Abstract
APK Advanced Medical Technologies (Atlanta, GA) is developing a sutureless beating heart (SBH) left ventricular assist device (LVAD) connector system consisting of anchoring titanium coil, titanium cannula with integrated silicone hemostatic valve, coring and delivery tool, and LVAD locking mechanism to facilitate LVAD inflow surgical procedures. Feasibility testing was completed in human cadavers (n=4) under simulated normal and hypertensive conditions using saline to observe seal quality in degraded human tissue and assess anatomic fit; acutely in ischemic heart failure (IHF) bovine model (n=2) to investigate short-term performance and ease of use; and chronically for 30-days in healthy calves (n=2) implanted with HeartWare HVAD to evaluate performance and biocompatibility. Complete hemostasis was achieved in human cadavers and animals at LV pressures up to 170 mmHg. In animals, off pump (no cardiopulmonary bypass) anchoring of the connector was accomplished in less than 1 minute with no residual bleeding after full delivery and locking of the LVAD; and implant of connector and LVAD were successfully completed in under 10 minutes with total procedure blood loss less than 100mL. In chronic animals prior to necropsy, no signs of leakage or disruption at the attachment site were observed at systolic LV pressures >200 mmHg.
Keywords: Heart failure, LVAD, ventricular apical access, cardiac assist, minimally invasive
INTRODUCTION
Left ventricular assist devices (LVAD) restore cardiac output in patients with advanced heart failure (HF) and have been shown to improve patient outcomes as bridge-to-transplant or destination therapy.1–5 Currently, LVAD require a major surgical implant procedure (sternotomy or thoracotomy) and cardiopulmonary bypass (CPB) support.6–9 CPB increases surgical implantation time, potential blood loss, and risk of exposure to donor blood products. Additionally, each manufacturer has a proprietary apical cannulation connector unique to their LVAD system. Typically, apical cannulation is achieved by suturing a connector ring to the apex of the native left ventricle (LV). The LV apex is then cored during CPB and LVAD inflow is anchored to the apical connector ring, which is a highly invasive procedure with significant blood loss and may require extensive time to complete implant and achieve homeostasis. The complexity and invasiveness of LVAD therapy has resulted in mortality (7% 30-day mortality post-LVAD implant per INTERMACS),10 longer hospitalization (~33 days),11,12 and increased costs.12–14 To overcome some of these limitations, APK Advanced Medical Technologies (Atlanta, GA) has developed a sutureless beating heart (SBH) LVAD connector system to facilitate less invasive and sutureless deployment and retrieval of the LVAD inflow for most clinically approved LVAD systems. Its unique design may enable less invasive surgical approaches (subcostal, mini-thoracotomy) for LVAD implant without the need for CPB, which may significantly reduce implantation time and blood loss, shorten the length of hospitalization, and decrease costs. In this study, feasibility of a prototype SBH LVAD connector system was tested in human cadavers and an ischemic HF (IHF) bovine model15 to develop implant procedure, assess anatomic fit, investigate ability to achieve homeostasis, evaluate hemocompatibility, and quantify performance metrics.
METHODS
Device Design
The APK Advanced Medical Technologies SBH connector system consists of a conical titanium coil, titanium cannula with silicone hemostatic valve, coring and delivery tool, and flexible locking tool (Figure 1). The conical coil is used to anchor the SBH connector and create a seal around the LVAD inlet (Figure 2). The larger outer ring of the coil is introduced into the LV apex first, and rotated with each sequentially smaller loop following the same path resulting in three-dimensional transmyocardial placement with compression directed radially-inward against the LVAD inlet to create a tight seal. A coring tool is then used to deploy the cannula with the hemostatic valve providing a secure and stable coupling of the LVAD to the LV apex. The locking mechanism is actuated using a flexible tool to provide a compression fitting between the deployed SBH connector and LVAD inflow cannula (Figure 3).
Figure 1.
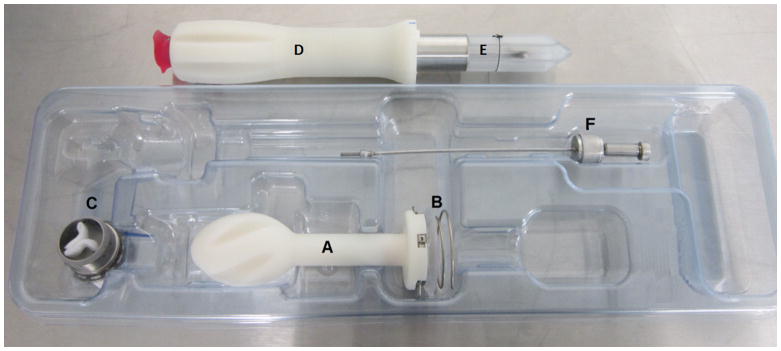
Photograph of the APK Sutureless Beating Heart (SBH) connector system tested in bovine animal model experiments that includes a locking tool (A); anchoring coil delivery handle (B); anchoring coil assembly (C); threaded titanium cannula with silicone valve (D); coring tool for extracting LV apical core (E); cannula loading protection plastic (F); flexible locking tool.
Figure 2.

(Left) Close up photograph of the SBH connector system with titanium cannula integrated with a one-way silicone valve, conical titanium coil assembly, coring tool and delivery handle. (Right). The elliptical titanium coil is inserted by rotating into the myocardium to provide secure coupling via radial compression forces (arrows).
Figure 3.

Fluoroscopy image of the SBH connector system with titanium conical coil embedded in LV myocardium providing a stable, hemostatically sealed platform for HeartWare HVAD in a chronic bovine model (left). Photo of the SBH system titanium connector with integrated silicone tri-leaflet valve placed in the LV apex of a human cadaver heart (right).
The anchoring coil assembly is composed of a titanium conical coil welded to a proximal titanium securing ring with a silicone flange interface and a radially locking screw (Figure 1B). The silicone flange serves as a soft and flexible apposition surface, which forms a seal with the epicardium. The delivery handle for the anchoring coil assembly is a Delrin® handle with a central pass though orifice used for passage of a centering guidewire, which serves as a rotational axle during deployment (Figure 1A). The delivery handle has a simple mechanical releasable interface to the anchoring coil assembly. The valved cannula consists of a silicone valve overmolded onto a titanium body with a bead-sintered tip. The coring tool has a distal piercing forward blade which is used to initially penetrate into the ventricle. Retractable semi-circular flaps are located above the piercing blade. When deployed in step two of the coring process, they serve as counter traction against the endocardial surface and secures the tissue plug within the coring blade. The deployed flaps also provide hemostasis after the initial piercing of the LV with the blade and allow for full thickness coring. Prior to the coring process, the valved cannula is pre-mounted in the operating room concentrically over the coring blade using a plastic loader. Clockwise rotation during the coring procedure attaches the valved cannula onto the anchoring coil assembly while simultaneously coring the ventricle. The LVAD inflow cannula is introduced into the ventricle through the valve cannula. Finally, a flexible stainless steel locking tool is used to rotate the integrated locking screw in the body of the anchoring coil securing both the valved cannula and LVAD inflow cannula as one single hemostatic attachment. The flexible shaft of the locking tool allows for transference of torque through the left thoracotomy window with the LVAD in place. Use of the SBH connector system is demonstrated in Supplemental Video Clip 1.
Study Objectives
Feasibility testing of the SBH connector system was completed in human cadavers (n=4) under simulated normal and hypertensive conditions using saline as working fluid to observe seal quality in degraded human tissue and assess anatomic fit. The SBH connector system was also tested acutely in an IHF bovine model15 (n=2) to investigate short-term performance and ease of use, and chronically for 30-days in healthy calves (n=2) implanted with HeartWare HVAD (HeartWare Inc., Miami Lakes, FL) to evaluate performance, biocompatibility, and hemocompatibility. The Institutional Animal Care and Use Committee at the University of Louisville approved the acute and chronic large animal study protocols.
Cadaver Study
An anatomical fit study of the SBH connector system was performed in human cadaver hearts (n=4) at the University of Louisville Fresh Tissue Lab. The duration, ease of use, and complexity of implanting the SBH connector system were evaluated using subcostal and mini-thoracotomy (intercostal space) surgical approaches. The heart was then excised from the cadaver with the SBH connector system to assess device placement and anatomical fit. The excised heart was filled with saline and instrumented with a fluid-filled pressure catheter (BD, Becton-Dickson, Franklin Lakes, NJ) placed in the left ventricle via insertion through the aortic root. Hypertension test conditions (> 150 mmHg) were simulated using a pressure infusor bag (Baxter, Deerfield, IL) to investigate valve integrity and hemostatic seal. A summary of step-by-step implant procedures in the human cadaver model is presented in Figure 4.
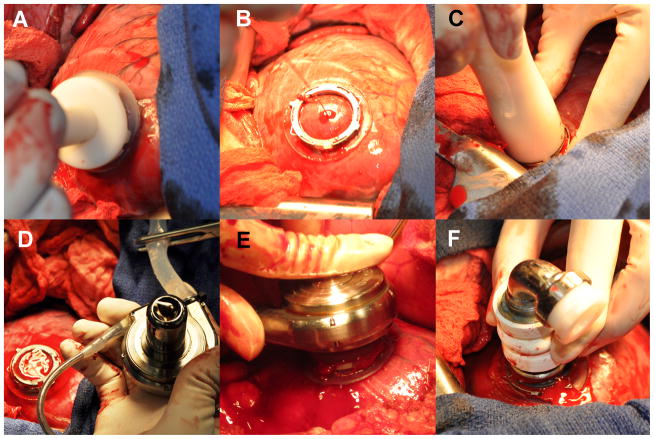
Figure 4.

Summary of the implant procedure for the SBH connector system as shown in a human cadaver model. A mini-thoracotomy is performed for exposure and access to the LV apex (A). Next, the titanium coil is passed over a guidewire and inserted into the LV apex with a deployment tool (B). Once fully engaged and secured (C), the blades (D) of the integrated coring tool (E) make an incision, remove an apical core, and deliver the hemostatic one-way valve (F) resulting in the fully implanted, hemostatically-sealed SBH connector system ready for the LVAD inflow cannula (G). The entire procedure was performed without CPB and with minimal blood loss.
Acute Experiments in IHF calves
The SBH connector system was tested acutely in an IHF bovine model15 (Jersey calves, 75,120 kg, n=2) to develop surgical implantation techniques, to investigate hemocompatibility and potential damage to the myocardium due to insertion of the titanium conical coil, and to assess placement of the titanium connector with integrated silicone hemostatic valve in the LV apex. Technical performance of the SBH connector system was also evaluated while using clinically approved axial (HeartMate II, Thoratec Corporation, Pleasanton, CA) and centrifugal (HeartWare HVAD) LVAD systems during steady-state hypertension test conditions (greater than 150 mmHg systolic pressure) via intravenous (IV) administration of phenylephrine (response dependent). LVAD systems were explanted at the end of feasibility testing to confirm integrity and function of the hemostatic valve. The rationale for using the IHF bovine model was to provide evaluation of the SBH connector system under clinically relevant hemodynamic test conditions with enlarged, thin-walled LV.
Chronic Experiments in Healthy Calves
The SBH connector system was tested chronically for 30 days in normal, healthy calves (Jersey calves, 85,90 kg, n=2). Technical performance, hemocompatibility, and biocompatibility with the SBH connector system and a clinically approved LVAD (HeartWare HVAD) were investigated. Venous blood samples were collected pre-implant (baseline) and post-implant daily (days 1–7) and weekly (days 8–30) for complete blood chemistry (CBC) analysis. The rationale for using normal, healthy calves was to evaluate the long-term technical performance, hemocompatibility, and biocompatibility to assess safety and reliability of the SBH connector system.
Surgical Procedures
The animals were anesthetized with 1–5% isoflurane and 100% oxygen. A left mini-thoracotomy (intercostal approach) was performed at the 5th intercostal space to provide access and exposure of the pulmonary artery, LV apex, and descending thoracic aorta. The SBH connector was prepared and implanted in a two-stage process using modified Seldinger technique. A needle and guidewire technique was used to place titanium connector with integrated silicone valve in the LV apex ensuring the implanted LVAD system when engaged points towards the mitral valve. First, the anchoring coil was rotated into the myocardium until the silicone flanges on its base were fully appossed onto the epicardial surface, a guidewire was used as centering pivot during rotation of the coil. The anchoring coil was delivered using clockwise rotation of the delivery handle. A shallow epicardial incision was made to simplify passage of the blades. The blades penetrated the myocardium and the wings were actuated and retracted by depressing buttons on the handle. A myocardial tissue plug was bored and the cannula was delivered by clockwise rotation of the boring tool. The delivery handle for the anchoring tool was then removed. Second, the titanimum cannula with integrated hemostatic valve was placed using the coring tool, which simulatneously performed cutting and extraction of full-thickness LV apical core and engaged hemostatic valve assembly onto the anchoring coil. The coring tool was then removed leaving the hemostatically sealed titanimum cannula and silicone valve across the LV apex. Once hemostasis was confirmed, heparin (100–300 u/kg) was administered to achieve an activated clotting time (ACT) of greater than 300 seconds. Implantation of the LVAD system was completed for acute and chronic experiments. A summary of the step-by-step implant procedures in the animal model is presented in Figure 5.
Figure 5.
Summary of the implant procedure for the SBH connector system with HVAD (HeartWare, Miami Lakes FL) as shown in a bovine model. Still shot from the overhead camera during an acute HF bovine experiment showing the different surgical steps during off-pump beating heart procedure. (A) Coil is anchored at LV apex using delivery handle. (B) No tearing or bleeding was observed following anchoring of coil. (C) No significant bleeding was observed during the coring procedure. Coring motion also enables delivery of valved cannula that is pre-mounted over the coring blade. (D) Valve maintains hemostasis during exchange of coring tool and connection to the LVAD. (E) HeartWare® HVAD® inlet is introduced though valve with complete hemostasis. (F) HVAD® is exchanged for Thoratec® HeartMate II® inlet cannula to show universality of system. High level of hemostasis is maintained thought the whole procedure. Complete hemostasis at 160mmHg.
Measurements and Analysis
In the acute and chronic animal experiments, arterial and LV pressures were monitored using high-fidelity pressure catheters (Millar Instruments, Houston, TX). Central venous pressure was monitored using a fluid-filled catheter (BD, Becton-Dickson, Franklin Lakes, NJ). Pulmonary artery flow was monitored using a transit-time flow probe (Transonic Systems, Ithaca, NY). A Phillips iE33 transthoracic echocardiography system with an S8-3 ultrasound probe was used to obtain M-mode and Doppler echocardiographic recordings to calculate LV end-diastolic volume, end-systolic volume, and ejection fraction. Fluoroscopy was performed in the acute and chronic animal experiments using an angiography catheter placed in the LV and aorta for injection of radioopaque dye (50–150 mL) to validate position and quantify deflection of the elliptical coil and for flow visualization. Fluoroscopy was also used to evaluate LVAD flow through the inflow cannula via the SBH connector system during implant and prior to euthanization.
Blood samples (15 mL) were collected to assess biocompatibility in the chronic bovine model. A baseline sample was collected pre-implantation and then samples were collected on a daily basis during the first post-operative week and on a weekly basis from post-operative days 8 through 30. Complete blood chemistry (CMP), complete blood count (CBC), liver enzymes, ACT, arterial blood gases, and plasma free hemoglobin (PfHb) were measured. Following animal euthanasia, necropsy and histopathologic analyses were performed with tissue blocks sectioned (5 μm thick) and prepared for staining with hematoxylin and eosin (H&E) for visualization of histopathological changes under light microscopy. The SBH connector system was also visually inspected for evidence of fractures or other defects, and full gross and histologic examination of the heart and end organs was completed. All tissue samples were analyzed by an external, independent veterinary pathologist (Mass Histology Services, Worcester, MA).
RESULTS
Cadaver fit study
The SBH connector system was implanted through subcostal (Figure 6A) and mini-thoracotomy (Figure 6B) surgical approaches in all cadaver studies (n=4). Feasibility of LVAD implant and explant were demonstrated with clinically approved HeartMate II (Figure 6C) and HVAD (Figure 6D) LVAD. The SBH LVAD connector system demonstrated complete hemostasis during simulated hypertension at left ventricular pressures greater than 150 mmHg, and anatomical fit with LVAD in the chest cavity. There was no fluid loss during the coil anchoring procedure, and less than 40 mL fluid loss during boring of the LV apical core. The valve has a minimum amount of seepage designed into it for de-airing purposes, which only allows drops of fluid to escape after it is delivered. The tri-leaflet valve provided hemostasis during exchange of the boring tool to the LVAD inlet in pressures exceeding 150mmHg. No device failures were observed up to 170 mmHg, which was the maximum pressure limit of the experimental setup, but not a threshold limit of the device. In three of the four hearts, hemostasis was achieved with the LVAD inlets fully seated into the myocardium though the cannula with pressures greater than 150 mmHg. In the fourth heart, both static and pulsatile pressures were simulated. Tissue failure occurred at 80 mmHg pulsatile pressure, but not at the site of implant. The failure occurred at the septum and through the right side of the heart due to heavily degraded pre-thawed tissue.
Figure 6.
Cadaver studies demonstrated the SBH connector system may be implanted via subcostal (A) or a mini-thoracotomy (B) incisions and using modified Seldinger technique. The SBH connector system also demonstrated feasibility of anatomic fit, ease of use, and hemostasis during simulated hypertension (pressures up to 170 mmHg) with clinically approved HeartMate II (C) and HVAD (D) LVAD.
Acute Large Animal Study
Hemostasis was successfully demonstrated in the IHF bovine model with thin ventricular walls as evidenced by stable ventricular and arterial pressures following deployment of the SBH connector system (Figure 7) and with LV volume unloading during LVAD support (Figure 8). The entire procedure was completed off pump (no CBP) with the anchoring of the SBH connector achieved in less than 1 minute and no residual bleeding after full delivery and secure locking to the LVAD (HeartMate II and HVAD). The time to complete implant of the connector and LVAD was less than 10 minutes, with total procedure blood loss (skin to skin) less than 100mL.
Figure 7.
The SBH connector system demonstrated stable hemostatic seal in acute the IHF bovine model as evidenced by maintenance of left ventricular peak systolic pressure (LVPpksys), LV end-diastolic pressure (LVPed), mean arterial pressure (ArtPmean), and arterial pulse pressure (ArtPpulse) post-SBH implantation and post-LVAD insertion compared to pre-SBH implant baseline.
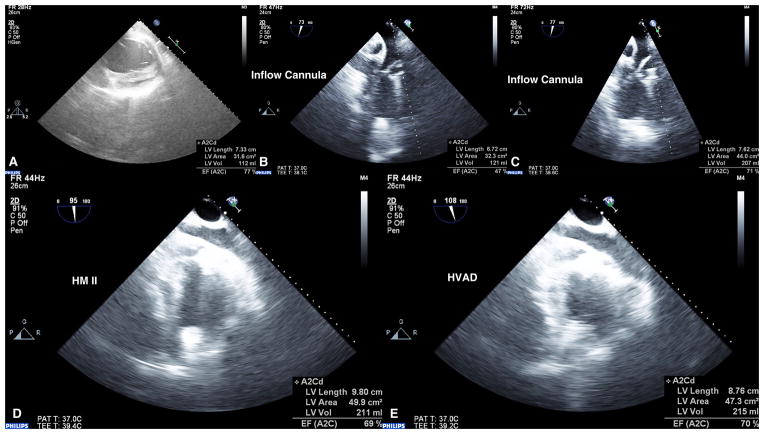
Figure 8.
Echocardiography images (3D iE33 Phillips Medical Systems) recorded at pre-implant baseline (A), post-implant of SBH connector system with HeartWare HVAD operating at maximum pump speed (B) and normal pump speed with animal in acute hypertension (C). Images of Thoratec HeartMate II (D) and HeartWare HVAD (E) LVAD with SBH connector system demonstrating hemostasis was maintained and LV volume unloading achieved.
Chronic Large Animal Study
Echocardiography (weekly evaluation) and fluoroscopy (implant and explant evaluation) verified the in vivo position of the SBH connector system and LVAD system over 30-day study period. On day 30, LV and arterial pressures demonstrated hemostatic seal was maintained as evidenced by LV and arterial pressures over a range of LVAD support and during simulated hypertension (Figure 9). There were no arrhythmias observed and ventricular function (% EF) was preserved (Animal 1: 77% Implant, 71% sacrifice; Animal 2: 73% implant, 72% sacrifice). There were no device failures and both calves completed the 30-day study. There were no significant changes in measurements of hepatic function (ALP, ALT) or renal function (creatinine) throughout the study period compared to baseline (Table 1), suggesting that the SBH connector system and LVAD did not negatively affect end organ function. Plasma free hemoglobin (pfHb), BUN, and creatinine levels were normal at all measured time points. All animals were electively sacrificed and a complete necropsy and histopathological analyses were performed on each animal, including examination of the SBH connector system and LVAD, all major abdominal and thoracic organs, and the brain. An independent pathologist (Mass Histology) examined all end-organ tissues and concluded that there were no notable histopathological changes when compared to other similar LVAD studies using calf models.
Figure 9.
The SBH connector system demonstrated stable hemostatic seal 30-days post-implant in normal calves as evidenced by left ventricular peak systolic pressure (LVPpksys), LV end-diastolic pressure (LVPed), mean aortic pressure (AoPmean), and aortic pulse pressure (AoPpulse) at optimal therapy pump speed (2900 RPM), maximum pump speed (3950 RPM), and acute hypertension (2900 RPM, mean systolic arterial pressure greater than 170mmHg).
Table 1.
Summary of complete blood count (CBC), hemobglobin (Hgb), hematocrit (Hct), white blood cell (WBC), platelet (PLT), renal (blood urea nitrogen (BUN), creatinine (Cr)), and blood trauma ((plasma free hemoglobin (PFHb), activated clotting time (ACT), international normalized ratio (INR)) markers sampled at pre-implant (baseline), 24 hours post-implant, and at weekly intervals thereafter in 30-day chronic bovine models (n=2). Reported values are means ± standard deviations. Values without reported standard deviations reflect data for a single subject (n=1).
| Clinical Marker | Pre-Implant | 24-hour | 1-week | 2-week | 3-week | 4-week |
|---|---|---|---|---|---|---|
| Hemoglobin, Hgb (g/dL) | 10.3 ± 2.0 | 10.4 ± 2.2 | 8.5 ± 0.1 | 8.5 ± 0.1 | 9.4 ± 0.6 | 9.2 ± 0.3 |
| Hematocrit, Hct (%) | 33.2 ± 0.1 | 37.0 ± 6.5 | 30.6 ± 1.3 | 28.9 ± 1.7 | 33.5 ± 3.6 | 33.1 ± 1.3 |
| White blood cell, WBC (K/3μ) | 8.7 ± 1.5 | 11.9 ± 1.3 | 8.0 ± 1.1 | 7.6 ± 0.1 | 8.5 ± 0.6 | 8.0 ± 0.0 |
| Platelet, PLT (K/μL) | 347 ± 2 | 242 ± 68 | 335 ± 66 | 292 ± 76 | 278 ± 85 | 305 ± 57 |
| Blood Urea Nitrogen, BUN (mg/dL) | 5 ± 3 | 11 ± 4 | 6 ± 4 | 8 ± 3 | 6 ± 4 | 10 ± 3 |
| Creatinine, Cr (mg/dL) | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| Plasma Free Hemoglobin, PFHb (mg/dL) | 5 ± 7 | 5 ± 0 | 0 ± 0 | 3 ± 4 | 3 ± 4 | 3 ± 4 |
| International Normalized Ratio, INR | 1.53 ± 0.08 | 2.78 | 4.02 ± 0.86 | 4.04 ± 0.01 | 3.43 ± 0.21 | 3.85 ± 0.91 |
| Activated Clotting Time, ACT (sec) | 168 ± 17 | 203 ± 1 | 250 | --- | 212 | 315 ± 33 |
DISCUSSION
The SBH connector system uses a novel method to implant LVAD without the need for an inflow cannula-sewing ring and improve ‘pullout’ force tolerance. This procedure has many potential benefits over conventional LVAD implantation: 1) surgical procedures may be simplified, shortening patient recovery times, which may improve patient outcomes and decrease the hospital length of stay and cost; 2) implantation of LVAD using the SBH connector system may be performed ‘off pump’ (without the need for CPB), which reduces length of surgery as well as reduces potential risks and complications associated with CPB. In this feasibility study, we demonstrated that the SBH connector system may be implanted with minimally invasive surgical approaches (subcostal and mini-thoracotomy) for secure connection to LVAD inflow with relative ease of use, minimal blood loss, and hemostatic seal even in a hypertensive state. The SBH connector system also provided inflow access to LVAD to achieve flows of up to 6 L/min without device failure, thrombosis, or hemolysis.
The conical coil geometry of the SBH connector system provides superior anchoring stability compared to current sutured connectors. Currently, LVAD implants require 10–14 pledgeted sutures to be used for securing the apical sewing ring to the LV. Since sutures have a thin cross sectional area and bite into a single-plane geometry, they act as a knife cutting through the tissue when pulled axially. By comparison, the elliptical coil has a thicker cross section, is less sharp, and each loop of the coil is on a different geometric plane distributing forces in 3-dimensions, thereby minimizing risk of cutting or axial expulsion.
LVAD implantation may also require significant blood transfusion at a cost of $1,600–$2,400 per unit of packed red blood cells (PRBC). The economic drain on the hospital resulting in a total transfusion cost per patient is estimated to be between $43,000 and $65,000.16 The SBH connector system’s design that minimizes blood loss and enables off-pump (no CPB) implantation may significantly reduce PRBC transfusions associated with LVAD implantation and reduce the demand on national blood supply. The SBH system may also reduce costs for length of stay, which are significant at an average of $3,200 per day in an intensive care unit room, and costs for reoperation due to postoperative bleeding complications.16
Conventional LVAD implantation techniques for long-term circulatory support require a median sternotomy and CPB.6–9 Cardiac surgery via median sternotomy have been associated with decreased lung volumes, reduced thoracic motion, and a more restrictive respiratory pattern.17–19 CPB has been associated with activation of inflammatory mediators and coagulation cascades, larger volume of blood loss, longer durations until extubation, and coagulopathy.20,21 Further, most patients with advanced-stage HF have additional co-morbidities (liver congestion, renal insufficiency, and pulmonary edema), which may worsen with LVAD implant surgery and CPB. We hypothesize that less invasive surgical approaches (i.e. subcostal, mini-thoracotomy) will result in improved preservation of end-organ function, decreased intra-operative blood loss, shortened intubation periods, and reduced manipulation of inflammatory and coagulative properties compared to current surgical approaches using median sternotomy with CPB.
Many clinical investigators have proposed alternative approaches for insertion that are less invasive and/or can be completed off-pump.20, 22–25 For instance, off-pump implantation of the HeartWare HVAD through a minimal J sternotomy and mini-thoracotomy has been reported.23 Although this surgical approach exposes the LV apex cannulation site without cardiac manipulation/displacement, it still requires pledgeted sutures to secure a sewing ring to the LV apex and significant blood loss was observed requiring reinfusion of packed red blood cells and fresh frozen plasma. This technique also has the potential risk of generating massive air emboli between diastolic and systolic pumping phases with an open transapical hole. The SBH connector system offers the same advantages of less invasive, off-pump surgery with the added benefit of deploying a secure, sutureless sewing ring.
Conclusion
APK has developed a novel SBH LVAD connector system that can be implanted using a minimally invasive subcostal or mini-thoracotomy surgical approach, does not require CPB, and minimizes the potential for significant blood loss during implant (and retrieval) procedures.
Supplementary Material
Acknowledgments
The Phase I program (NIH-SBIR phase I grant 1R44HL117426-01) began on March 1, 2013 and was completed November 30, 2013. The objective of this proposal was to develop a minimally invasive surgical implant procedure and complete feasibility testing of a sutureless beating heart (SBH) connector system to facilitate LVAD implantation for the treatment of advanced HF patients. The authors thank the following individuals for their support of this feasibility study: Paul Linsky MD, Karen Lott, Laura Lott, Regina Turner, and Cary Woolard.
Footnotes
Disclosures
Funding for this study was supported by NIH SBIR phase I-II 1R44HL117426-01 (PI: Jorge Jimenez; subcontract co-I: Guruprasad Giridharan, Steven Koenig, Mark Slaughter, Kevin Soucy). Jorge Jimenez and Seth West are employees of APK Advanced Medical Technologies. For the remaining authors, no conflict of interest was declared.
References
- 1.Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125:3191–3200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal S, Pagani FD. Bridge to transplantation: current outcomes. J Card Surg. 2010;25:455–461. doi: 10.1111/j.1540-8191.2010.00996.x. [DOI] [PubMed] [Google Scholar]
- 3.Pagani FD, Miller LW, Russel SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardio. 2009;54:312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Fang JC. Rise of the machines--left ventricular assist devices as permanent therapy for advanced heart failure. N Engl J Med. 2009;361:2282–2285. doi: 10.1056/NEJMe0910394. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 6.Radovancevic B, Frazier OH, Duncan JM. Implantation technique for the HeartMate left ventricular assist device. J Card Surg. 1992;7:203–207. doi: 10.1111/j.1540-8191.1992.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 7.Frazier OH, Delgado RM, III, Kar B, Patel V, Gregoric ID, Myers TJ. First clinical use of the redesigned HeartMate II left ventricular assist system in the United States: a case report. Tex Heart Inst J. 2004;31:157–159. [PMC free article] [PubMed] [Google Scholar]
- 8.Westaby S, Frazier OH, Pigott DW, Saito S, Jarvik RK. Implant technique for the Jarvik 2000 Heart. Ann Throrac Surg. 2002;73:1337–1340. doi: 10.1016/s0003-4975(01)03599-8. [DOI] [PubMed] [Google Scholar]
- 9.Slaughter MS. Implantation of the HeartWare left ventricular assist device. Semin Thorac Card Surg. 2011;23:245–247. doi: 10.1053/j.semtcvs.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Kirklin JK, Naftel DC, Kormos RL, et al. Second INTERMACS annual report: more than 100 primary left ventricular assist device implants. J Heart Lung Transplant. 2010;29:1–10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams ML, Trivedi JR, McCants KC, et al. Heart transplant vs left ventricular assist device in heart transplant-eligible patients. Ann Thor Surg. 2011;91:1330–1334. doi: 10.1016/j.athoracsur.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 12.Miller LW, Nelson KE, Bostic RR, Tong K, Slaughter MS, Long JW. Hospital costs for left ventricular assist devices for destination therapy: lower costs for implantation in the post-REMATCH era. J Heart Lung Transplant. 2006;25:778–784. doi: 10.1016/j.healun.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Rogers JG, Bostic RR, Tong KB, Adamson R, Russo M, Slaughter MS. Cost-effectiveness analysis of continuous-flow left ventricular assist devices as destination therapy. Circ Heart Fail. 2012;5:10–16. doi: 10.1161/CIRCHEARTFAILURE.111.962951. [DOI] [PubMed] [Google Scholar]
- 14.Mulloy DP, Bhamidipati CM, Stone ML, Ailawadi G, Kron IL, Kern JA. Orthotopic heart transplant versus left ventricular assist device: a national comparison of cost and survival. J Thorac Cardiovasc Surg. 2013;145:566–573. doi: 10.1016/j.jtcvs.2012.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartoli CR, Sherwood LC, Giridharan GA. Bovine model of chronic ischemic cardiomyopathy: implications for ventricular assist device research. Artif Organs. 2013;37:2013 E202–14. doi: 10.1111/aor.12129. [DOI] [PubMed] [Google Scholar]
- 16.Basha J, Dewitt RC, Cable D, Jones GP. Transfusions and their costs: managing patients needs and hospitals economics. The Internet Journal of Emergency and Intensive Care Medicine. 2006;9:5. [Google Scholar]
- 17.Ragnarsdottir M, Kristjansdottir A, Ingvarsdottir I, Hannesson P, Torfason B, Cahalin L. Short-term changes in pulmonary function and respiratory movements after cardiac surgery via median sternotomy. Scand Cardiovasc J. 2004;38:46–52. doi: 10.1080/14017430310016658. [DOI] [PubMed] [Google Scholar]
- 18.Kristjansdottir A, Ragnarsdottir M, Hannesson P, Beck HJ, Torfason B. Respiratory movements are altered three months and one year following cardiac surgery. Scand Cardiovasc J. 2004;38:98–103. doi: 10.1080/14017430410028492. [DOI] [PubMed] [Google Scholar]
- 19.Westerdahl E, Lindmark B, Bryngelsson I, Tenling A. Pulmonary function 4 months after coronary artery bypass surgery. Respir Med. 2003;97:317–322. doi: 10.1053/rmed.2002.1424. [DOI] [PubMed] [Google Scholar]
- 20.Gregoric I, Francesca S, Myers T, et al. A less invasive approach to axial flow pump insertion. Int J Heart Lung Trans. 2008;27:423–426. doi: 10.1016/j.healun.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Pintar T, Collard CD. The systemic inflammatory response to cardiopulmonary bypass. Anesthesiol Clin N Am. 2003;21:453–464. doi: 10.1016/s0889-8537(03)00039-7. [DOI] [PubMed] [Google Scholar]
- 22.Sun BC, Firstenberg MS, Louis LB, et al. Placement of long-term implantable ventricular assist device without the use of cardiopulmonary bypass. Journal of Heart and Lung Transplantation. 2008;27:718–721. doi: 10.1016/j.healun.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Cheung A, Lamarche Y, Kaan A, et al. Off-pump implantation of the HeartWare HVAD left ventricular assist device through minimally invasive incisions. Ann Thorac Surg. 2011;91:1294–1296. doi: 10.1016/j.athoracsur.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 24.Frazier OH. Implantation of the Jarvik 2000 left ventricular assist device without use of cardiopulmonary bypass. Ann Thorac Surg. 2003;75:1028–1030. doi: 10.1016/s0003-4975(02)04304-7. [DOI] [PubMed] [Google Scholar]
- 25.Anyanwu AC, Fischer GW, Plotkina I, Pinney S, Adams DH. Off-pump implant of the Jarvik 2000 ventricular assist device through median sternotomy. Ann Thorac Surg. 2007;84:1405–1407. doi: 10.1016/j.athoracsur.2007.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.