Abstract
Rationale
Receptor mechanisms underlying the in vivo effects of nicotinic acetylcholine receptor (nAChR) drugs need to be determined to better understand possible differences in therapeutic potential.
Objective
This study compared the effects of agonists that are reported either to differ in intrinsic activity (i.e., efficacy) at α4β2 nAChR in vitro or to have in vivo effects consistent with differences in efficacy. The drugs included nicotine, varenicline, cytisine, epibatidine, and three novel epibatidine derivatives 2′-fluoro-(4-nitrophenyl) deschloro-epibatidine (RTI-7527-102), 2′-fluorodeschloroepibatidine (RTI-7527-36), and 3′-(3″-dimethylaminophenyl)-epibatidine (RTI-7527-76).
Methods
Mice discriminated nicotine base (1 mg/kg base) from saline; other mice were used to measure rectal temperature.
Results
In the nicotine discrimination assay, the maximum percentage of nicotine-appropriate responding varied: 92% for nicotine, 84% for epibatidine, 77% for RTI-7527-36, 71% for varenicline, and significantly less for RTI-7527-76 (58%), RTI-7527-102 (46%), and cytisine (33%). Each drug markedly decreased rectal temperature by as much as 12 °C. The rank order potency in the discrimination and hypothermia assays was epibatidine > RTI-7527-36 > nicotine > RTI-7527-102 > varenicline = cytisine = RTI-7527-76. The nAChR antagonist mecamylamine (3.2 mg/kg) antagonized the discriminative stimulus effects of epibatidine and RTI-7527-102, as well as the hypothermic effects of every drug except cytisine. The β2-subunit selective nAChR antagonist dihydro-β-erythroidine (DHβE; up to 10 mg/kg) antagonized hypothermic effects, but less effectively so than mecamylamine.
Conclusions
The marked hypothermic effects of all drugs except cytisine are due in part to agonism at nAChR containing β2 subunits. Differential substitution for the nicotine discriminative stimulus is consistent with differences in α4β2 nAChR efficacy; however, these results suggest that multiple nAChR receptor subtypes mediate the effects of the agonists.
Introduction
Nicotinic acetylcholine receptors (nAChR) are comprised of five protein subunits that form an ion channel and exist in multiple subtypes according to the composition and configuration of various isoforms of the protein subunits. The most prevalent nAChR subtypes in brain include α4β2, α6β2, α3β4, and α7 and subunits (Gotti et al. 2006). Nicotine is reported to have equally high efficacy at all four subtypes and binds with highest affinity at receptors containing β subunits (Grady et al. 2010). The results of studies with antagonists that are selective for receptors containing a β2-subunit, as well as studies in transgenic mice lacking β2-subunits, suggest that β2-subunits mediate the abuse and dependence liability of nicotine (Le Foll and Goldberg 2009; McGranahan et al. 2011).
Smoking cessation aids include nicotine products and other nAChR agonists including cytisine (Tabex®; Etter et al. 2008) and varenicline (Chantix®; Oncken et al. 2006). Nicotine, varenicline, and cytisine bind with high affinity at α4β2 nAChR receptors (Grady et al. 2010; Rollema et al. 2010). However, varenicline and cytisine have lower efficacy than nicotine at α4β2 nAChR receptors as evidenced by differences in maximum current evoked in cells transfected with human α4β2 nAChR receptors (Coe et al. 2005). Differences in α4β2 nAChR receptor efficacy are suggested to account for differences in the magnitude of effects on dopamine turnover (Coe et al. 2005) and discriminative stimulus effects in rodents trained to discriminate nicotine from saline (LeSage et al. 2009; Jutkiewicz et al. 2011; Cunningham and McMahon 2013). However, a contribution of nAChR subtypes other than α4β2 to the behavioral effects of nicotine, varenicline, and cytisine cannot be excluded.
Epibatidine, a naturally derived alkaloid with high affinity for α4β2 nAChR (Damaj et al. 1994), is the chemical template for the synthesis of novel nAChR ligands including 2′-fluorodeschloroepibatidine (RTI-7527-36), 3′-(3″-dimethylaminophenyl) epibatidine (RTI-7527-76), and 2′-fluoro-(4-nitrophenyl) deschloro-epibatidine (RTI-7527-102). These epibatidine derivatives were developed as high-affinity α4β2 nAChR ligands with potentially lower efficacy than epibatidine. In pre-clinical assays of analgesic and hypothermic effects in mice, RTI-7527-36 was more effective than RTI-7527-76 and RTI-7527-102 in producing antinociceptive effects, whereas all three epibatidine derivatives were equally effective in producing hypothermia (Carroll et al. 2004, 2005, 2010). When tested in a nicotine discrimination assay in rats, RTI-7527-102 did not mimic the effects of nicotine (Tobey et al. 2012). These effects in mice are consistent with in vitro data showing that RTI-7527-102 has low efficacy, as evidenced by electrophysiological responses at both human and rat α4β2 nAChR expressed in Xenopus oocytes (Abdrakhmanova et al. 2006; Ondachi et al. 2012). The efficacy of RTI-7527-36 and RTI-7527-76 at α4β2 nAChR in vitro has not been established.
The current study examined the extent to which the epibatidine derivatives RTI-7527-36, RTI-7527-76, and RTI-7527-102 share effects with prototypic high efficacy nAChR agonists (nicotine and epibatidine) or low efficacy α4β2 nAChR agonists (varenicline and cytisine) in male C57BL/6J mice. The nicotine discrimination assay used here is selective for nAChR agonism in vivo and is sensitive to the partial effects of varenicline and cytisine (Cunningham and McMahon, 2013). Moreover, nicotine discriminations also have utility for measuring pharmacological mechanisms underlying nicotine abuse. The marked hypothermic effects of nicotine and other nAChR agonists (Ankier et al. 1971; Hall and Myers 1971) were used in this study as a convenient method of determining a time course for each drug and to further examine nAChR mechanisms underlying discrimination versus hypothermia. Drugs were combined with the non-competitive nAChR antagonist mecamylamine and the α4β2 receptor-selective antagonist dihydro-β-erythroidine (DHβE).
Materials and Methods
Subjects
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were purchased for drug discrimination (n=12) and hypothermia (n=40) experiments at 8 weeks of age (approximately 15 g) and were housed individually (discrimination study) or 4 per cage (28 × 18 cm; height = 13 cm) (hypothermia study) on a 14/10-h light/dark cycle (lights on at 0600 h). Following habituation to the colony room for 7 days, mice used for the discrimination study were maintained at 85% of free-feeding weight and received up to 0.6 cc of 50% condensed milk during experimental sessions as well as 2.5 g of food (Dustless Precision Pellets 500 mg, Rodent Grain-Based Diet, Bio-Serv, Frenchtown, NJ) per day after sessions. Mice used for the hypothermia study had access to food (Harlan, Teklad 7912, Houston, TX) continuously in the home cage. Water was continuously available in the home cage for all mice. All experiments were conducted during the light period. Mice were maintained, and experiments were conducted in accordance with, the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio and the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, 2011).
Apparatus
Drug discrimination experiments were conducted in ventilated and sound-attenuating mouse operant conditioning chambers (MedAssociates, St. Albans, VT). The ceiling of each chamber contained a light. There were four recessed holes (each 2.2-cm diameter), three evenly spaced (5.5 cm apart) on one wall and the fourth in the center of the opposite wall. The center of each hole was positioned 1.6 cm from the floor. The wall with the single hole contained a dipper that could be filled with 0.01 cc of condensed milk. Each hole on the opposite wall contained a photo beam and a light. An interface (MedAssociates) connected the operant conditioning chambers to a computer, and experimental events were controlled and recorded with Med-PC software (Med Associates). Rectal temperature was measured with a probe (RET-3) attached to a Microcomputer Thermometer (7001H) (Physitemp Instruments, Inc., Clifton, NJ).
Drug discrimination
Experimental sessions were conducted once daily, 5-7 days per week. The session began with a 10-min timeout; responding during the timeout had no programmed consequence. The timeout was followed by a 15-min period of responding under a fixed ratio 10 of food delivery that was signaled by illumination of the lights inside the left and right nose-poke holes. Responding on the center hole had no programmed consequence. Ten responses (i.e., disruptions of the photobeam) on the correct hole resulted in 10-s access to 0.01 cc of milk from the hole on the opposite wall. During milk availability, the lights inside the nose-poke holes were extinguished, the ceiling light was illuminated, and disruption of the photobeams had no programmed consequence. The correct hole was determined by administration of nicotine or saline at the beginning of the sessions. Responses on the incorrect hole had no programmed consequence. The nicotine- and saline-associated holes were right and left, respectively, for six mice and the assignment was reversed for the other six mice. These assignments remained the same for an individual mouse throughout the study. The training sequence alternated between two consecutive days of nicotine and two consecutive days of saline. Periodically, training conditions were alternated daily for 3-4 days.
The first drug discrimination test was conducted after mice satisfied the test criteria, defined as five consecutive or six out of seven training sessions with at least 80% of the total responses occurring in the correct hole and fewer than ten responses occurring in the incorrect hole prior to delivery of the first reinforcer. Subsequent tests were conducted after performance satisfied the test criteria for 3 consecutive training sessions, including one saline and one nicotine training session. Test sessions were identical to training sessions except that ten responses in either hole resulted in access to milk and mice received saline, a dose of nicotine, or dose of test drug at the beginning of the timeout.
For novel experimental drugs, direct observation of behavior (i.e., immobility) was used to estimate behaviorally active doses. Dose-effect functions for nicotine, varenicline, cytisine, epibatidine, RTI-7527-36, RTI-7527-76, and RTI-7527-102 were determined in one group of mice (n=6) by administering all doses for an agonist in non-systematic order before determining the dose-effect function for the next agonist. The order of testing with agonists across mice was non-systematic. At the end of the study, the nicotine dose-effect function was determined a second time. In a second group of mice (n=6), dose-response functions for epibatidine and RTI-7527-102 were determined after administration of saline or mecamylamine (3.2 mg/kg). Saline or mecamylamine was administered 5 min before the timeout. Drugs were administered from ineffective doses up to doses that produced nicotine-appropriate responding or decreased response rate to less than 20% of the individual control.
Hypothermia
Drug tests were conducted in groups of 6 mice selected non-systematically from the group of 40 mice designated for the hypothermia study. The same mouse could not be selected twice for the same test to ensure that each test was conducted in 6 different mice. Individual mice were tested with drugs on an average of 17 times (range 8 – 28), and no mouse received the same drug test twice. Mice were tested in groups of 8 once every 7 days. Subjects were separated into individual containers for 1 h prior to measurement of rectal temperature. The ambient room temperature was 23 °C. Temperature was measured by inserting the probe 2 cm into the rectum. Following the baseline temperature reading, saline or a dose of DHβE was administered immediately followed by a second injection of saline or another drug. Mecamylamine was administered 5 min before the baseline temperature. Following post-baseline drug administration, temperature was measured at 10, 30, 60, 90, 120, 240, and 360 min. The order of testing with drugs was non-systematic with no more than two subjects receiving the same dose of drug on any test day. For each drug or drug combination, six mice were tested with each dose including at least one ineffective dose. If a drug or drug combination resulted in lethality, then larger doses were not studied. Some mice died after 10 mg/kg of cytisine alone or in combination with 3.2 mg/kg of DHβE, resulting in final sample sizes of 3 and 4, respectively.
Drugs
Figure 1 shows the chemical structure of each drug, except nicotine, included in the study. (–)-Nicotine hydrogen tartrate salt, cytisine and epibatidine dihydrochloride were obtained from Sigma Chemical (St. Louis, MO). Varenicline dihydrochloride was obtained from the Research Technology Branch of the National Institute on Drug Abuse (Rockville, MD), mecamylamine from Waterstone Technology (Camel, IN) and dihydro-β-erythroidine hydrobromide from Tocris (Minneapolis, MN). 2′-Fluorodeschloroepibatidine (RTI-7527-36), 3′-(3″-dimethylaminophenyl) epibatidine (RTI-7527-76), and 2′-fluoro-(4-nitrophenyl) deschloroepibatidine (RTI-7527-102) were synthesized at the Center for Organic and Medicinal Chemistry, Research Triangle Institute (Research Triangle Park, NC) according to methods previously described (Carroll et al., 2004, 2005, 2010). Drugs were dissolved in 0.9% physiological saline and administered s.c. (except mecamylamine which was administered i.p.) in a volume of 10 ml/kg. Nicotine dose is expressed in terms of the weight of the free base; dose of other drugs is expressed as the weight of the base and salt.
Figure 1.
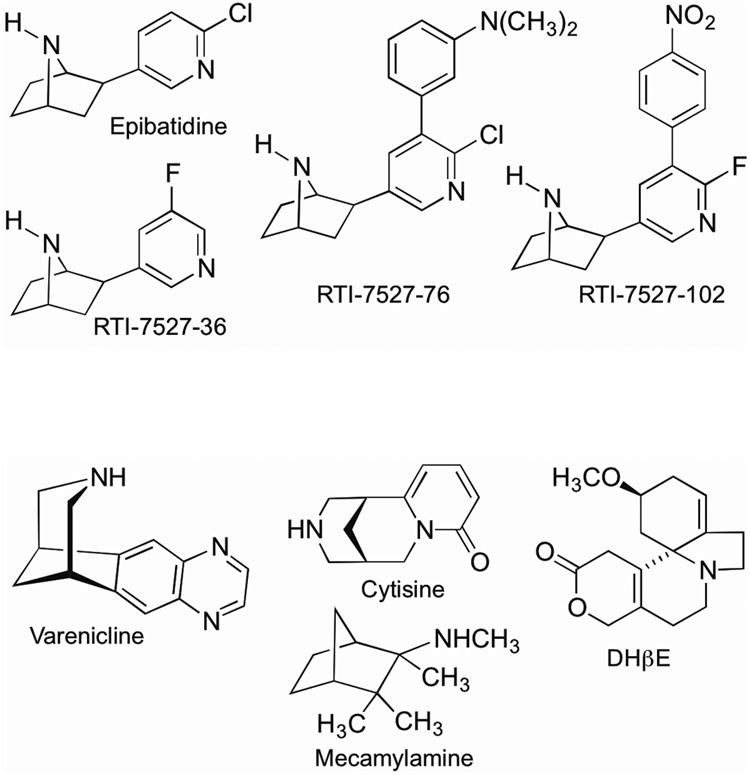
Structures of epibatidine, epibatidine analogs (RTI-7527-36, RTI-7527-76, and RTI-7527-102), varenicline, cytisine, mecamylamine, and DHβE.
Data analyses
All data were expressed as a mean ± S.E.M. Discrimination data were expressed as a percentage of nicotine-appropriate responses out of the total number of vehicle- and nicotine-appropriate responses. Response rate was expressed as the mean percentage of control. Control rate of responding (responses per s when milk was available under the FR10 schedule excluding responses during the 10-s period of milk availability) was calculated as the average rate for the preceding 5 saline training sessions. Response rate was expressed and analyzed as a percentage of the control for individual mice. Discrimination data for an individual subject were not included for analyses when response rate was less than 20% of the control for that subject; however, response rate data were always included in the group average.
Body temperature was plotted in °C and expressed as a function of dose or time. The maximum effect of each drug was selected from the mean time course data (0-120 min) and individual data at that time point were used for further analysis. To examine the antagonism of hypothermic effects by mecamylamine or DHβE, the area calculated from the hypothermia time course was calculated for each dose of drug, both when the dose was studied alone and when the dose was studied in combination with a dose of antagonist. The area was calculated from 0-120 min of the hypothermia time course and included the space between the saline control line and the line defined by a dose of drug. For each drug, the largest area calculated from the hypothermia time course at any dose (e.g., usually the largest dose studied) was defined as the maximum effect for that drug and the area for smaller doses was expressed as a percentage of the maximum.
Individual dose-response data were simultaneously fitted with straight lines by means of GraphPad Prism version 5.0 for Windows (San Diego, CA) with linear regression. The slopes of dose-effect curves were compared with an F-ratio test using GraphPad. If the slopes were not significantly different, then parallel line analysis of data from individual subjects with the common, best-fitting slope was used to estimate potency, potency ratios, and 95% confidence limits (Kenakin 1997; Tallarida 2000). For drug discrimination and response rate data, ED50 values were calculated for drugs producing a minimum of 50% effect. For hypothermic effects, potency was calculated as the dose decreasing temperature to 34 °C (i.e., ED34°C value). Potencies were considered significantly different when the 95% confidence limits of the potency ratio did not include 1. Maximum hypothermic and discriminative stimulus effects were compared with one-way analysis of variance and Tukey's post hoc test. To determine whether mecamylamine or DHβE significantly modified dose-response functions of another drug, an F-ratio test was conducted to examine whether a single line could be fit to all of the data combined (i.e., no antagonism) or two lines were needed to describe the data (i.e., significant antagonism).
Results
Discriminative stimulus effects and rates of operant responding
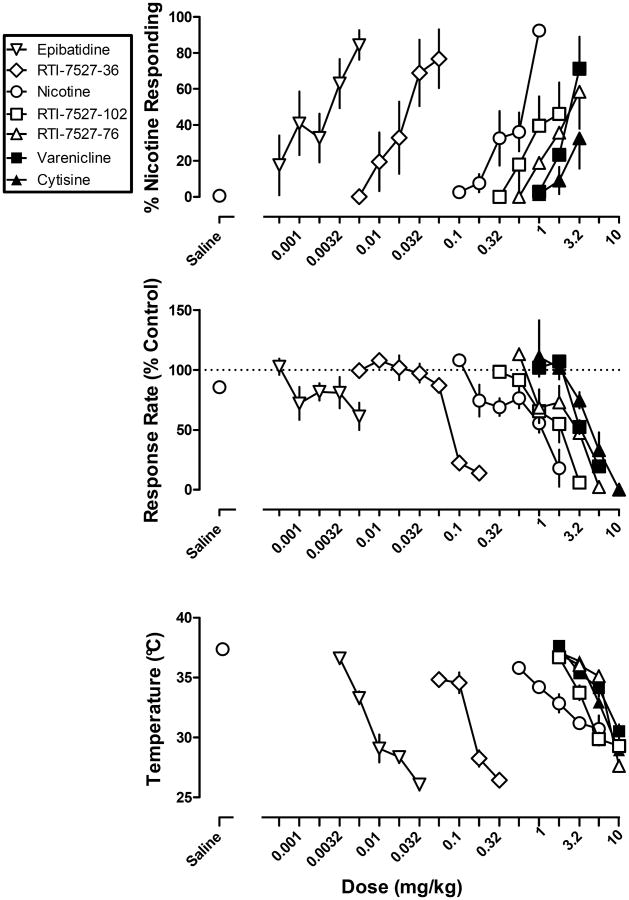
Mice acquired the discrimination of 1 mg/kg nicotine base from saline in a mean of 36 sessions, (range 16-75) including both nicotine and saline training sessions. Nicotine dose-dependently increased the percentage of responses on the nicotine-appropriate nose-poke hole to 92% (Figure 2 top, circles), whereas saline produced 1% nicotine-appropriate responses (Figure 2 top, Saline). The ED50 value (95% confidence limits) of nicotine to produce discriminative stimulus effects was 0.44 (0.26-0.74) mg/kg. Nicotine dose-dependently decreased response rate (Figure 2 middle, circles); the ED50 value (95% confidence limits) of nicotine to decrease response rate was 0.91 (0.61-1.35) mg/kg (Table 1).
Figure 2.
Discriminative stimulus effects (top), rate-decreasing effects (middle), and hypothermic effects (bottom) of nAChR drugs. Abscissae: dose in milligram per kilogram body weight. Ordinates: percentage of nicotine-appropriate responses (top), response rate expressed as a percentage of the control rate of responding (middle), and rectal temperature measured in °C (bottom).
Table 1.
ED50 values for discriminative stimulus, rate-decreasing effects, and ED34°C values for hypothermia and corresponding 95% confidence limits. Potency ratios are calculated with epibatidine as a reference, e.g., ED34°C values were divided by the ED34°C values for epibatidine.
| % Nicotine responding | Decreases in response rate | Hypothermia | ||||
|---|---|---|---|---|---|---|
| Drug | ED50 values in mg/kg (95% confidence limits) | Potency ratio (95% confidence limits) | ED50 values in mg/kg (95% confidence limits) | Potency ratio (95% confidence limits) | ED34°C values in mg/kg (95% confidence limits) | Potency ratio (95% confidence limits) |
| Epibatidine | 0.002 (0.001-0.003) | 0.004 (0.002-0.008) | 0.005 (0.004-0.006) | |||
| RTI-7527-36 | 0.023 (0.008-0.062) | 13.0 (3.4-59.2) | 0.11 (0.05-0.22) | 26.4 (14.9-54.6) | 0.07 (0.06-0.09) | 14.3 (11.5-17.8) |
| Nicotine | 0.44 (0.26-0.74) | 248 (108-606) | 0.91 (0.61-1.35) | 217 (116-378) | 1.3 (1.1-1.6) | 347 (263-474) |
| RTI-7527-102 | n/a | n/a | 1.50 (1.01-2.24) | 359 (160-642) | 3.0 (2.6-3.5) | 608 (507-739) |
| RTI-7527-76 | 2.29 (0.80-6.51) | 1290.9 (429.0-7312.7) | 2.52 (1.62-3.92) | 601 (251-1106) | 4.2 (3.5-5.0) | 846 (674-1090) |
| Cytisine | n/a | n/a | 5.62 (3.36-9.38) | 1341 (739-2435) | 4.2 (3.8-4.6) | 850 (709-1040) |
| Varenicline | 2.53 (0.98-6.51) | 1427 (413-7727) | 2.75 (1.55-4.88) | 657 (338-2435) | 4.6 (4.1-5.2) | 994 (775-1160) |
Epibatidine, RTI-7527-36, varenicline, and RTI-7527-76 dose-dependently increased nicotine-appropriate responding to a maximum of 84% at 0.0056 mg/kg of epibatidine, 77% at 0.056 mg/kg of RTI-7527-36, 71% at 3.2 mg/kg of varenicline, and 58% at 3.2 mg/kg of RTI-7527-76. The ED50 values for nicotine-like discriminative stimulus effects were 0.002 mg/kg for epibatidine, 0.023 mg/kg for RTI-7527-36, 2.53 mg/kg for varenicline and 2.29 mg/kg for RTI-7527-76 (Table 1). The maximum percentage of nicotine-appropriate responding produced by RTI-7527-102 and cytisine was 46% and 33%, respectively; ED50 values were not calculated for these two drugs. Nicotine, epibatidine, and RTI-7527-36 produced significantly more percent nicotine- appropriate responding than varenicline, RTI-7527-76, -102 and cytisine (F(6, 41)=3.69, p<0.01). All of the drugs, with the exception of epibatidine, were tested up to doses that decreased rate of responding to less than 20% control (Figure 2 middle). The ED50 values for rate-decreasing effects were 0.004 mg/kg for epibatidine, 0.11 mg/kg for RTI-7527-36, 1.50 mg/kg for RTI-7527-102, 2.52 mg/kg for RTI-7527-76, 2.75 mg/kg for varenicline and 5.62 mg/kg for cytisine.
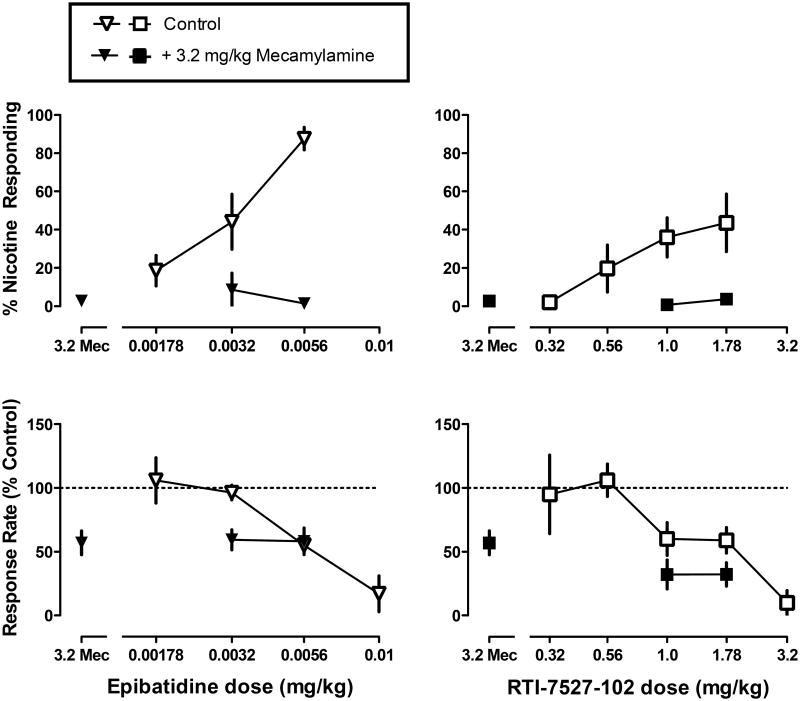
When administered alone, mecamylamine (3.2 mg/kg) produced 3% nicotine-appropriate responding and significantly decreased response rate to 57% of control (Figure 3 top and bottom, respectively, data above 3.2 Mec). Mecamylamine significantly antagonized the discriminative stimulus effects of epibatidine and RTI-102 (p<0.05), but not the rate-decreasing effects of epibatidine and RTI-102 (Figure 3 left and right, respectively).
Figure 3.
Discriminative stimulus effects (top) and rate-decreasing effects (bottom) of epibatidine (left) and RTI-7527-102 (right), alone and in combination with mecamylamine. Abscissae: mecamylamine (Mec) alone (3.2 mg/kg) or dose in milligram per kilogram body weight. Ordinates: percentage of nicotine-appropriate responses (top) and response rate expressed as a percentage of the control rate of responding (bottom).
Hypothermic effects: potency and time course
Epibatidine, RTI-7527-36, nicotine, RTI-7527-102, cytisine, varenicline, and RTI-7527-76 dose-dependently decreased rectal temperature (Figure 2 bottom). Up to the largest doses that were not lethal, epibatidine and RTI-7527-36 produced significantly greater hypothermia (F(6, 137)=12.75, p<0.0001) (maximum decrease of 12 °C) than nicotine (7.3 °C), RTI-7527-102 (8.7 °C), cytisine (9.1 °C), varenicline (7.5 °C), and RTI-7527-76 (10 °C). The slopes of the dose-effect functions expressed as absolute rectal temperature were not significantly different from each other. The rank order potency was epibatidine > RTI-7527-36 > nicotine > RTI-7527-102 (Table 1). These drugs were more potent than varenicline, cytisine, and RTI-7527-76, which were not different in potency from each other. In general, there was no systematic relationship between lethality and the capacity of a drug to produce hypothermia.
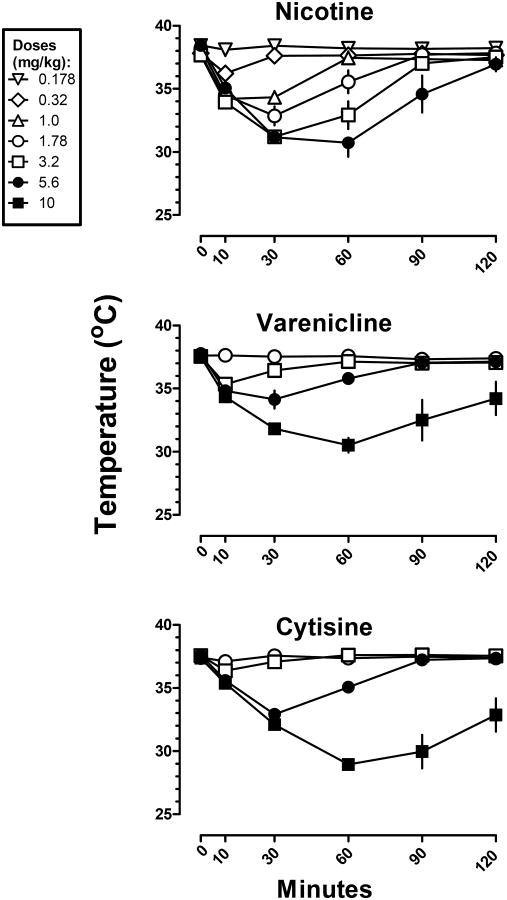
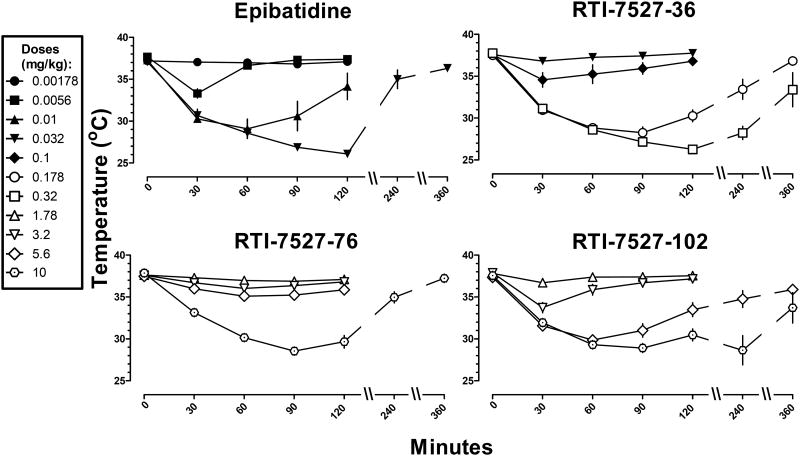
When examining hypothermic effects over time, there was a positive relationship between dose, duration of action and, in some cases, latency to maximum effect (Figures 4 and 5). Nicotine was ineffective at a dose of 0.178 mg/kg of (Figure 4 top). The next largest dose (0.32 mg/kg) of nicotine decreased temperature by 2° C at 10 min, and significant hypothermia at this dose was no longer observed at 30 min. The next larger doses (1 and 1.78 mg/kg) of nicotine increased both the magnitude (decrease of 4-5 ° C) and duration (at least 30 min) of hypothermia. The largest doses (3.2 and 5.6 mg/kg) of nicotine not only further increased the magnitude and duration of action of hypothermia, but also increased the latency to the maximum effect from 10 min to 30 min. Similar relationships between dose, magnitude, duration and latency to maximum effect were observed for all of the other compounds. When comparing equally effective doses, the duration of action of nicotine (3.2 and 5.6 mg/kg; Figure 4 top) was shorter than all of the other drugs including varenicline (10 mg/kg; Figure 4 middle) and cytisine (10 mg/kg; Figure 4 bottom). Epibatidine, RTI-7527-36, RTI-7527-76, and RTI-7527-102 had the longest delay to onset of maximum effect and duration of action (Figure 5).
Figure 4.
Time course of rectal temperature at various doses of nicotine, varenicline, and cytisine. Abscissae: time in minutes. Ordinates: rectal temperature measured in °C. Time 0 is rectal temperature measured immediately before drug administration.
Figure 5.
Time course of rectal temperature at various doses of epibatidine, RTI-7527-36, RTI-7527-76, and RTI-7527-102. Abscissae: time in minutes. Ordinates: rectal temperature measured in °C. Time 0 is rectal temperature measured immediately before drug administration.
Antagonism of hypothermic effects by mecamylamine and DHβE
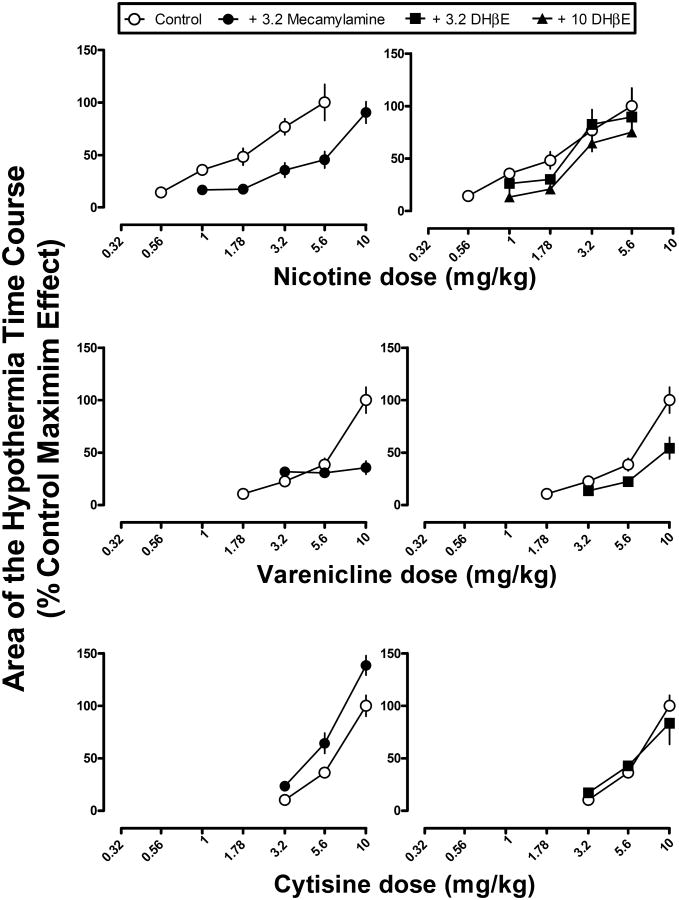
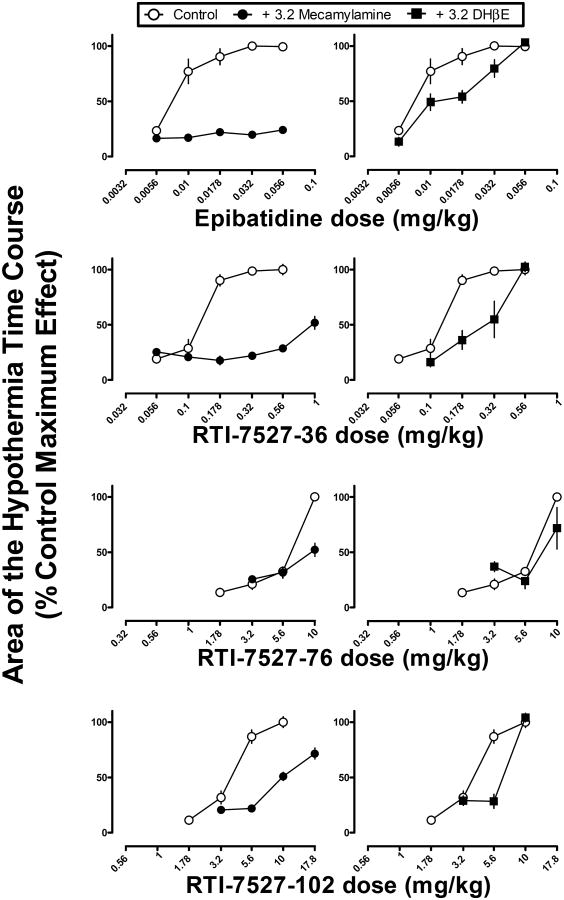
When studied alone, mecamylamine (3.2 mg/kg) and DHβE (3.2 or 10 mg/kg) did not modify temperature relative to saline (F(3,23)=1.42, p=0.27) (data not shown), although there was a tendency for mecamylamine to decrease body temperature by 0.9 °C at 30 min. Mecamylamine (3.2 mg/kg) antagonized the hypothermic effects of some but not all of the drugs. Mecamylamine produced a significant, parallel rightward shift in the nicotine dose-response curve (Figure 6 top left), as evidenced by a significant difference in the intercepts and not the slopes of the dose-response curves. A single line was not sufficient to fit the dose-effect data for nicotine alone and in combination with mecamylamine (Table 2). The ED50 values (95% confidence limits) calculated for the effect area were 1.5 (1.2-2.0) mg/kg for nicotine alone and 4.1 (3.5-5.2) mg/kg for nicotine in combination with mecamylamine (3.2 mg/kg). The potency ratio (95% confidence limits) was 2.7 (1.9-3.9). Mecamylamine (3.2 mg/kg) also significantly antagonized the hypothermic effects of varenicline, epibatidine, RTI-7527-36, RTI-7527-76, and RTI-7527-102 (Figures 6 and 7, left panels), as evidenced by significant F-ratio tests showing that the dose-effect functions were significantly different from each other for each respective drug. However, potency ratios were not calculated due to significant differences in slope in each case, i.e., the dose-response curves were not parallel. While the slopes and intercepts for cytisine in combination with mecamylamine were significantly different from each other, this was due to a leftward shift in the cytisine dose response curve (Figure 6, bottom left).
Figure 6.
Hypothermic effects of nicotine, varenicline, and cytisine alone and in combination with mecamylamine (left) or DHβE (right). Abscissae: dose of nicotine, varenicline, or cytisine in milligram per kilogram body weight. Ordinates: the area calculated from the hypothermia time course in Figure 3 and expressed as a percentage of the maximum effect for each respective drug.
Table 2.
The results of F-ratio tests examining the effects of mecamylamine or DHβE to modify the hypothermic effects of nicotine and related drugs. The F-ratio tests determine whether the two dose-response functions, i.e. the drug alone versus the same drug in combination with mecamylamine or DHβE, can be fitted with a single line. A significant F-value indicates that a single line cannot be used to fit the dose-response data, i.e. there is a significant effect of mecamylamine or DHβE to modify the position of the dose-response curve of another drug.
| F-ratio test of a significant shift in the dose-response curve for hypothermic effects | ||
|---|---|---|
| + Mecamylamine (3.2 mg/kg) | + DHβE (3.2 mg/kg) | |
| Nicotine | F(2,68)=18.78 p<0.05* | F(2,62)=2.91 p>0.05 |
| Varenicline | F(2,38)=16.37 p<0.05* | F(2,38)=9.13 p<0.05* |
| Cytisine | F(2,35)=15.13 p<0.05* | F(2,33)=0.59 p>0.05 |
| Epibatidine | F(2,74)=146.4 p<0.05* | F(2,68)=9.87 p<0.05* |
| RTI-7527-36 | F(2,68)=68.62 p<0.05* | F(2,56)=13.71 p<0.05* |
| RTI-7527-76 | F(2,38)=23.03 p<0.05* | F(2,38)=1.37 p>0.05 |
| RTI-7527-102 | F(2,50)=59.92 p<0.05* | F(2,38)=6.69 p<0.05* |
| Nicotine** | F(2,50)=8.86 p<0.05* | |
significant shift in dose-effect function
+ 10 mg/kg of DHβE
Figure 7.
Hypothermic effects of epibatidine, RTI-7527-36, RTI-7527-76 and RTI-7527-102 alone and in combination with mecamylamine (left) or DHβE (right). Abscissae: dose of epibatidine, RTI-7527-36, RTI-7527-76 or RTI-7527-102 in milligram per kilogram body weight. Ordinates: the area calculated from the hypothermia time course in Figure 4 and expressed as a percentage of the maximum effect for each respective drug.
Overall, DHβE was a less effective antagonist of hypothermic effects as compared with mecamylamine. A dose of 3.2 mg/kg of DHβE significantly antagonized the hypothermic effects of varenicline, epibatidine, RTI-7527-36, and RTI-7527-102 (Figures 6 and 7, righ panels and Table 2). However, inspection of the data shows that the magnitude of antagonism was less than that produced by mecamylamine (Figures 6 and 7, compare left and right panels within rows). For nicotine, a larger dose (10 mg/kg) of DHβE was needed to produce significant antagonism of hypothermic effects (Figure 6 top right). DHβE (3.2 mg/kg) did not significantly modify the hypothermic effects of cytisine or RTI-7527-76. Further tests with 10 mg/kg of DHβE were not conducted due to lethal effects of DHβE when studied in combination with cytisine.
Discussion
Nicotine, epibatidine, RTI-7527-36, and varenicline produced significantly higher percentages of drug-appropriate responding than RTI-7527-76, RTI-7527-102 and cytisine in male C57BL/6J mice discriminating nicotine (1 mg/kg). The discrimination data are consistent with the available data showing differences in efficacy at α4β2 nAChR (Abdrakhmanova et al. 2006; Rollema et al. 2010; Ondachi et al. 2012). Although in vitro electrophysiological estimates of nAChR efficacy are lacking for RTI-7527-36 and RTI-7527-76, the discrimination data suggest that they have relatively high and low α4β2 nAChR efficacy, respectively. Alternatively, differences in substitution could be due to involvement of multiple nAChR subtypes, a hypothesis that requires further evaluation with nAChR subtype-selective antagonists. Every agonist produced marked hypothermia. DHβE antagonized the hypothermic effects of every drug except cytisine and RTI-7527-36, suggesting that the hypothermic effects of most of the drugs were mediated at least in part by nAChR containing β2 subunits. The discrimination data suggest that differences in efficacy at nAChR containing β2 subunits could be a determinant of some behavioral effects; however, the strikingly similar effects of the agonists under some conditions, and limited antagonism of those effects by DHβE, suggest that an additional nAChR subtype mediates the effects of agonists.
A primary goal of the current study was to examine the extent to which the in vivo effects of nAChR drugs vary as a function of α4β2 nAChR agonist efficacy. Substitution for the nicotine discriminative stimulus was consistent with efficacy estimates in vitro. However, the magnitude of hypothermia did not vary with α4β2 nAChR efficacy estimates in vitro. Evidence for overlap in the nAChR receptor(s) mechanisms mediating discrimination and hypothermia is provided by the similar relative potency for producing both effects (epibatidine > RTI-7527-36 > nicotine > RTI-7527-76 ≈ varenicline) and antagonism of both effects by mecamylamine. Mecamylamine antagonized the hypothermic effects of all drugs except cytisine, as well as the discriminative stimulus effects of epibatidine and RTI-7527-102 in the current study and those of nicotine, varenicline, and cytisine in a previous study (Cunningham and McMahon 2013). The current results are not consistent with a single receptor type mediating discriminative stimulus and hypothermic effects. Hypothermia required larger doses and in turn higher nAChR efficacy than discrimination. Assuming mediation by a single receptor type, the greatest difference in maxima would have been expected under conditions of highest efficacy demand (Bergman et al. 2000). Instead, the current results suggest that different nAChR subtypes mediated the discriminative stimulus and hypothermic effects of the agonists.
The epibatidine derivatives tested here were less potent than epibatidine; there was a 14-fold increase in the ED50 value of RTI-7527-36 and marked increases in the ED50 values of RTI-7527-102 (608-fold) and RTI-7527-76 (846-fold). However, epibatidine and its derivatives had the same duration of action (4-6 h), which was longer relative to nicotine, varenicline, and cytisine. Epibatidine has a 7-azabicyclo[2.2.1]heptane structure to which is attached an exo-5-(2′-chloropyridinyl) substituent. RTI-7527-36 has the 2′-chloropyridinyl substituent in epibatidine replaced with a 3′-fluoropyridinyl group. RTI-7527-76 has a 3-dimethylaminophenyl substituent added to the 3′-position of the 2′-chloropyridinyl group in epibatidine. For RTI-7527-102, the 2′-chloropyridinyl substituent is replaced with a 2′-fluoropyridinyl group containing a 3′-(4-nitrophenyl). In vitro, RTI-7527-36 and RTI-7527-102 have higher binding affinity at αβ-containing nAChR subtypes as compared with epibatidine, and RTI-7527-76 is more selective than epibatidine for heteromeric αβ as compared with homomeric α7 nAChR subtypes (Carroll et al. 2004; 2005; 2010). The decreased potency of the epibatidine derivatives, as compared with epibatidine, appears to reflect lower nAChR efficacy and/or pharmacokinetics that result in lesser amounts in the brain. On the other hand, differences in substitution for the nicotine discriminative stimulus among drugs appears to be unrelated to pharmacokinetics inasmuch as all of the drugs produced a similar magnitude of hypothermia and had similar relative potency for producing hypothermia and decreases in operant response rate.
In mice trained to discriminate nicotine base (1 mg/kg), epibatidine (maximum 84% nicotine-appropriate responding), RTI-7527-36 (77%), and varenicline (71%) produced significantly greater nicotine-appropriate responding than RTI-7527-76 (58%), RTI-7527-102 (46%), and cytisine (33%). The current results with RTI-7527-102 concur with and extend previous findings in rats discriminating nicotine (Tobey et al. 2012). Varenicline, cytisine, and RTI-7527-102 have all been demonstrated to have lower efficacy than nicotine and epibatidine at α4β2 nAChR in vitro (Abdrakhmanova et al. 2006; Rollema et al. 2010; Ondachi et al. 2012). Moreover, the antinociceptive effects of RTI-7527-76 and RTI-7527-102, as compared with nicotine, are consistent with low efficacy (Carroll et al. 2010; 2004). One interpretation of the current results is that differences in α4β2 nAChR efficacy underlie varying degrees of substitution for the discriminative stimulus effects of nicotine, i.e., the lower efficacy of RTI-7527-76 and RTI-7527-102 as compared with nicotine and epibatidine, is an important determinant of in vivo effects. However, this interpretation is difficult to reconcile with the failure of DHβE to antagonize the discriminative stimulus effects of nicotine at the current training dose (1 mg/kg) of nicotine base, as demonstrated previously (Cunningham and McMahon, 2013). DHβE is assumed to be a competitive antagonist of nicotine at α4β2 nAChR. Therefore, nAChR receptors in addition to α4β2 could have differentially mediated the discriminative stimulus effects of the nAChR agonists tested here.
In vitro estimates of efficacy are often determined in cell lines transfected with a single subtype of nAChR and relatively large concentrations of drugs can be studied to establish the asymptote (i.e., maximal effect) of the response. There are multiple subtypes of nAChR in brain, nAChR ligands differ in binding affinity and efficacy at the various subtypes, and the relative contribution of any given subtype to a in vivo effect remains to be established. In drug discrimination experiments, a partial effect can be obtained at doses that also decrease response rate (Cunningham and McMahon 2013; Holtzman 2000; Jutkiewicz et al. 2011; LeSage et al. 2009), as was the case in the current study. For many drugs, decreases in response rate can be mediated by a receptor subtype(s) different from the subtype(s) mediating discriminative stimulus effects, as evidenced by antagonism of discriminative stimulus effects but not the rate-decreasing effects of drugs (Koek et al. 2004; Jutkiewicz et al. 2011; Platt and Bano 2011). Mecamylamine differentially antagonized the discriminative stimulus and rate-decreasing effects of nAChR agonists (current results; Cunningham and McMahon, 2013), implicating different underlying receptor mechanisms. While efficacy might be sufficiently high for all of the agonists to fully share discriminative stimulus effects, disruption of operant behavior by actions at a second nAChR prevents full substitution from being observed.
The failure of mecamylamine and DHβE to antagonize the hypothermic effects of cytisine was unexpected. The relative potency of cytisine to produce discriminative stimulus, rate-decreasing, and hypothermic effects was the same as that for all of the other nAChR agonists studied here. Moreover, mecamylamine was demonstrated previously to antagonize both the discriminative stimulus and rate-decreasing effects of cytisine (Cunningham and McMahon 2011; 2013). The mechanism responsible for the hypothermic effects of cytisine could be of some interest inasmuch as non-nicotinic mechanisms are involved in the effectiveness of cytisine as a smoking cessation aid.
The differential affinity and efficacy of nicotine and other drugs at multiple subtypes of nAChR provides a basis for developing drugs that differ in effect and perhaps therapeutic indication. However, firm relationships between nAChR subtypes and in vivo effects have been difficult to establish due, in part, to the lack of nAChR subtype-selective antagonists. By comparing the potency and maxima of nAChR agonists to produce in vivo effects, systematic relationships between in vivo effects, binding affinity, and efficacy might emerge. Here, the level of substitution for a nicotine discriminative stimulus was apparently related to differences in efficacy (intrinsic activity) at α4β2 nAChR; however, differential involvement of multiple nAChR subtypes cannot be excluded. The therapeutic utility of some smoking cessation drugs (e.g., varenicline) is proposed to be due to low efficacy at α4β2 nAChR. The current data suggest that RTI-7527-102 might exert a similar therapeutic profile. Moreover, given that nicotine has higher efficacy at α4β2 nAChR and is an effective smoking cessation aid, it is not unreasonable to consider the therapeutic potential of other high efficacy α4β2 nAChR agonists, especially epibatidine derivatives with lower potency and perhaps increased safety relative to epibatidine.
Acknowledgments
Funding: DA025267 to LRM
Abbreviations
- DHβE
dihydro-β-erythroidine
- nAChR
nicotinic acetylcholine receptor
- RTI-7527-36
2′-fluorodeschloroepibatidine
- RTI-7527-76
3′-(3″-dimethylaminophenyl)-epibatidine
- RTI-7527-102
2′-fluoro-(4-nitrophenyl) deschloro-epibatidine
- ANOVA
analysis of variance
Footnotes
Conflict of interest disclosure: All authors declare no conflict of interest.
References
- Abdrakhmanova GR, Damaj MI, Carroll FI, Martin BR. 2-Fluoro-3-(4-nitro-phenyl) deschloroepibatidine is a novel potent competitive antagonist of human neuronal alpha4beta2 nAChRs. Mol Pharmacol. 2006;69:1945–1952. doi: 10.1124/mol.105.021782. [DOI] [PubMed] [Google Scholar]
- Ankier SI, Brittain RT, Jack D. Investigation of central cholinergic mechanisms in the conscious mouse. Br J Pharmacol. 1971;42:127–136. doi: 10.1111/j.1476-5381.1971.tb07092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, France CP, Holtzman SG, Katz JL, Koek W, Stephens DN. Agonist efficacy, drug dependence, and medications development: preclinical evaluation of opioid, dopaminergic, and GABAA-ergic ligands. Psychopharmacology. 2000;153(1):67–84. doi: 10.1007/s002130000567. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Ware R, Brieaddy LE, Navarro HA, Damaj MI, Martin BR. Synthesis, nicotinic acetylcholine receptor binding, and antinociceptive properties of 2′-fluoro-3′-(substituted phenyl) deschloroepibatidine analogues. Novel nicotinic antagonist. J Med Chem. 2004;47:4588–4594. doi: 10.1021/jm040078g. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Brieaddy LE, Navarro HA, Damaj MI, Martin BR. Synthesis and pharmacological characterization of exo-2-(2′-chloro-5-pyridinyl)-7-(endo and exo)-aminobicyclo[2.2.1]heptanes as novel epibatidine analogues. J Med Chem. 2005;48:7491–7495. doi: 10.1021/jm058243v. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Ma W, Deng L, Navarro HA, Damaj MI, Martin BR. Synthesis, nicotinic acetylcholine receptor binding, and antinociceptive properties of 3′-(substituted phenyl) epibatidine analogues. Nicotinic partial agonists. J Nat Prod. 2010;73:306–312. doi: 10.1021/np9006124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Cunningham CS, McMahon LR. The effects of nicotine, varenicline, and cytisine on schedule-controlled responding in mice: differences in α4β2 nicotinic receptor activation. Eur J Pharmacol. 2011;654:47–52. doi: 10.1016/j.ejphar.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CS, McMahon LR. Multiple nicotine training doses in mice as a basis for differentiating the effects of smoking cessation aids. Psychopharmacology (Berl) 2013;228:321–333. doi: 10.1007/s00213-013-3037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Creasy KR, Grove AD, Rosecrans JA, Martin BR. Pharmacological effects of epibatidine optical enantiomers. Brain Res. 1994;664:34–40. doi: 10.1016/0006-8993(94)91950-x. [DOI] [PubMed] [Google Scholar]
- Etter JF, Lukas RJ, Benowitz NL, West R, Dresler CM. Cytisine for smoking cessation: a research agenda. Drug Alcohol Depend. 2008;92:3–8. doi: 10.1016/j.drugalcdep.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb Exp Pharmacol. 2009;192:335–367. doi: 10.1007/978-3-540-69248-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Riganti L, Vailati S, Clementi F. Brain neuronal nicotinic receptors as new targets for drug discovery. Curr Pharm Des. 2006;12:407–428. doi: 10.2174/138161206775474486. [DOI] [PubMed] [Google Scholar]
- Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Fedorov NB, McKinney S, Whiteaker P, Bencherif M, Lester HA, et al. Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 6 beta 2*-, alpha 3 beta 4*- and alpha 7-nicotine acetylcholine receptors. Neuropharmacology. 2010;58:1054–1066. doi: 10.1016/j.neuropharm.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GH, Myers RD. Hypothermia produced by nicotine perfused through the cerabral ventricles of the unanaesthetized monkey. Neuropharmacology. 1971;10:391–398. doi: 10.1016/0028-3908(71)90067-0. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Further characterization of the discriminative stimulus effects of spiradoline. Pharmacol Biochem Behav. 2000;66:517–522. doi: 10.1016/s0091-3057(00)00172-6. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Brooks EA, Kynaston AD, Rice KC, Woods JH. Patterns of nicotinic receptor antagonism: nicotine discrimination studies. J Pharmacol Exp Ther. 2011;339:194–202. doi: 10.1124/jpet.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin . Pharmacologic Analysis of Drug-Receptor Interaction. Lippincott Williams & Wilkins; New York: 1997. [Google Scholar]
- Koek W, Flores LR, Carter LP, Lamb RJ, Chen W, Wu H, Coop A, France CP. Discriminative stimulus effects of gamma-hydroxybutyrate in pigeons: role of diazepam-sensitive and -insensitive GABA(A) and GABA(B) receptors. J Pharmacol Exp Ther. 2004;308(3):904–11. doi: 10.1124/jpet.103.056093. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Ross JT, Carroll FI, Corrigall WA. Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacol Biochem Behav. 2009;91:461–467. doi: 10.1016/j.pbb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. α4β2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. J Neurosci. 2011;31:10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, Anziano R, Reeves K. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166:1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Ondachi P, Castro A, Luetje CW, Damaj MI, Mascarella SW, Navarro HA, Carroll FI. Synthesis and nicotinic acetylcholine receptor in vitro and in vivo pharmacological properties of 2′-fluoro-3′-(substituted phenyl) deschloroepibatidine analogues of 2′-fluoro-3′-(4-nitrophenyl) deschloroepibatidine. J Med Chem. 2012;55:6512–6522. doi: 10.1021/jm300575y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DM, Bano KM. Opioid receptors and the discriminative stimulus effects of ethanol in squirrel monkeys: Mu and delta opioid receptor mechanisms. Eur J Pharmacol. 2011;650(1):233–9. doi: 10.1016/j.ejphar.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Shrikhande A, Ward KM, Tingley FD, 3rd, Coe JW, O'Neill BT, Tseng E, Wang EQ, Mather RJ, Hurst RS, et al. Pre-clinical properties of the alpha4beta2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol. 2010;160:334–345. doi: 10.1111/j.1476-5381.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose-Effect Data Analysis. Chapman Hall/CRC Press; Boca Raton, FL.: 2000. [Google Scholar]
- Tobey KM, Walentiny DM, Wiley JL, Carroll FI, Damaj MI, Azar MR, Koob GF, George O, Harris LS, Vann RE. Effects of the specific α4β2 nAChR antagonist, 2-fluoro-3-(4-nitrophenyl) deschloroepibatidine, on nicotine reward-related behaviors in rats and mice. Psychopharmacology. 2012;223(2):159–168. doi: 10.1007/s00213-012-2703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]