Lesson
Swimming-induced pulmonary oedema/edema (SIPO/SIPE) is likely to become commoner with increasing popularity of endurance sports meaning an increased awareness by participants, organisers and medical personnel is important, especially as individuals are at increased risk of future life threatening episodes and drowning if an accurate diagnosis and appropriate advice are not given. The most important risk factors we identified are a highly trained individual, competitive exercise, hypertension and cold environment.
Keywords: Cardiovascular medicine, emergency medicine, sports and exercise medicine
Introduction
Swimming-induced pulmonary oedema (SIPO) is an important complication of exercise with the possibility of misdiagnosis. The condition is important as it may recur, is potentially fatal and is becoming more common with the increase in participation in endurance sports such as triathlons. The pathophysiological processes are poorly understood. We describe two patients who recently presented to our hospital, review the published literature and propose a novel pathophysiological mechanism.
Background
Swimming-induced pulmonary oedema/edema (SIPO/SIPE) is a well-recognised but relatively rare condition that may be misdiagnosed. The mechanism(s) involved in the development of the disorder are not understood. We present two cases of probable SIPO.
Case 1
A 60-year-old man became severely breathless while taking part in the swimming phase of an Ironman triathlon. He is a fit individual with no medical history and an active outdoor job. Thirty minutes into the open water swim, wearing a wetsuit, with a lake temperature of 13°C, he developed sudden onset dyspnoea. He had not inhaled water. A doctor at the scene found widespread respiratory crackles, a systemic arterial oxygen saturation of 86% on air, a blood pressure of 131/82, respiratory rate 20 min−1 and heart rate of 77 bpm. Jugular venous pressure was not elevated and there was no peripheral oedema. Furosemide 50 mg IV, glyceryl trinitrate and a salbutamol nebuliser were administered with symptomatic improvement. On arrival at hospital, he had an oxygen saturation of 95% on air with mid-zone crackles bilaterally. A further furosemide 40 mg IV and salbutamol nebuliser were administered. Complete recovery was observed within a few hours. He was initially diagnosed as an acute coronary syndrome and admitted to the coronary care unit.
Electrocardiogram (ECG) showed non-pathological Q waves and saddle T waves with no ischaemic changes. A chest X-ray and routine bloods tests were normal with a C-reactive protein (CRP) of <1 mg/L. A 12-hour troponin was mildly elevated at 72 ng/L (normal <40). An echocardiogram showed a left ventricular (LV) anteroseptal wall thickness of 14 mm, inferolateral wall of 12 mm, LV ejection fraction of >55% and a mildly dilated left atrium of 29 cm2. He completed nearly 16 min of a Bruce protocol reaching 92% of his maximum predicted heart rate. A coronary computed tomography was normal. A diagnosis of probable SIPO was made.
He was discharged the following day with no on-going treatment and advice about the risk of recurrence.
Case 2
A 55-year-old female triathlete, who regularly competed at world championship level, presented following sudden onset of shortness of breath, chest tightness and coughing up pink frothy sputum while undertaking a training swim in open water. She had not aspirated. She had no relevant medical history and no medications. Initially, in the Emergency Department, she had end-expiratory crackles bilaterally, with a systemic arterial oxygen saturation of 95% on air and no peripheral oedema. She was given a salbutamol nebuliser and transferred to the medical admissions unit. On arrival she had a clear chest on auscultation. A chest X-ray was normal. Her ECG, CRP, D-dimer and Troponin were normal. She had a pO2 of 12 KPa on an arterial blood gas. She was discharged home the same day without a specific diagnosis.
Ten days later she was reviewed in the cardiology clinic. An echocardiogram was normal with a LV ejection fraction of 57% by biplane Simpson’s method and LV wall thickness of 9 mm. The patient completed 16.5 min of an exercise tolerance test using the Bruce protocol reaching her target heart rate. Home monitoring of blood pressure showed borderline hypertension.
No further treatment was given, and the patient was advised of future recurrence risk. She began training again after this episode. She was given a presumptive diagnosis of swimming-induced pulmonary oedema.
Discussion
Increasing numbers of cases of SIPO/SIPE are being reported in army trainees1 and community triathletes with an incidence of 1.4%.2 Episodes are more likely to occur in highly fit individuals undertaking strenuous or competitive swims, particularly in cold water. Many have swum before in the same conditions without experiencing symptoms.
Wilmshurst et al.3 proposed in 1989 that a combination of reactivity of blood vessels and hypertension was the underlying cause of SIPE. A recent study of American triathletes also found an association with hypertension.2 Other risk factors included fish oil use, long course triathlons, wearing a tight wetsuit and female sex.2 The condition is potentially fatal in the absence of being rescued and can recur.4
Pathophysiology
It has been suggested that cold water immersion produces peripheral vasoconstriction with a decrease in core body temperature causing a redistribution of blood from peripheral to thoracic vessels. The resulting increased preload and afterload was proposed to cause an increased pulmonary artery pressure, alveolar oedema and progressive breakdown of the capillary-alveolar barrier.5,6
We suggest a modification of this explanation. It is hypothesised that the unique combination of strenuous swimming, cold water and a highly trained individual leads to right and LV stroke volume mismatch potentially similar to that seen in other forms of acute heart failure.7 Triathletes train to increase both right and LV stroke volumes significantly, particularly during cycling and running secondary to physiological remodelling.8 Cold water immersion causes both peripheral vasoconstriction and physical compression (due to a higher pressure) leading to increased afterload. The left side of the heart is therefore pumping against a higher peripheral vascular resistance leading to a limited increase in LV stroke volume with effort. The change in LV stroke volume may diminish further in the presence of systemic hypertension due to an increased peripheral vascular resistance or the presence of LV hypertrophy.9,10
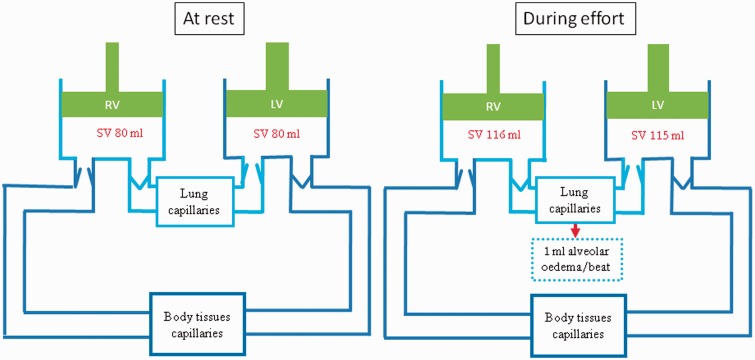
The right ventricular stroke volume is able to increase unhindered as there is no equivalent increase in pulmonary vascular resistance. The Frank–Starling mechanism, normally designed to redress the potential stroke volume imbalance, is likely to have already reached its physiological limit. The result is a difference in the left and right stroke volumes and the ensuing mismatch must inevitably lead to accumulation of fluid in the lungs (see Figure 1). Systemic venous constriction and redistribution of blood from the peripheral circulation will maintain the right ventricular filling pressures. It will also account for the redistribution of fluid from the systemic circulation into the lungs. Pulmonary oedema will result from an ongoing stroke volume difference particularly if the athlete is in open water and unable or unwilling to rest.
Figure 1.
The proposed schematic representation of the pathophysiological process involved in SIPE. Assumes ejection fraction of 55% at rest and 80% during exercise. Alveolar oedema results from a mismatch between right and left ventricular stroke volumes. Peripheral vasoconstriction accompanies the redistribution of fluid from the systemic circulation into the alveoli (see text for further details).
In summary, we suggest that SIPO might be explained by a limited increase in LV stroke volume compared with a relatively greater rise in right ventricular stroke volume during exertion. An increased peripheral vascular resistance due to immersion, a cold environment and systemic hypertension may exacerbate this stroke volume mismatch.
Declarations
Competing interests
None declared
Funding
None declared
Ethical approval
Written informed consent for publication was obtained from the patient.
Guarantor
DM
Contributorship
All authors have made substantial contributions to the manuscript. DM developed the original idea for the report. HC and AGD wrote the first draft that was revised by DM.
Acknowledgements
We would like to thank the whole cardiology unit at Musgrove Park Hospital for the care they provided.
Provenance
Not commissioned; peer-reviewed by Andrew Clarke
References
- 1.Shupak A, Guralnik L, Keynan Y, Yanir Y, Adir Y. Pulmonary edema following closed-circuit oxygen diving and strenuous swimming. Aviat Space Environ Med 2003; 74: 1201–1204. [PubMed] [Google Scholar]
- 2.Miller CC, III, Calder-Becker K, Modave F. Swimming-induced pulmonary edema in triathletes. Am J Emerg Med 2010; 28: 941–946. [DOI] [PubMed] [Google Scholar]
- 3.Wilmshurst PT, Nuri M, Crowther A, Webb-Peploe MM. Cold-induced pulmonary oedema in scuba divers and swimmers and subsequent development of hypertension. Lancet 1989; 1: 62–65. [DOI] [PubMed] [Google Scholar]
- 4.Adir Y, Shupak A, Gil A, et al. Swimming-induced pulmonary edema: clinical presentation and serial lung function. Chest 2004; 126: 394–399. [DOI] [PubMed] [Google Scholar]
- 5.Koehle MS, Lepawsky M, McKenzie DC. Pulmonary oedema of immersion. Sports Med 2005; 35: 183–190. [DOI] [PubMed] [Google Scholar]
- 6.Biswas R, Shibu PK, James CM. Pulmonary oedema precipitated by cold water swimming. Br J Sports Med 2004; 38: e36–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacIver DH, Dayer MJ, Harrison AJI. A general theory of acute and chronic heart failure. Int J Cardiol 2013; 165: 25–34. [DOI] [PubMed] [Google Scholar]
- 8.MacIver DH. Is remodeling the dominant compensatory mechanism in both chronic heart failure with preserved and reduced left ventricular ejection fraction? Basic Res Cardiol 2010; 105: 227–234. [DOI] [PubMed] [Google Scholar]
- 9.MacIver DH, Townsend M. A novel mechanism of heart failure with normal ejection fraction. Heart 2008; 94: 446–449. [DOI] [PubMed] [Google Scholar]
- 10.MacIver DH, Dayer MJ. An alternative approach to understanding the pathophysiological mechanisms of chronic heart failure. Int J Cardiol 2012; 154: 102–110. [DOI] [PubMed] [Google Scholar]