Abstract
Vascular endothelial growth factor (VEGF-A) is one of the most important regulatory factors in pathological and physiological angiogenesis. Alternative splicing is a complicated molecular process in VEGF-A gene expression which adds complexity to VEGF-A biology. Among all VEGF-A exons, alternative splicing of exon 8 is the key determinant of isoform switching from pro-angio-genic VEGF-xxx to anti-angiogenic VEGF-xxxb. This is known as a key molecular switching in many pathological situations. In fact, the balance between VEGF-xxx and VEGF-xxxb isoforms is a critical controlling switch in both conditions of health and disease. Here, the properties of VEGF-xxx and VEGF-xxxb isoforms were discussed and their regulatory mechanism and their roles in certain pathological processes were evaluated. In summary, it was suggested that C-terminal VEGF-A alternative splicing can provide a new treatment opportunity in angiogenic diseases.
Keywords: Alternative splicing, Angoigenesis, Disease, Health, Vascular endothelial growth factor A
Introduction
Vascular Endothelial Growth Factor A (VEGF-A) is the most specific and prominent angiogenic factor among all VEGF family members. It has become a center of interest due to its important role in physiological and pathological processes such as embryonic development, wound healing, female reproductive cycle, cancer, cardiovascular disease, etc. (1–3). This unique growth factor is required for the proper function of adult blood vessels and regulation of endothelial cell differentiation during embryonic development (4, 5). VEGF spans 16,272 bp of chromosome 6p12 and encodes a signaling 46 kDa glycoprotein involved in the regulation of angiogenesis and vasculo-genesis as extremely tightly regulated processes (6, 7). Modest changes in VEGF expression level result in embryonic lethality caused by unsuitable vascularization. Accordingly, VEGF gene regulation is an important biological process which is too complex. As a result, many researchers have tried to determine the conservative regulatory elements involved in VEGF gene regulation in multiple levels such as transcriptional level and also, mRNA stabilization, splicing and translational levels (8). Among all of the biological complexities of VEGF, the alternative splicing and the multiple isoforms generated by this process has been discussed as a basic regulatory factor in health and disease recently.
The investigation of VEGF isoform regulation might be the key for controlling and targeting the angiogenesis balance in pathologies, and also provides a link between angiogenesis, treatment of pathologies and the alternative splicing process (8, 9). In this article, the importance of VEGF alternative splicing process was evaluated in physiological and pathological situations.
VEGF gene structure
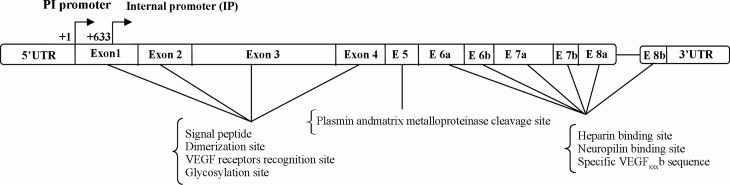
The VEGF gene consists of eight exons and both of its exons and introns are highly conserved in different species (10). The domains encoded by each exon, result in specific properties in the disulfide-bonded growth factor. The first four exons encode the signal peptide, dimerization site, VEGF receptors (VEGF-R1 and VEGF-R2) recognition site and glycosylation site. The signal peptide which is cleaved to create a mature secretive protein is encoded by exon 1 and four residues of exon 2. Exon 5 encodes a specific sequence which consists of the plasmin and matrix metalloproteinase cleavage sites. Eventually, exons 6, 7 and 8, respectively encode for the heparin binding site, the neuropilin binding site and the unique VEGF sequence (11, 12). The VEGF gene consists of a first TATA-less promoter region (+1) which includs Hypoxia Responsive Element (HRE), and a second internal promoter (+633) which leads to truncated mRNA transcription (13, 14). The VEGF regulatory 5’- untranslated region (5'UTR) spans 1038 nucleotides in human beings and consists of three CUG start codons, a classical AUG initiation codon and two internal ribosome entry sites (IRESes) which control VEGF translation in different situations (15). The 3’- untranslated region (3'UTR) is another important regulatory region and consists of multiple polyadenylation signals and AU-rich element response to various stabilizing and destabilizing proteins (16) (Figure 1).
Figure 1.
Structure of VEGF exons between 5'UTR and 3'UTR (The description is given in the text)
Alternative splicing and VEGF-A molecular diversity
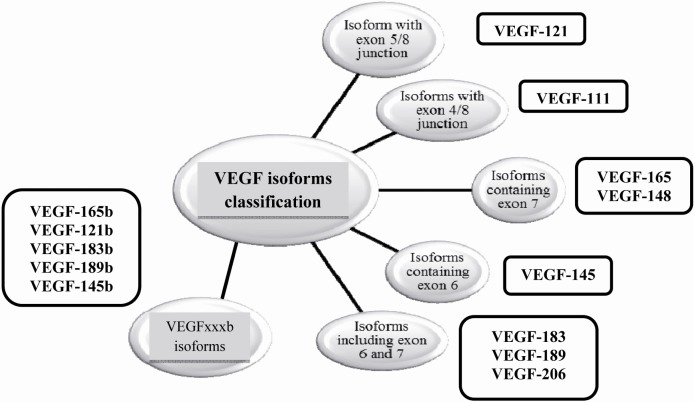
Alternative splicing is a regulated post-transcriptional process that leads to the production of several proteins by one gene (17). In this process and in order to create multiple mRNA variants from a single pre-mRNA, exons are either included in the mRNA or targeted to be excluded in different modes such as exon skipping or cassette exon, mutually exclusive exons, alternative donor site, etc. In humans, at least 70% of approximately 25,000 genes (95% of multiexonic genes) are alternatively spliced. It is clear that abnormal alternative splicing should be associated with a large proportion of human genetic deficiencies as the result of imbalanced distribution of mRNA variants in different tissues (18). Therefore, alternative splicing is a key regulatory molecular mechanism of VEGF regulation that leads to the production of different iso-forms. These isoforms, on the other hand, showed variations in their expression patterns, their binding to heparan sulfate proteoglycans and their interaction with neuropilins 1 and 2 (Nrp-1 and Nrp-2) co-receptors. This molecular diversity of VEGF-A is a regulator of its bioavailability and biological activity (19). It is known that alternative splicing of VEGF occurs in exons 6, 7 and 8; therefore, all iso-forms contain exon 1-5. The only exception is VEGF-111 which is a newly identified VEGF isoform containing exons1-4 and 8 and is important in VEGFR-1 and VEGFR-2 interactions (Figure 2). VEGF-111 was discovered by Mineur et al in several types of cultured cells upon treatment through UV-B irradiation and genotoxic drugs. The lack of exon 5 in VEGF-111 makes the isoform resistant to plasmin and matrix metalloproteinase, the feature that may be presented as a novel therapeutic property for treatment of ischemic diseases (20). Exons 6a and 7 encode Heparin Binding Domains (HBDs) in containing iso-forms, and enable these molecules to bind cell surface and Nrp co-receptor. Base on these properties, VEGF isoforms are categorized as soluble and diffusible isoforms (VEGF-121), cell-bounded isoforms (VEGF-189, VEGF-206) and moderate isoforms which have both soluble and cell-bounded properties (VEGF-165). Heparin binding property of VEGF is really important in health and disease (8, 21, 22). VEGF variants were considered to be pro-angiogenic until 2002, when Bates et al described a novel VEGF-165 named VEGF-165b (23).
Figure 2.
The classification of VEGF isoforms
Nowadays, molecular researchers believe that there are two major VEGF subfamily isoforms including VEGF-xxx (VEGF-xxxa) and VEGF-xxxb. VEGF-xxx isoforms are pro-angiogenic and VEGF-xxxb are anti-angiogenic and as it is known, xxx demonstrates the amino acid number of mature proteins. These subfamilies differ in the sequence of exon 8 which leads to two sub-exons termed exon 8a (tgtgacaagccgaggcggtga) and exon 8b (tctctcaccaggaaagactga). These findings add more complexities to VEGF biology and show that angiogenesis is the outcome of pro-angiogenic and anti-angiogenic isoform activity and their balance levels are really important in health and disease (23, 24).
In the next part of the study, alternative splicing and its regulation will be described particularly in exon 8. Then, the role of angiogenesis and VEGF molecular switching from pro-angiogenic to anti-angiogenic will be discussed in health and disease.
The regulation of VEGF C terminal alternative splicing
Although the regulatory mechanism of VEGF transcription is well-known, the regulation of multiple isoform production through alternative splicing needs more clarification. Scientists focus on molecular pathway especially exon 8 alternative splicing, because of its potent roles in physiological and pathological angiogenesis. Splicing and growth factors differentially regulate VEGF switching from pro-angiogenic to anti-angiogenic isoforms. Specific growth factors induce signal transduction pathway which can affect alternative splicing and different expression of VEGF-xxx and VEGF-xxxb isoforms (8, 25, 26). These signaling pathways mediate the stimulation of particular Splicing Factors (SFs), which select 3’ Distal Splicing Site (3’ DSS) or 3’ Proximal Splicing Site (3’ PSS) variously. Differential selection of 3’ PSS or 3’ DSS, primarily differentiated the splicing results and finally led tothe production of VEGF-xxx or VEGF-xxxb sub-families, respectively.
Despite wide investigations used to detect the growth factors and to uncover the details of the molecular cascades which were involv-ed in VEGF splice site selection, our information is still very scarce. Known elements in these pathways are divided into four categories including: Growth Factors (GF), Cell Signaling Molecules (CSM), SF and SF Kinases (SFK). SFs interact with C terminal domain of RNA polymerase II and recruit it to the specific cis-acting splicing sequences. It must be mentioned that each of the SF is regulated by a special SFK, that in return down-regulated by various CSM, and these CSM are controlled by different GF (17, 24). Some of the elements in this molecular switching process are determined by bioinformatics and in vivo studies.
Bioinformatics researches indicate the existence of some specific SF-binding sites on VEGF pre-mRNA which are adjacent to 3’ PSS or 3’ DSS and which recruit particular SF. Some in vivo analyses have been performed to investigate the role of GF, CSM and SFK in this specific splicing site selection through main transduction cascades. GF involving insulin-like growth factor 1 (IGF-1), transforming growth factor β1 (TGFβ1), tumor necrosis factor α (TNFα), etc. increase the total VEGF expression level, but differentially influence pro-angiogenic and antiangiogenic isoforms. In spite of wide studies in the field of these regulatory elements, only the role of a small group of GF and SF has been described so far. At the initial step of 3’ PSS selection pathway, IGF-1 induces Protein Kinase C (PKC) which leads to SFK (SRPK1/2) activation. Then, SRPK1/2 phosphorylate the ASF-SF2 splicing factor at the end of the 3’ PSS selection pathway. Therefore, 3’ PSS is selected especially through a particular pathway which results in VEGF-xxx expression. Beside several transcription factors such as Hypoxia-Inducible Factor (HIF) and also many SF participated in the previously described pathway, there are so many othersthat have not been clearly identified. In fact, IGF-1 induces VEGF expression through different signaling pathways, but the role of the mentioned kinase cascade has not been confirmed by in vivo studies (15, 27, 28).
In contrast, TGFβ1 is located at the initial point of 3’ DSS selection through the activation of p38-mitogen activated protein kinase. Then, CLK1 and CLK4 induction occure and subsequently, these SFK phosphorylate the SRp55 splicing factor. SRp55 in turn, leads to selection of 3’ DSS and the expression of anti-angiogenic isoforms. But this molecular mechanism is highly complex and many other factors such as chromatin modification and structure may influence it. In summary, the regulation of VEGF C terminal alternative splicing is a critical process and more accurate insilco and in vitro researches are required for its clarification. Understanding these molecular mechanisms would improve our knowledge of angiogenesis in normal and pathological situations. Finally, the manipulation of splicing machinery related to VEGF switching, can be used as a new opportunity in treatment of various diseases (24, 26).
VEGF roles in pathological angiogenesis
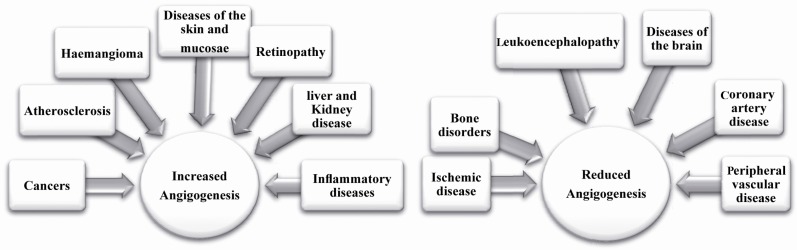
Angiogenesis is an important biological process in many pathological states (angiogenic disease). The increase or decrease of angiogenesis is related to various diseases in different stages of life. The potent role of VEGF-A in pathological angiogeneses, such as tumor angiogenesis has been widely described in the last decade. VEGF is a key angiogenic growth factor and its level of expression is a critical marker for detection of diseases (29). Generally, a high or low level of VEGF expression results in two different pathological situations: diseases which show an increase in angiogenesis and pathological states which show reduction in angiogenesis (Figure 3). Many studies have demonstrated the differential roles of VEGF isoforms in physiological and pathological angiogenesis which have added complexity to VEGF biology. Multiple VEGF isoforms are not functionally equal and all isoforms are required for normal vascularization. These researches used isoform-specific knock-in mice and suggested a selective targeting of VEGF isoforms by specific drugs in different pathological states. However, the role of various VEGF isoforms in many angiogenic diseases still remains unidentified and needs more investigation (30, 31). Recently, scientists have focused on VEGF C terminal alternative splicing and the role of two major VEGF families in different pathologies, as will be described.
Figure 3.
Two different pathological situations caused by increased or reduced angiogenesis
The expression of pro-angiogenic and anti-angiogenic VEGF isoforms in diseases
VEGF-xxx and VEGF-xxxb isoforms have become the center of interest due to their basic switching in health and disease. VEGF-xxx isoforms are pro-angiogenic and are up-regulated in many pathological situations related to increased angiogenesis. In contrast, VEGF-xxxb isoforms are anti-angiogenic and are down-regulated in tumors and other angiogenic conditions (32). The expression pattern of VEGF isoforms are changed during tumorigenesis and many cancers are associated with anti- to pro-angiogenic switching which may be an initial step in the metastasis process. In a previous study, the level of VEGF expression was analyezed through Real time quantitative PCR like other important genes in many cancers (33).
Some studies which analyzed the level of multiple VEGF isoform expression have dem-onstrated that VEGF-xxxb isoforms are more than or near half of the total VEGF in normal tissue (except placenta). These anti-angio-genic isoforms are down- regulated in many angiogenic diseases such as various cancers, arthritis and cardiovascular diseases (33–35). In contrast, VEGF-xxx isoforms constitute the majority of expressed VEGF in these angio-genic situations. So, this is an attractive molecular switching in many diseases from anti to pro-angiogenic or from pro to anti-angio-genic VEGF isoforms. In fact, the balance of pro and anti-angiogenic isoforms is the critical molecular mechanism in normal tissue which is affected in many pathological situations related to increased or decreased angiogenesis (36–38).
The overexpression of VEGF-xxx and down-regulation of VEGF-xxxb iso-forms has been shown in prostate, bladder, renal and melanoma cancers and other diseases related to excess angiogenesis such as diseases of the eye including diabetic retinopathy and Age-related Macular Degeneration (AMD). Increasing the level of anti-angiogenic isoforms in these pathological situations is a new therapeutic opportunity (15, 38–40). Some other studies which altered the level of VEGF-xxx and VEGF-xxxb isoforms in different reproductive tissues, have demonstrated that the balance between these two VEGF subfamilies may be involved in fertility. The placenta is the only normal tissue which has a low level of VEGF-xxxb expression and the alteration in this level may be related to some genetic disorders (41–43). VEGF is a key regulatory factor in renal health and some researches confirmed the role of specific VEGF isoforms in the glumerolar function of mice. In these studies, the overexpression of specific iso-forms results in different glomerulopathy options and some isoforms do not have any important effects on this tissue (44–47). Taken together, the accurate expression level of anti and pro-angiogenic VEGF isoforms in different physiological and pathological states has not been analyzed so far and molecular assessments could open doors to new opportunities in angiogenic pathologies.
Conclusion
Generally, molecular studies of VEGF splicing, especially VEGF C-terminal alternative splicing are consistent with a disease-associated switching from anti to pro-angio-genic or from pro to anti-angiogenic isoform expression. The heterogeneity of VEGF mRNA distal or proximal splice site selection is a critical marker in angiogenic diseases. In recent years, many researchers have focused on this field of study and our knowledge has been improved about VEGF C-terminal alternative splicing and the regulatory agents in this process, but more investigation is needed to better understand this complicated molecular mechanism. Accurate researches and analyses about VEGF-xxx and VEGF-xxxb isoform expression patterns are crucial in finding new methods for angiogenic disease treatment.
References
- 1.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 2.Kajdaniuk D, Marek B, Borgiel-Marek H, Kos-Kudla B. Vascular endothelial growth factor (VEGF) part 1: in physiology and pathophysiology. Endokrynol Pol. 2011;62(5):444–455. [PubMed] [Google Scholar]
- 3.Giacca M. Non-redundant functions of the protein isoforms arising from alternative splicing of the VEGF-A pre-mRNA. Transcription. 2010;1(3):149–153. doi: 10.4161/trns.1.3.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 6.Vincenti V, Cassano C, Rocchi M, Persico G. Assignment of the vascular endothelial growth factor gene to human chromosome 6p21.3. Circulation. 1996;93(8):1493–1495. doi: 10.1161/01.cir.93.8.1493. [DOI] [PubMed] [Google Scholar]
- 7.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266(18):11947–11954. [PubMed] [Google Scholar]
- 8.Simons M. Silky, sticky chimeras-designer VEGFs display their wares. Circ Res. 2007;100(10):1402–1404. doi: 10.1161/01.RES.0000269333.10849.9e. [DOI] [PubMed] [Google Scholar]
- 9.Dehghanian F, Hojati Z. Synthesis, cloning and mRNA expression of human VEGF-111 and VEGF -111b recombinant cDNA; (Unpublished data) [Google Scholar]
- 10.Claffey KP, Senger DR, Spiegelman BM. Structural requirements for dimerization, glycosylation, secretion, and biological function of VPF/VEGF. Biochim Biophys Acta. 1995;1246(1):1–9. doi: 10.1016/0167-4838(94)00144-6. [DOI] [PubMed] [Google Scholar]
- 11.Keyt BA, Berleau LT, Nguyen HV, Chen H, Heinsohn H, Vandlen R, et al. The carboxyl-terminal domain (111-165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem. 1996;271(13):7788–7795. doi: 10.1074/jbc.271.13.7788. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix me-talloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169(4):681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacal PM, Ruffini F, Pagani E, D'Atri S. An auto-crine loop directed by the vascular endothelial growth factor promotes invasiveness of human melanoma cells. Int J Oncol. 2005;27(6):1625–1632. [PubMed] [Google Scholar]
- 14.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, et al. Spatially restricted patterning cuesprovided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16(20):2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA. VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularisation in mice. Mol Vis. 2006;12:626–632. [PubMed] [Google Scholar]
- 16.Seifi T, Tanhaei S, Tavassoli M, Baharvand H, Ghaedi K, Hojati Z, et al. Amplification of GC-rich putative mouse PeP promoter using betaine and DMSO in ammonium sulfate polymerase chain reaction buffer. Avicenna J Med Biotechnol. 2012;4(4):206–209. [PMC free article] [PubMed] [Google Scholar]
- 17.Matlin AJ, Clark F, Smith CW. Understanding alternative splicing: towards a cellular code. Nat Rev Mol Cell Biol. 2005;6(5):386–398. doi: 10.1038/nrm1645. [DOI] [PubMed] [Google Scholar]
- 18.Black DL. Mechanisms of alternative pre-messen-ger RNA splicing. Annu Rev Biochem. 2003;72(1):291–336. doi: 10.1146/annurev.biochem.72.121801.161720. [DOI] [PubMed] [Google Scholar]
- 19.Hojati Z, Dehghanian F. Enhanced production of bioactive recombinant VEGF-111 protein in mammalian cell lines; Int J Biochem Cell Biol; (Unpublished data) [DOI] [PubMed] [Google Scholar]
- 20.Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5(12):1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 21.Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol Biol Cell. 2010;21(5):687–690. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krilleke D, Ng YS, Shima DT. The heparin-bind-ing domain confers diverse functions of VEGF-A in development and disease: a structure-function study. Biochem Soc Trans. 2009;37(Pt 6):1201–1206. doi: 10.1042/BST0371201. [DOI] [PubMed] [Google Scholar]
- 23.Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62(14):4123–4131. [PubMed] [Google Scholar]
- 24.Delcombel R, Janssen L, Vassy R, Gammons M, Haddad O, Richard B, et al. New prospects in the roles of the C-terminal domains of VEGF-A and their cooperation for ligand binding, cellular signaling and vessels formation. Angiogenesis. 2013;16(2):353–371. doi: 10.1007/s10456-012-9320-y. [DOI] [PubMed] [Google Scholar]
- 25.Gu F, Li X, Kong J, Pan B, Sun M, Zheng L, et al. VEGF111b, a new member of VEGFxxxb isoforms and induced by mitomycin C, inhibits angiogenesis. Biochem Biophys Res Commun. 2013;441(1):18–24. doi: 10.1016/j.bbrc.2013.09.144. [DOI] [PubMed] [Google Scholar]
- 26.Dehghanian F, Hojati Z. Molecular cloning and expression of VEGF-111b: A newly identified anti-angiogenic VEGF-A isoform; (Unpublished data) [DOI] [PubMed] [Google Scholar]
- 27.Cébe Suarez S, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, et al. A VEGF-A splice variant defective for heparan sulfate and neuro-pilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci. 2006;63(17):2067–2077. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dehghanian F, Hojati Z. A new approach to synthesize recombinant cDNA of VEGF-A gene by Eco31I enzyme. Genetics in the Third Millennium. 2013;11(1):2980–2989. [Google Scholar]
- 29.Blaustein M, Pelisch F, Tanos T, Munoz MJ, Wengier D, Quadrana L, et al. Concerted regulation of nuclear and cytoplasmic activities of SR proteins by AKT. Nat Struct Mol Biol. 2005;12(12):1037–1044. doi: 10.1038/nsmb1020. [DOI] [PubMed] [Google Scholar]
- 30.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 31.Hirose Y, Tacke R, Manley JL. Phosphorylated RNA polymerase II stimulates pre-mRNA splicing. Genes Dev. 1999;13(10):1234–1239. doi: 10.1101/gad.13.10.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109(3):227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 34.Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109(3):327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu Y, Hoareau-Aveilla C, Oltean S, Harper SJ, Bates DO. The anti-angiogenic isoforms of VEGF in health and disease. Biochem Soc Trans. 2009;37(Pt 6):1207–1213. doi: 10.1042/BST0371207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabatabaeian H, Hojati Z. Assessment of HER-2 gene overexpression in Isfahan province breast cancer patients using real time RT-PCR and immunohistochemistry. Gene. 2013;531(1):39–43. doi: 10.1016/j.gene.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 37.Hojati Z, Dehghanian F. Regulatory mechanisms of VEGF-A gene expression in angiogenesis and treat-ment of disease. Tashkhis. 2012:22–30. [Google Scholar]
- 38.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8(11):880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64(21):7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 40.Rennel ES, Waine E, Guan H, Schüler Y, Leenders W, Woolard J, et al. The endogenous anti-angio-genic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br J Cancer. 2008;98(7):1250–1257. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varey AH, Rennel ES, Qiu Q, Bevan HS, Perrin RM, Raffy S, et al. VEGF165b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A iso-forms has implications for therapy. Br J Cancer. 2008;98(8):1366–1379. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pepper MS, Baetens D, Mandriota SJ, Di Sanza C, Oikemus S, Lane TF, et al. Regulation of VEGF and VEGF receptor expression in the rodent mammary gland during pregnancy, lactation, and involution. Dev Dyn. 2000;218:507–524. doi: 10.1002/1097-0177(200007)218:3<507::AID-DVDY1012>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 43.Woolard J, Bevan HS, Harper SJ, Bates DO. Molecular diversity of VEGF-A as a regulator of its biological activity. Microcirculation. 2009;16(7):572–592. doi: 10.1080/10739680902997333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111(5):707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 46.Faustino NA, Cooper TA. Pre-mRNA splicing and human disease. Genes Dev. 2003;17(4):419–437. doi: 10.1101/gad.1048803. [DOI] [PubMed] [Google Scholar]
- 47.Arcondéguy T, Lacazette E, Millevoi S, Prats H, Touriol C. VEGF-A mRNA processing, stability and translation: a paradigm for intricate regulation of gene expression at the post-transcriptional level. Nucleic Acids Res. 2013;41(17):7997–8010. doi: 10.1093/nar/gkt539. [DOI] [PMC free article] [PubMed] [Google Scholar]