential increases in PDA were found for the IMI compared to the SMI condition. Concordance between self-reported drug use and urine screens ranged from 86.5% to 90% across data collection time points.
Table 2.
Outcomes by treatment group and gender and estimates of the adjusted longitudinal effect
| Time of Assessment Unadjusted Means |
||||||
|---|---|---|---|---|---|---|
| Primary Outcome Measure | Baseline Mean (SE) |
2 Months Mean (SE) |
4 Months Mean (SE) |
6 Months Mean (SE) |
Adjusted Effect& (SE) |
|
| Percent Days Abstinent | ||||||
| Standard MI (MI1) | Overall | .55 (.04) | .74 (.04) | .76 (.03) | .78 (.03) | .22††† (.04) |
| Men | .63 (.05) | .80 (.05) | .82 (.04) | .83 (.04) | .20††† (.05) | |
| Women | .46 (.05) | .67 (.05) | .71 (.05) | .73 (.05) | .24††† (.06) | |
| Intensive MI (MI9) | Overall | .56 (.04) | .74 (.03) | .75 (.03) | .75 (.03) | .20††† (.04) |
| Men | .66 (.05 | .82 (.04) | .81 (.04) | .82 (.03) | .17††† (.04) | |
| Women | .45 (.05) | .65 (.05) | .70 (.05) | .67 (.05) | .23††† (.06) | |
| ASI Drug Score | ||||||
| Standard MI (MI1) | Overall | .26 (.01) | .19 (.01) | .17 (.01) | .18 (.01) | −.08††† (.01) |
| Men | .23 (.01) | .16 (.02) | .14 (.02) | .15 (.02) | −.08††† (.02) | |
| Women | .29 (.01) | .22 (.02) | .20 (.02) | .21 (.02) | −.07††† (.02) | |
| Intensive MI (MI9) | Overall | .27 (.01) | .22 (.01) | .19 (.01) | .18 (.01) | −.06††† (.01) |
| Men | .24 (.01) | .19 (.01) | .17 (.01) | .15 (.01) | −.06††† (.02) | |
| Women | .30 (.01) | .24 (.02) | .22 (.02) | .22 (.02) | −.07††† (.02) | |
| ASI Psychiatric Status Score | ||||||
| Standard MI (MI1) | Overall | .31*** (.02) | .32 (.02) | .29 (.01) | .28 (.01) | −.01 (.02) |
| Men | .26* (.03) | .29 (.03) | .24 (.03) | .23 (.03) | −.01 (.03) | |
| Women | .35** (.03) | .34 (.03) | .33 (.03) | .32 (.03) | −.02 (.03) | |
| Intensive MI (MI9) | Overall | .40 (.02) | .37 (.02) | .34 (.02) | .32 (.02) | −.06† (.02) |
| Men | .36 (.03) | .32 (.03) | .28 (.03) | .27 (.03) | −.07† (.03) | |
| Women | .44 (.02) | .42 (.03) | .40 (.03) | .37 (.03) | −.05 (.03) | |
Adjusted treatment effect was estimated as the average outcome difference between the separate 2, 4, and 6 month follow-ups and baseline controlling for gender (in overall models), age, number of outpatient sessions attended, number of MI sessions, and time-varying ASI psychiatric severity (except for analyses of ASI psych as the outcome). No differential adjusted effects between MI1 and MI9 treatments were observed.
Indicates a significant difference, either overall or within gender, between treatment conditions for a specific interview at the .05, .01, .001 level
Indicates a significant adjusted within-treatment effect averaged over time at the .05, .01, .001 level
Similar effects were found for the ASI drug outcome as were found for PDA. At each interview, no univariate average differences between SMI and IMI conditions were found either overall or within gender. Differences from baseline were also found to be homogeneous for each of the 2-, 4-, and 6-month interviews for both the SMI and IMI conditions overall as well as by gender. In addition, average ASI drug significantly decreased from baseline to each of the 2-, 4-, and 6-month follow-ups for each of the SMI and IMI conditions but with no differential reduction across the two treatment groups.
3.3 Psychiatric symptoms
Somewhat different results were found for ASI psychiatric status, where both men and women in the IMI condition were significantly more severe than corresponding study participants in the SMI condition at baseline. No subsequent differences between conditions were found at the 2-, 4-, and 6-month interviews. This observed difference prompted the inclusion of ASI psychiatric score as a control variable in the longitudinal analyses of PDA and ASI drug reported above. Longitudinal adjusted effect estimates indicated that ASI psychiatric score was significantly reduced from baseline to follow-ups for the IMI but not the SMI group, a result found to be driven primarily by the men.
Analogous results for the secondary outcomes used anxiety and depression status as well as the number of days the respondent experienced psychiatric problems. For anxiety status, no differences between SMI and IMI conditions were found at any interview. In addition, no adjusted longitudinal effect was found for either condition.
Women in the IMI condition were significantly more likely to report experiencing depression at baseline and men more likely to report depression at the first follow-up. Adjusted longitudinal effect estimates combining men and women together (overall analysis) indicated a significant reduction in depression for those assigned to the IMI condition. No reduction of depression was found for among participants in the SMI condition. In fact, there was a directional increase in depression among women assigned to the SMI condition. When we compared women in the two study conditions we found the likelihood of depression was significantly lower in the IMI than the SMI condition. However, men in both conditions showed significant improvement on depression and there were no between condition effects.
Finally, the number of days the respondent experienced psychiatric problems was found to be significantly higher at baseline in the IMI than the SMI conditions (mirroring the finding for ASI psychiatric score). In addition, a significant reduction in the days of psychiatric problems was found for both men and women in the IMI (but not the SMI) condition and the corresponding reductions were found to be significantly larger in the IMI compared to the SMI condition.
4. Discussion
Our primary hypotheses suggested IMI would reduce days of MA use, overall drug severity, and psychiatric severity more than SMI combined with nutritional education. However, there were no significant differences between the two study conditions on MA use or drug severity. Individuals in both study conditions significantly reduced days of MA use and had lower ASI drug severity scores at follow-up than at baseline.
There was significant improvement on the ASI psychiatric severity scale for individuals assigned to IMI but not for those assigned to SMI and nutrition education. When we examined psychiatric severity further we found a significant reduction in number of days experiencing depression over the past 30 days for individuals assigned to IMI but not those assigned to SMI and nutrition. Significant reduction of depression among men in IMI and increases in depression among women in the SMI condition accounted for these differences. There was also a significant reduction in number of days experiencing any psychiatric symptoms among participants assigned to IMI but not among those assigned to SMI plus nutrition education. Differences between the two study conditions were significant on this measure.
Although our primary hypotheses centered on reductions in MA use, ASI drug severity, and psychiatric severity, we also compared IMI and SMI with other ASI measures and found no significant differences between the two MI conditions. The lack of findings for IMI for ASI alcohol severity contrasts with a previous analysis that examined alcohol problems among women who also presented with MA dependence.31 Results from that analysis examined only participants with alcohol problems, defined as ASI score>0 at baseline or one positive symptom for alcohol dependence on the DSM-IV, and found that women receiving IMI showed significantly larger reductions in drinking and drinking related problems at 6-month follow-up compared to women in the SMI condition.
4.1 Treatment as Usual
The improvements in days of MA use and severity of drug problems found in both study conditions are likely due in part to the effects of both interventions, but also in part to the effects of the standard outpatient treatment that all study participants received. The intervention used (CIM) has been previously studied and shown to be effective for the treatment of MA dependence. CIM was one of the treatment interventions used in a multi-center study of treatment effectiveness for MA dependence.32 Individuals assigned to the CIM condition showed significant longitudinal improvements on a variety of measures, including MA use, that were comparable to the Matrix Model intervention being studied.
Although CIM is quite different from MI based interventions, the treatment staff who delivered CIM had training in MI and used MI-based techniques in the group when they determined it would be helpful. Thus, to some degree all study participants might have benefited from MI as it transpired in group sessions. To enhance attendance and engagement participants were contacted by phone or mail when they missed group, IMI, or nutrition education sessions and encouraged to attend the next session. These contacts appeared to be helpful in terms of increasing involvement in treatment and they were often experienced as supportive.
4.2 Assessment Reactivity
Assessment reactivity refers to the contention that assessment interviews for research purposes can have a therapeutic effect and thereby reduce differences between interventions being studied. This idea has received increasing attention in recent years and empirical investigations examining assessment reactivity have provided evidence of clinical benefits resulting from research interviews.33 Assessment reactivity may be particularly salient in our study because of the frequency, comprehensiveness and quality of the research interviews. Research staff interviewed participants weekly during the active phase of treatment (8 weeks) to assess MA use. Extensive interviews assessing a wide range of substance use and related areas were discussed at baseline and 2-, 4-, and 6-month follow-up. Research interviewers were trained to interact with participants in a nonjudgmental, supportive manner that is consistent with an MI style of counseling. In addition to collecting data, research interviewers were helpful to participants in terms of coordinating times for them to meet with study therapists and referring them to outside services as needed.
4.3 Standard MI
Our study design did not have a “standard treatment only” condition. It is therefore not possible to accurately parse out the relative effects of IMI and SMI on participant improvement separate from standard treatment. However, one possibility is that SMI had a substantive beneficial impact on reduction of MA use and related problems that was not improved by the more intensive IMI intervention. Originally, MI was developed as a brief intervention designed to enhance motivation for change among problem drinkers early in the treatment process. Miller34 suggested that a brief dose of MI for problem drinkers, even a single session, can have a substantive therapeutic impact. He also posited that increasing the intensity of MI does little to improve drinking outcome. One explanation for our findings is that this might be the case for MA use as well.
Comments from a focus group of study therapists and adherence monitors participating in this project supported the view that a single session of MI can be helpful.35 They indicated that SMI was able to condense a wealth of important material into a session that appeared to be well received by participants. On the other hand, they noted that many clients assigned to SMI were disappointed they could not receive more sessions. When we asked participants at 6 months whether the number of individual sessions they received was about right, too many, or too few, 76% of those in IMI and 80% of those in SMI indicated too few.
Another way to interpret the lack of an effect of IMI on MA use is that it adds to a growing body of literature showing mixed findings for MI-based interventions for drug problems. Although meta-analyses1,2 have found MI-based interventions to be effective for both alcohol and drug problems, the findings for alcohol are stronger. Further, a number of large randomized trials of MI-based interventions for populations that included illicit drug disorders did not find an effect on drug use.10,15,11,12,14 To the extent that MI does not impact illicit drug use it might not matter if the intensity increases.
4.4 Nutrition Education
In addition to factual information about nutrition, the nutrition education intervention included discussions about weight management, diet, and exercise. Although we did not collect data relevant to changes in diet and exercise, anecdotal observations from the group leader and other treatment staff suggest some participants made significant lifestyle changes in these areas. MA users tend to have poor dietary and oral hygiene habits36 and the nutrition sessions may have been helpful in addressing these issues. Staff members observed that some participants, particularly women, used MA to lose or maintain weight. As they decreased MA use they needed alternative weight management strategies and the group may have been helpful in that regard. Thus, the nutrition education intervention might have had unanticipated benefits for some participants. The nutrition education intervention might have been helpful for other body related issues as well. In a previous study of psychiatric disorders among individuals with MA dependence we found women reported high rates of somatoform disorders, bulimia and hypochondriasis relative to men. It is conceivable that these disorders play a role in driving MA use and that an intervention addressing nutrition and diet could reduce their influence on MA use.37 Investigating interventions designed to address issues related to diet and health, such as our nutrition intervention, warrants further study.
4.5 Limitations
One limitation of the study was attrition between screening and the baseline assessment. About 55% of those found to be eligible attended the baseline interview and we do not know the characteristics of others who did not attend or how they may have differed from those who were ultimately randomized.
Other limitations of the study relate to the design issues discussed above that made it difficult to find between condition effects. One example includes the use of a very strong treatment as usual intervention that all study participants in both conditions received. Another is the effect of assessment reactivity, which was pronounced because research interviews were conducted on a weekly basis.
There are other limitations as well. First, outcomes represent MA dependent individuals in the greater San Francisco Bay Area and might not generalize generalization to other geographic areas. Second, although outpatient treatment was provided free of charge by the study, participants had to be willing and able to travel to attend treatment and research interviews at least three times per week. Third, assessment of MA use was restricted to self-report and the length of follow-up assessment was limited to 6-months. Follow up interviews at later time points might result in different findings. Finally, psychiatric severity was higher at baseline for individuals assigned to the IMI condition, which left more room for improvement on psychiatric measures.
4.6 Differential Effects of IMI
One of the most concerning aspects of MA dependence is the severity of psychiatric problems that accompany it. Our findings suggest that these problems can be substantively improved by providing IMI to MA dependent clients. A limitation of SMI is that it did not result in improvement of psychiatric problems. This finding is consistent with other studies of MI for substance misuse and serious psychiatric comorbidity.38–40 The importance of addressing psychiatric disorders among MA dependent persons is evident in the high rates of psychiatric problems among persons seeking help for MA dependence and the finding that severity of these problems is associated with outcome.41
Our results showing that a more intensive dose of MI improved psychiatric problems more than a lower dose is consistent with studies from the general psychotherapy literature showing dose effects for psychotherapy and psychiatric disorders.42–44 However, IMI appears to have inconsistent effects across various problems. Contrary to our expectations, there was no effect of IMI on MA use, ASI drug severity, or other ASI scales (medical, legal, employment, and family). Nevertheless, previous analyses of these data showed women with coexisting alcohol problems and MA dependence reduced alcohol problems more if they were assigned to IMI than the comparison condition.31 Taken together, the findings suggest additional research needs to be conducted to decipher when IMI does and does not add benefit over SMI and for whom.
Table 3.
Secondary Outcomes by treatment group and gender and estimates of the adjusted longitudinal effect
| Time of Assessment Unadjusted Means |
||||||
|---|---|---|---|---|---|---|
| Secondary Outcome Measure | Baseline Mean (SE) |
2 Months Mean (SE) |
4 Months Mean (SE) |
6 Months Mean (SE) |
Adjusted Effect& (SE) |
|
| Anxiety Status | ||||||
| Standard MI (MI1) | Overall | .55 (.05) | .63 (.05) | .57 (.05) | .48 (.05) | .06 (.28) |
| Men | .51 (.07) | .60 (.07) | .51 (.07) | .44 (.07) | −.01 (.39) | |
| Women | .58 (.07) | .65 (.07) | .62 (.07) | .53 (.07) | .12 (.41) | |
| Intensive MI (MI9) | Overall | .58 (.05) | .67 (.05) | .59 (.05) | .55 (.05) | .13 (.27) |
| Men | .54 (.07) | .59 (.07) | .56 (.07) | .54 (.07) | .08 (.37) | |
| Women | .61 (.07) | .75 (.07) | .62 (.07) | .57 (.07) | .20 (.40) | |
| Depression Status | ||||||
| Standard MI (MI1) | Overall | .55 (.05) | .37* (.05) | .35 (.05) | .36 (.05) | −.16 (.30) |
| Men | .38 (.07) | .27* (.07) | .30 (.07) | .23 (.06) | −.95† (.46) | |
| Women | .38** (.07) | .47 (.07) | .40 (.07) | .49 (.07) | .45 (.40)a | |
| Intensive MI (MI9) | Overall | .58 (.05) | .51 (.05) | .43 (.05) | .43 (.05) | −.67††(.28) |
| Men | .51 (.07) | .50 (.07) | .36 (.07) | .33 (.07) | −.72# (.39) | |
| Women | .63 (.07) | .53 (.07) | .50 (.07) | .53 (.07) | −.64 (.40) | |
| # Days Had Psych Problems | ||||||
| Standard MI (MI1) | Overall | 11.40** (1.11) | 11.76 (1.17) | 11.36 (1.14) | 11.88 (1.27) | .26 (1.30)b |
| Men | 8.55* (1.48) | 9.71 (1.54) | 10.02 (1.67) | 10.98 (1.87) | 1.63 (1.80)b | |
| Women | 14.25* (1.57) | 13.81 (1.73) | 12.62 (1.56) | 12.76 (1.74) | −1.13 (1.88) | |
| Intensive MI (MI9) | Overall | 15.70 (1.08) | 12.50 (1.18) | 12.72 (1.22) | 10.80 (1.08) | −3.67†† (1.27) |
| Men | 12.93 (1.47) | 10.46 (1.63) | 9.60 (1.51) | 7.98 (1.29) | −3.59† (1.73) | |
| Women | 18.63 (1.50) | 14.67 (1.67) | 16.04 (1.84) | 13.74 (1.67) | −3.77† (1.87) | |
Adjusted treatment effect was estimated as the average outcome difference between the separate 2, 4, and 6 month follow-ups and baseline controlling for gender (in overall models), age, number of outpatient sessions attended, number of MI sessions, and time-varying ASI psychiatric severity (except for analyses of ASI psych as the outcome).
Indicates a significant difference, either overall or within gender, between treatment conditions for a specific interview at the .10, .05, .01, .001 level
Indicates a significant adjusted within-treatment effect averaged over time at the .10, .05, .01, .001 level
Indicates a significant difference between adjusted treatment effects across treatment at the .10, .05 level
Acknowledgement
This work was supported by NIDA grant #R01 DA024714.
References
- 1.Burke BL, Arkowitz H, Menchola The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71:843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- 2.Hettema J, Steele J, Miller WR. Motivational interviewing. Ann Rev Clin Psychol. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- 3.Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65:1232–1245. doi: 10.1002/jclp.20638. [DOI] [PubMed] [Google Scholar]
- 4.Heather N. The public health and brief interventions for excessive alcohol consumption: the British experience. Addict Behav. 1996;21:857–868. doi: 10.1016/0306-4603(96)00040-8. [DOI] [PubMed] [Google Scholar]
- 5.Project MATCH Research Group. Matching alcoholism treatment to client heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- 6.Sellman JD, Sullivan PF, Dore GM, Adamson SJ, MacEwan I. A randomized controlled trial of motivational enhancement therapy (MET) for mild to moderate alcohol dependence. J Stud Alcohol. 2001;62:389–396. doi: 10.15288/jsa.2001.62.389. [DOI] [PubMed] [Google Scholar]
- 7.Bien TH, II, Miller WR, Tonigan JS. Brief interventions for alcohol problems: a review. Addiction. 1993;88:315–336. doi: 10.1111/j.1360-0443.1993.tb00820.x. [DOI] [PubMed] [Google Scholar]
- 8.Brown JM, Miller WR. Impact of motivational interviewing on participation and outcome in residential alcoholism treatment. Psychol Addict Behav. 1993;7:211–218. [Google Scholar]
- 9.Dunn C, Deroo L, Rivera FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction. 2001;96:1149–1160. doi: 10.1046/j.1360-0443.2001.961217253.x. [DOI] [PubMed] [Google Scholar]
- 10.Donovan DM, Rosengren DB, Downey L, Cox GB, Sloan KL. Attrition prevention with individuals awaiting publicly funded drug treatment. Addiction. 2001;96:1149–1160. doi: 10.1046/j.1360-0443.2001.96811498.x. [DOI] [PubMed] [Google Scholar]
- 11.Miller WR, Yahne CE, Tonigan JS. Motivational interviewing in drug abuse services: a randomized trial. J Consult Clin Psychol. 2003;71:754–763. doi: 10.1037/0022-006x.71.4.754. [DOI] [PubMed] [Google Scholar]
- 12.Mullins SM, Suerez M, Ondersman SJ, Page MC. The impact of motivational interviewing on substance abuse treatment retention in a randomized control trial of women involved with child welfare. J Subst Abuse Treat. 2004;27:51–58. doi: 10.1016/j.jsat.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Schneider RJ, Casey J, Kohn R. Motivational versus confrontational interviewing: a comparison of substance abuse assessment practices at employee assistance programs. J Behav Health Serv Res. 2000;27:60–74. doi: 10.1007/BF02287804. [DOI] [PubMed] [Google Scholar]
- 14.Winhusen T, Kropp F, Babcock D, et al. Motivational enhancement therapy to improve treatment utilization and outcome in pregnant substance users. J Subst Abuse Treat. 2008;35:161–173. doi: 10.1016/j.jsat.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll KM, Ball SA, Nich C, et al. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: a multisite effectiveness study. Drug Alcohol Depend. 2006;81:301–312. doi: 10.1016/j.drugalcdep.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polcin DL, Galloway GP, Palmer J, Mains W. The case for high-dose Motivational Enhancement Therapy. Substance Use and Misuse. 2004;39:331–343. doi: 10.1081/ja-120028494. [DOI] [PubMed] [Google Scholar]
- 17.Polcin DL, Brown M, Galloway GP. Intensive Motivational Enhancement Therapy Manual. Berkeley, CA: Alcohol Research Group; 2005. [Google Scholar]
- 18.Ball SA, Martino S, Nich C, et al. Site matters: multisite randomized trial of motivational enhancement therapy in community drug abuse clinics. J Consult Clin Psychol. 2007;75:556–567. doi: 10.1037/0022-006X.75.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galloway GP, Polcin DL, Kielstein A, Brown M, Mendelson J. J Psychoactive Drugs. SARC; 2007. A nine session manual of motivational enhancement therapy for methamphetamine dependence: adherence and efficacy; pp. 393–400. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 21.Martino S, Ball SA, Gallon SL, et al. Motivational Interviewing Assessment: Supervisory tools for enhancing proficiency Salem, OR: Northwest Frontier Addiction Technology Transfer Center, Oregon Health and Science University. 2006 [Accessed: 2013-02-05. Archived by WebCite® at http://www.webcitation.org/6EDD4BNKM]; [Google Scholar]
- 22.Nuro KF, Maccarelli L, Martino S, et al. Yale Adherence and Competence Scale (YACSII) Guidelines. West Haven, CT: Yale University Psychotherapy Development Center; 2005. [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4th ed. Washington, DC: American Psychiatric Publishing, Inc.; 2000. [Google Scholar]
- 24.Forman RF, Sivikis D, Montoya ID, Blaine J. Selection of a substance use disorder diagnostic instrument by the National Drug Abuse Treatment Clinical Trials Network. J Subst Abuse Treat. 2004;27:1–8. doi: 10.1016/j.jsat.2004.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobell LC, Sobell MB, Buchan G, Cleland PA, Fedoroff IC, Leo GI. Association for the Advancement of Behavior Therapy. New York, NY: 1996. The reliability of the Timeline Followback method applied to drug, cigarette, and cannabis use. [Google Scholar]
- 26.Cacciola JS, Alterman AI, McLellan AT, Lin Y-T, Lynch KG. Initial evidence for the reliability and validity of a "Lite" version of the Addiction Severity Index. Drug Alcohol Depend. 2007;87:297–302. doi: 10.1016/j.drugalcdep.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 27.McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: the Addiction Severity Index. The Journal of Nervous and Mental Disease. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 28.SPSS Inc. PASW Statistics 18. Chicago, IL: SPSS, Inc.; 2009. [Google Scholar]
- 29.Stata Corp. Stata Statistical Software: Release 13.0. College Station, TX: Stata Corporation; 2013. [Google Scholar]
- 30.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. 2nd ed. Oxford, UK: Oxford University Press; 2002. [Google Scholar]
- 31.Korcha RA, Polcin DL, Evans K, Bond JC, Galloway GP. Intensive motivational interviewing for women with concurrent alcohol problems and methamphetamine dependence. J Subst Abuse Treat. 2014;46:113–119. doi: 10.1016/j.jsat.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawson RA, Marinelli-Casey P, Burke C, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99:708–717. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 33.Clifford PR, Maisto SA, Davis CM. Alcohol treatment research assessment exposure subject reactivity effects: Part 1. Alcohol use and related consequences. J Stud Alcohol Drugs. 2007;68:519–528. doi: 10.15288/jsad.2007.68.519. [DOI] [PubMed] [Google Scholar]
- 34.Miller WR. Rediscovering fire: small interventions, large effects. Psychol Addict Behav. 2000;14:6–18. [PubMed] [Google Scholar]
- 35.Polcin DL, Sterling J, Brown T, Brown M, Buscemi R. [working paper] Client and therapist views about intensive and standard motivational interviewing. Emeryville, CA: Alcohol Research Group; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall TA, Cunningham M, Guzman-Armstrong S. Dietary habits of methamphetamine users. Journal of the American Dietetic Association. 2010;110:A44–A. [Google Scholar]
- 37.Polcin DL, Buscemi R, Nayak M, Korcha R, Galloway G. Sex differences in psychiatric symptoms among methamphetamine-dependent residents in sober living houses. Addict Disord Their Treat. 2012;11:53–63. doi: 10.1097/ADT.0b013e3182213ef1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cleary M, Hunt GE, Matheson S, Walter G. Psychosocial treatments for people with co-occurring severe mental illness and substance misuse: systematic review. Jounral of Advanced Nursing. 2009;65:238–258. doi: 10.1111/j.1365-2648.2008.04879.x. [DOI] [PubMed] [Google Scholar]
- 39.Cleary M, Hunt G, Matheson S, Siegfried N, Walter G. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database Systematic Reviews. 2008:CD001088. doi: 10.1002/14651858.CD001088.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addictive Behaviors. 2012;37:11–24. doi: 10.1016/j.addbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse MP, Ang A, Rawson RA. Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug Alcohol Rev. 2010;29:12–20. doi: 10.1111/j.1465-3362.2009.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howard KI, Kopta SM, Krause MS, Orlinsky DE. The dose-effect relationship in psychotherapy. Am Psychol. 1986;41:159–164. [PubMed] [Google Scholar]
- 43.Seligman MEP. The effectiveness of psychotherapy: The Consumer Reports study. Am Psychol. 1995;50:965–974. doi: 10.1037//0003-066x.50.12.965. [DOI] [PubMed] [Google Scholar]
- 44.Shadish WR, Matt GE, Navarro AM, Phillips G. The effects of psychological therapies under clinically representative conditions: a meta-analysis. Psychol Bull. 2000;126:512–529. doi: 10.1037/0033-2909.126.4.512. [DOI] [PubMed] [Google Scholar]