Abstract
A single bout of acute exercise increases oxidative stress and stimulates a transient increase in antioxidant enzymes. We asked whether this response would induce protection from a subsequent oxidative challenge, different from that of exercise, and whether the effects were affected by aging. We compared young (20 ± 1 years, n = 8) and older (58 ± 6 years, n = 9) healthy men and women. Resistance to oxidative stress was measured by the F2-isoprostane response to forearm ischemia/reperfusion (I/R) trial. Each participant underwent the I/R trial twice, in random order; once after performing 45 min of cycling on the preceding day (IRX) and a control trial without any physical activity (IRC). Baseline F2-isoprostane levels were significantly lower at IRX compared to IRC (P < 0.05) and not different between groups. F2-isoprostane response to IRX was significantly lower compared to IRC in young (P < 0.05) but not different in the older group. Superoxide dismutase activity in response to acute exercise was significantly higher in young compared to older adults (P < 0.05). These data suggest that signal transduction of acute exercise may be impaired with aging. Repeated bouts of transient reactive oxygen species production as seen with regular exercise may be needed to increase resistance to oxidative stress in older individuals.
Keywords: Acute exercise, Aging, Oxidative stress, F2-isoprostanes, Superoxide dismutase
Introduction
Oxidative stress is strongly implicated in aging and in the etiology of age-related disease such as cardiovascular disease, type 2 diabetes, cancer, and Alzheimer’s disease (Gradinaru et al. 2013; Mecocci et al. 1994; Totter 1980). Resistance to oxidative stress and oxidative damage may play an important role in the prevention of age-related disease and promotion of increased health span. While regular exercise has been shown to reduce risks and complications of chronic diseases associated with oxidative stress, the mechanisms of resistance to oxidative stress demonstrated in animal studies have not been well translated to humans (Goto et al. 2004; Koltai et al. 2012).
Previous studies have shown that a single bout of exercise induces oxidative stress while also increasing antioxidant enzyme activity in both trained and sedentary subjects (El Abed et al. 2011). In addition, animal studies have shown that gene expression and protein content can be upregulated by a single bout of exercise (Hollander et al. 2001; Muthusamy et al. 2012). These responses to the stimulus of exercise can be attributed to cell signaling effects of reactive oxygen species (ROS) leading to activation of pathways involved in transcription of phase II enzymes increasing resistance to cellular stress (Syu et al. 2011). The length of time that these effects last from a single bout of exercise is not well characterized, but it is likely limited unless the stimulus is applied repeatedly as would occur with regular exercise training. Even then, an acute stimulus may be required to see the adaptations. This was shown in a study of older men that exhibited lower oxidative stress and greater antioxidant capacity after a 16-week exercise intervention (Fatouros et al. 2004); however, many of the effects of the exercise intervention were only shown when measured in response to a maximal exercise test and were not evident at rest.
The existing literature on oxidative stress and acute exercise has mainly focused on measurements immediately before and after the exercise bout. What is not known is whether these acute responses to exercise provide a sustained protection to subsequent oxidative stressors, especially those that are not produced by exercise. In addition, data from animal studies show that aging is associated with reduced ability to translate stress signals such as acute exercise or ischemic preconditioning (induced brief periods of ischemia prior to ischemic event) into improved protection (Fenton et al. 2000; McArdle et al. 2002; van den Munckhof et al. 2013). The goal of this study was to investigate whether resistance to oxidative stress was increased by preceding acute exercise and to examine the effect of aging on this response. Resistance to oxidative stress was measured by the plasma F2-isoprostane response to forearm ischemia/reperfusion (I/R trial), a challenge known to induce oxidative stress (Davies et al. 2009; Traustadóttir et al. 2009; Traustadóttir et al. 2012). In addition, we explored potential mechanisms contributing to resistance to oxidative stress by measuring changes in activity of the antioxidant enzyme superoxide dismutase (SOD) in response to acute exercise and the first hour of the I/R trial. We hypothesized that acute exercise would increase protection against a subsequent oxidative challenge and that this protective effect would be attenuated with age.
Materials and methods
Participants
Nine young (18–25 years) and nine older (≥50 years) men and women were recruited from the community. Study participants were non-smokers, were not taking antioxidant supplementation in excess of a multivitamin or equivalent, were generally healthy, were not overly obese (BMI ≤ 33.0 kg/m2), and had no known cardiovascular, pulmonary, or metabolic disease. Individuals were excluded if they had experienced a myocardial infarction within the last 6 months or any history of angina, if there was any clinically significant arrhythmia during resting electrocardiogram (EKG), or if there were significant EKG changes during the maximal oxygen consumption (VO2max) test. Any condition that would contraindicate maximal exercise testing, including elevated blood pressure at rest (systolic BP > 140 and/or diastolic BP > 90 mmHg) or musculoskeletal problems, excluded subjects from participating in the study. Subjects were not included in the study if their maximal oxygen consumption was above the 70th percentile for age and gender, based on American College of Sports Medicine (ACSM) guidelines because physical fitness has previously been shown to alter the response to the forearm I/R trial (Traustadóttir et al. 2012). Older women were post-menopausal and were not taking any hormone replacement therapy. Younger women were not taking any form of oral contraceptives and were tested during the early-follicular phase of the menstrual cycle to avoid any confounding effects of estrogen (Razmara et al. 2007; Strehlow et al. 2003). All participants signed an informed consent approved by the Northern Arizona University Institutional Review Board.
Study design
The study employed a randomized crossover counterbalanced design (see Fig. 1), where half of the participants completed the trial with acute exercise followed by the forearm ischemia reperfusion trial (IRX) first and then the control trial (IRC). The other half completed the IRC first and then the IRX. Prior to these trials, the participants went through a screening visit and a test of maximal oxygen consumption. A predetermined time of at least 7 days separated VO2 max from the first study trial (regardless of condition) to control for any confounding effects of the maximal exercise.
Fig. 1.
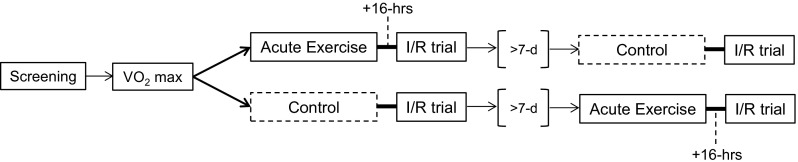
Study design
Screening visit
Prior to any exercise testing, the participants completed a health history questionnaire. Additionally, height, weight, and resting blood pressure were measured and a 12-lead supine resting EKG was obtained.
Maximal oxygen consumption test
Maximal oxygen consumption (VO2max) was measured with a graded exercise test performed on a cycle ergometer as previously described (Traustadóttir et al. 2012; Traustadóttir et al. 2008). The starting workload was selected based on the predicted maximal workload for each individual, and the workload was increased every minute by 15 or 20 W until volitional exhaustion. Participants were instructed to maintain a pedaling rate of 60–70 rpm throughout the test. Oxygen consumption was measured using indirect calorimetry using a metabolic measurement cart (Vmax29, CareFusion, Yorba Linda, CA). Heart function was monitored with continuous 12-lead EKG. VO2 max was considered achieved if two of the following three criteria were met: (1) a plateau in VO2 with an increase in workload, (2) a respiratory exchange ratio (RER) ≥1.10, and (3) heart rate within 10 beats of age-predicted maximal heart rate (220-age) (Kohrt et al. 1991). Standard contraindications to exercise testing and termination criteria outlined by ACSM were followed at all times.
Acute exercise trial
The participants completed 45 min of cycling at a workload predicted to elicit approximately 60 % of VO2max. The duration and intensity of the acute exercise trial were determined from a pilot study performed in our lab that compared different exercise intensities and durations. The trial selected elicited the highest oxidative stress response as measured by F2-isoprostanes. Prior to exercise, a blood draw was obtained and two additional blood samples were drawn 10 and 30 min post-exercise. The participants returned the following day (16 h later) to complete the I/R trial (IRX).
Forearm ischemia/reperfusion trial
Study participants reported to the laboratory 16 h post-exercise or on the control day to perform the forearm ischemia/reperfusion trial as previously described (Davies et al. 2009; Traustadóttir et al. 2009; Traustadóttir et al. 2012). Briefly, an intravenous catheter was inserted into the arm and a baseline blood sample was collected (pre). The catheter was kept in situ with a slow saline drip throughout the trial. A blood pressure cuff was placed on the same arm, inflated to 200 mmHg and kept inflated for 10 min then released for 2 min. This inflation procedure was repeated twice more (total time 34 min). During each inflation, approximately 1–2 mL of heparin flush was injected into the blood sample tubing to prevent clotting of the catheter. After the three ischemia/reperfusion periods, additional blood samples were obtained at 15, 30, 60, 120, and 180 min after the final cuff deflation.
F2-isoprostanes analyses
Samples for F2-isoprostane analyses were collected into SST Vacutainer tubes and kept at room temperature for 30 min to clot, then placed in a refrigerator (4 °C) until being centrifuged at 3000 rpm for 15 min. Plasma was aliquoted and stored at −80 °C. Time of storage did not exceed 6 months prior to shipment to Vanderbilt University CORE center for analysis. Free F2-isoprostanes in plasma were quantified, after purification and derivatization, using gas chromatography/negative ion chemical ionization–mass spectrometry with [2H4]15-F2t-isoprostane as an internal standard (Morrow and Roberts 1999). Compounds were analyzed as pentafluorobenzyl ester, trimethylsilyl ether derivatives by monitoring mass-to-charge ratios of 569 and 573 for endogenous F2-isoprostanes and the [2H4]15-F2t-isoprostane internal standard, respectively.
Superoxide dismutase activity
Blood samples were collected into EDTA Vacutainer tubes and placed on ice for 5 min prior to being centrifuged at 2200 rpm for 10 min. Following removal of plasma and leukocytes, 250 μL of erythrocytes were lysed with 1000 μL of HPLC-grade water and placed on ice for 2 min before centrifugation for 15 min at 10,000g. Samples were stored at −80 °C until analysis. Superoxide dismutase (SOD) activity was measured using commercially available kits (Cayman Chemical, Ann Arbor, MI, USA). SOD activity is measured using a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. The definition of one unit of SOD is the amount of enzyme needed to exhibit 50 % dismutation of the superoxide radical. The SOD activity was quantified by measuring the decrease in absorbance at 450 nm.
Statistical analyses
Subject demographics were compared between the two groups (young and older) using independent samples t test. The plasma F2-isoprostane response across time comparing IRX and IRC was analyzed by 2 × 6 repeated-measures ANOVA (trial × time point), both for the whole cohort and each age group separately. SOD activity was analyzed by 2 × 3 repeated measures ANOVA (trial × time point for the I/R trial and group × time point for the acute exercise). The integrated F2-isoprostane responses were calculated for each individual as area under the curve (AUC) and area under the response curve (AURC) by the method of the trapezoidal rule. AUC is calculated with reference to ground (zero), and AURC is calculated in reference to the individual baseline value. Mean AURC and AUC responses were analyzed by paired t test. Assumption of normality was tested using the Shapiro–Wilk test, and Mauchly’s test of sphericity was used to test equality of distribution. All comparisons were considered significant at P < 0.05. All data shown are means ± SE. The statistical analyses were conducted using IBM SPSS Statistics 22 software.
Results
Thirty-six subjects were screened to participate in the study; of those, 18 had to be excluded due to having maximal oxygen consumption above the 70th percentile, the use of oral contraceptives, or other exclusion criteria. There were missing data for one young individual due to difficulty with blood draws, precluding any meaningful analyses. The final cohort therefore included 17 participants, 8 young and 9 older men and women. Subject characteristics are shown in Table 1. As designed, the groups differed in age, but there were no significant differences in anthropometric measures such as height, weight, and body mass index. The older group had a significantly higher resting diastolic blood pressure and, as expected, a significantly lower maximal heart rate, despite no group differences in maximal oxygen consumption or maximal work load attained during the graded exercise test.
Table 1.
Participant characteristics
| Young (n = 8) | Older (n = 9) | P value | |
|---|---|---|---|
| Age (year) | 20 ± 1 | 58 ± 2 | <0.001 |
| Gender ratio (M/W) | 3/5 | 3/6 | – |
| Height (cm) | 173 ± 4 | 174 ± 3 | NS |
| Weight (kg) | 71.8 ± 4.0 | 82.2 ± 3.6 | NS |
| BMI (kg/m2) | 24.1 ± 0.9 | 26.9 ± 1.0 | NS |
| SBP (mmHg) | 113 ± 4 | 125 ± 6 | NS |
| DBP (mmHg) | 71 ± 2 | 82 ± 3 | 0.011 |
| VO2 max (mL/kg/min) | 39.5 ± 2.6 | 32.7 ± 3.3 | NS |
| Workloadmax (watts) | 231 ± 20 | 203 ± 20 | NS |
| HRmax (bpm) | 191 ± 4 | 163 ± 3 | <0.001 |
| RERmax | 1.19 ± 0.02 | 1.17 ± 0.03 | NS |
Data are means ± SEM
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, RER respiratory exchange ratio
The goal intensity for the acute exercise bout was 60 % relative to each individual’s maximal capacity. Table 2 shows the mean data from the acute exercise trial. The actual intensity during the trial was 67 % in the study cohort as a whole, with no significant difference between the young and older individuals. Additionally, there were no group differences in relative VO2 max, absolute workload, or relative workload (Table 2).
Table 2.
Acute exercise data
| Young (n = 8) | Older (n = 9) | P value | |
|---|---|---|---|
| VO2 (mL/kg/min) | 27.3 ± 2.3 | 21.6 ± 2.3 | NS |
| VO2 (% of max) | 69 ± 3 | 66 ± 2 | NS |
| Workload (watts) | 119 ± 14 | 102 ± 14 | NS |
| Workload (% of max) | 51 ± 2 | 49 ± 3 | NS |
Data are means ± SEM
Response to forearm I/R trial
The I/R trial significantly increased F2-isoprostane levels in the whole study cohort (main effect of time, P = 0.001). Comparing the two trials regardless of age, the F2-isoprostane response was significantly lower in the IRX trial (I/R trial preceded by acute exercise) as compared to the IRC trial (control) (main effect of trial, P = 0.029). There was no significant interaction between time and trial. The IRX baseline value was significantly lower than the IRC baseline value (36 ± 2 vs. 32 ± 2 pg/mL, respectively, P = 0.032). The overall F2-isoprostane concentration, indicated by the AUC, was significantly lower during IRX as compared to IRC (8149 ± 587 vs. 8967 ± 563 pg/mL/min, respectively, P = 0.04). The integrated response taking into account baseline values, calculated by area under the response curve (AURC), was not different between the two trials (IRX 1625 ± 255, IRC 1616 ± 320 pg/mL/min).
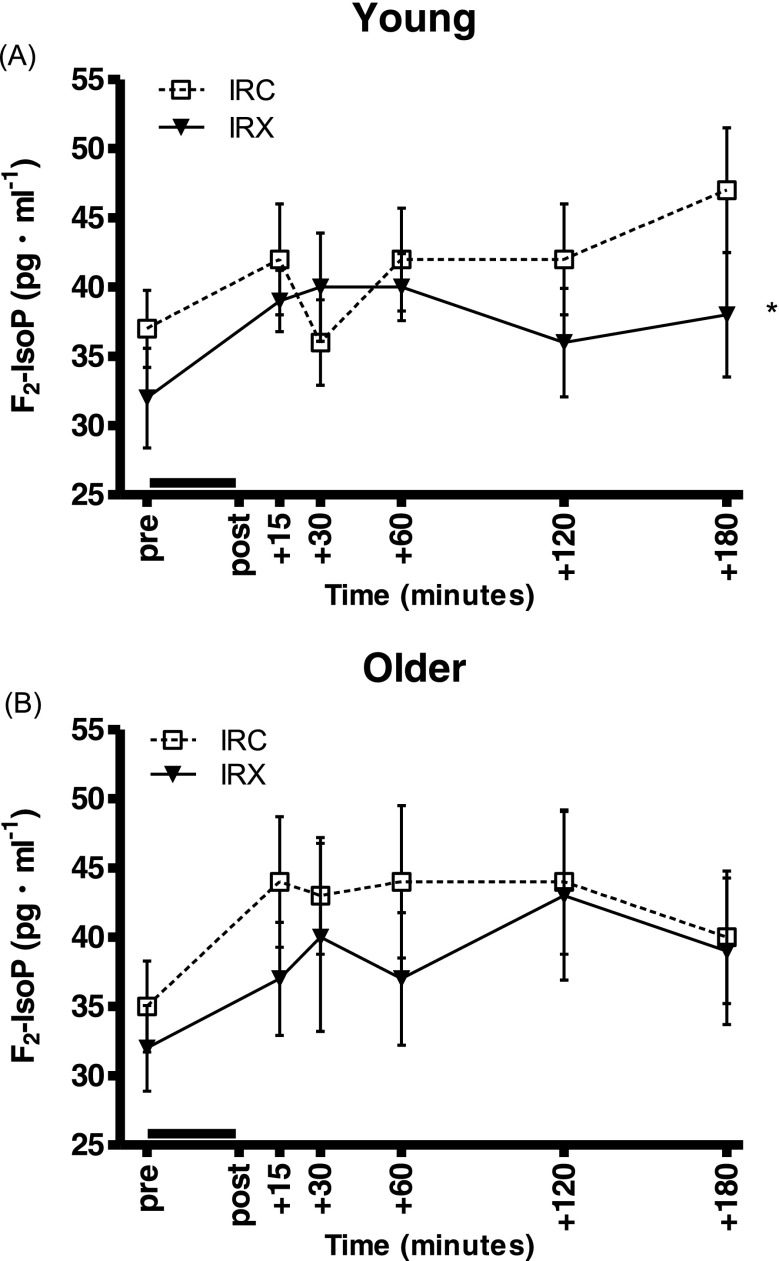
When the age groups were analyzed separately, only the young group exhibited a significantly lower F2-isoprostane response in IRX as compared to the IRC (P = 0.015, Fig. 2a), whereas there was no significant difference between the trials in the older group (Fig. 2b). Similarly, the overall integrated F2-isoprostane concentration (AUC) was significantly lower in IRX compared to IRC in the young (8001 ± 550 vs. 8857 ± 616 pg/mL/min, respectively, P = 0.026) but not the older group (IRX 8280 ± 1031, IRC 9065 ± 951 pg/mL/min). There were no significant differences in baseline values between the trials or AURC responses in either group (young: IRX 1717 ± 333, IRC 1484 ± 450; older: IRX 1544 ± 398, IRC 1733 ± 450, all pg/mL/min).
Fig. 2.
Plasma F2-isoprostane responses to the I/R trial with preceding exercise (IRX; black triangles, solid line) and the control I/R trial (IRC; open squares, dotted line). Values are means ± SEM. The black line above the x-axis denotes the time of forearm ischemia/reperfusion. The asterisk denotes a significant difference between trials. a Young adults (n = 8): there was a significantly lower response to IRX compared to IRC (P = 0.015). b Older adults (n = 9): the responses were not different between IRX and IRC (P = 0.226)
Antioxidant activity during I/R trials
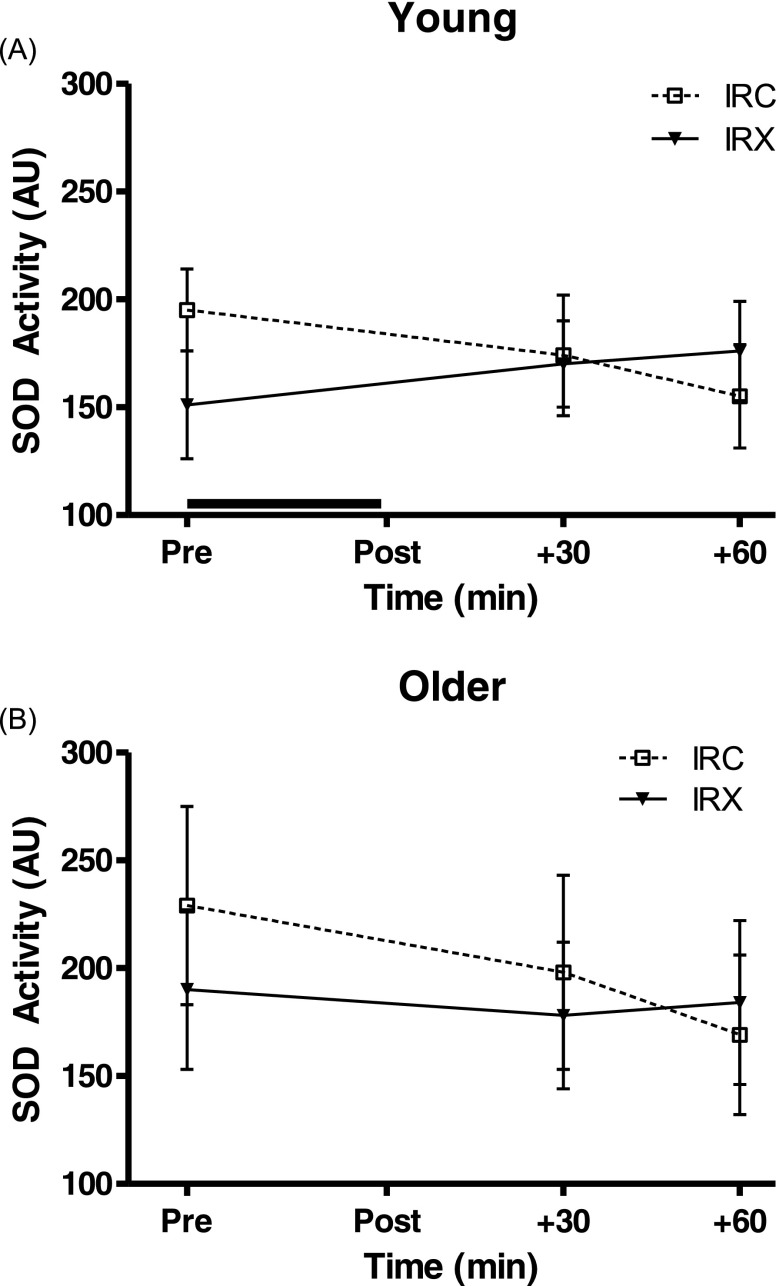
There was a significant interaction between time and trial for SOD activity (P = 0.013) in the study cohort as a whole, with no significant main effects. When the age groups were analyzed separately, the young cohort only displayed a trend in the interaction between time and trial (P = 0.079) with no significant main effects and the older group had no significant interaction or main effects. In general, the interaction occurred with baseline SOD activity being slightly higher during IRC than IRX and the activity then decreasing over the 60 min in IRC while being maintained during IRX (Fig. 3).
Fig. 3.
Superoxide dismutase (SOD) activity responses during the first hour of the I/R trial with preceding exercise (IRX; black triangles, solid line) and the control I/R trial (IRC; open squares, dotted line) in the young (a; n = 8) and older (b; n = 9) groups. Values are means ± SEM. The black line above the x-axis denotes the time of forearm ischemia/reperfusion. There were no significant main effects or interaction effect between time and trial in either group
Antioxidant activity response to acute exercise
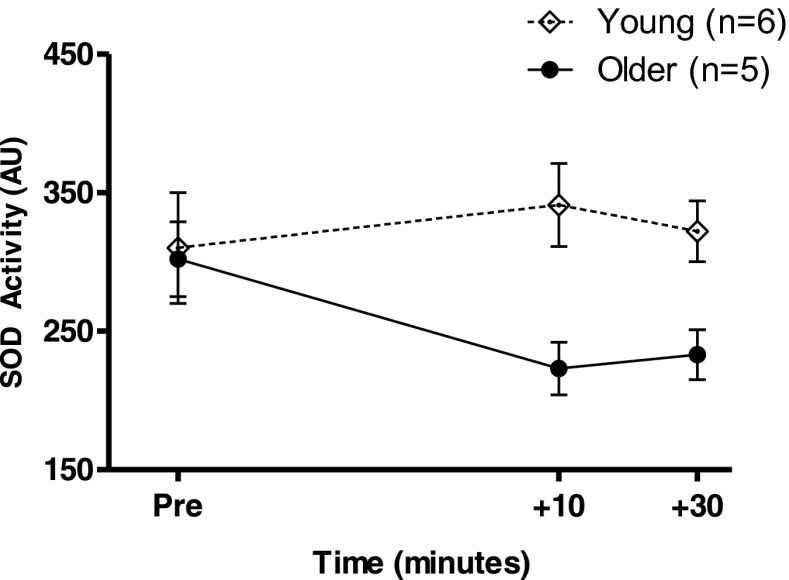
SOD activity in response to the acute exercise was analyzed in a subset of the total subject sample; six young and five older, due to funding constrains. There was a significant difference in the response between young and older adults (P = 0.048, Fig. 4).
Fig. 4.
Superoxide dismutase (SOD) activity in response to acute exercise in young (open diamonds, dotted line, n = 6) and older (black circles, solid line, n = 5) adults. Values are means ± SEM. There was a significant difference between the groups (P = 0.048). Note that these data are from a subset of the total study cohort where samples were available
Discussion
This study tested the hypotheses that acute exercise would increase resistance to a subsequent non-exercise oxidative challenge and that this protective effect would be attenuated with age. These hypotheses were supported as shown by significantly lower F2-isoprostane response to the I/R trial with preceding exercise (IRX) compared to the control (IRC) in the young but not the older individuals. The differences in quantified responses were only seen when analyzed as AUC and not when baseline was accounted for (AURC) indicating that the pattern of response was not different per se, but rather at a lower concentration. This could be due to either lower F2-isoprostane production or more efficient removal. A lower response to the forearm I/R trial with preceding exercise (IRX) compared to the control (IRC) indicates greater overall resistance to oxidative stress. The rationale for our hypothesis was based on previous literature indicating that ROS, induced by exercise, initiate cell signaling necessary for adaptation, such as antioxidant enzyme regulation that could therefore provide increased resistance to an oxidative insult (Berzosa et al. 2011; McClung et al. 2004; Muthusamy et al. 2012; Sachdev and Davies 2008). However, the functional translation of increased antioxidant enzyme activity has not been well established in humans.
Our findings of no differences between trials in the older adults support the existing data in animals where aging is associated with decreased ability to functionally translate a single bout of exercise into added protection against oxidative stress. Older mice have been shown to have impaired binding of the transcription factor Nrf-2, which is associated with gene regulation of antioxidant enzymes and could further explain impaired functional translation in older adults (Gounder et al. 2012). Our data suggest that a single bout of exercise is either insufficient in increasing antioxidants due to diminished cellular signaling or additional stimuli are necessary to promote added protection against oxidative stress because of the already elevated levels associated with normal aging. It is important to note that the exercise stimulus between the groups was equivalent as demonstrated by no significant group differences in relative intensity or workload. Nevertheless, there were significant age differences in the SOD response to the acute exercise, demonstrating that aging is associated with diminished ability to translate the increase in ROS from acute exercise to increased SOD activity. Animal models have demonstrated that chronic inhibition of SOD activity results in significantly higher F2-isoprostane levels (Lynch et al. 1997). Therefore, these lower levels of SOD activity following acute exercise in older adults, compared to young, could help explain the lack of protection from exercise against the oxidative challenge. These data are supported in rat and human models where aging attenuates the benefits of different forms of ischemic preconditioning against an I/R injury (Fenton et al. 2000; van den Munckhof et al. 2013).
The timing of the subsequent challenge was chosen to be on the following day, 16 h after the acute exercise bout. The rationale for this time interval was based on the concept that most people do not exercise more than once within a 24-h period and therefore any effects would need to stretch into that time frame in order to have clinical relevance. Additionally, previous findings show a significant increase in Nrf-2 gene expression at 12 and 18 h after acute exercise bout (Baar et al. 2002). Weiss et al. have previously shown that an acute 60-min bout of exercise performed 17 h prior to ingestion of a high sugar meal increased insulin sensitivity as assessed by flow-mediated dilation (FMD) of the brachial artery (Weiss et al. 2008). Collectively, these data suggest that acute exercise-induced stimulus can have protective effects lasting at least 17–18 h.
Limited data is available regarding the effects of aging on I/R protection in response to exercise. However, data from cardiac preconditioning suggest equivalent results to the present study regarding aging. Patients that displayed pre-infarct angina (a form of cardiac preconditioning) had smaller infarct size, lower incidence of mortality, and better prognosis (Kloner et al. 1995). These effects, however, were only seen in adult, non-elderly patients and did not extend to older adults, suggesting that the effects of preconditioning are lost or reduced with aging (Abete et al. 1997; Abete et al. 2011).
Similarly, preconditioning with blood pressure cuff at 200 mmHg did not alter the response of flow-mediated dilation of the brachial artery in older adults, whereas in young adults, there was a significant difference in endothelial function with preconditioning (van den Munckhof et al. 2013). Reasons for this increased rate of mortality and lack of preconditioning in older adults is not well understood; however, specific interest in cardiac protection has been placed on heat shock proteins, nitric oxide, and antioxidant enzymes (specifically SOD) due to cardiac protective properties and to further assess the mechanisms of age-associated decrease in the ability to handle I/R injury (Hamilton et al. 2001; Lennon et al. 2004; Starnes and Taylor 2007). We have previously shown using the forearm I/R model that older adults have decreased ability to withstand I/R insult (Davies et al. 2009; Traustadóttir et al. 2009). Furthermore, older adults were shown to have prolonged recovery to I/R injury compared to young adults (Devan et al. 2011). Collectively, these data suggest that aging affects the response to forearm I/R, as well as preconditioning which in the present study was implemented by acute exercise.
We found no differences in SOD activity between age groups in response to the I/R trial. However, it is evident as with data on ischemic preconditioning prior to I/R injury that protection against an oxidative insult would be provided through changes in the antioxidant system prior to rather than during the event. We therefore turned our attention to the changes in SOD activity in response to the acute exercise bout. F2-isoprostane response to the acute exercise was not measured because we had previously found that this protocol elicited a significant increase in F2-isoprostanes (unpublished pilot data). Additionally, it has been well established that acute exercise of equivalent time and intensity increases oxidative stress, via multiple markers in different tissues (Alessio et al. 1988; Davies et al. 1982; El Abed et al. 2011; Nikolaidis et al. 2011). The lack of a significant increase in SOD activity in response to the acute exercise in our study is not in agreement with other studies which have shown a significant increase in antioxidant defenses in response to acute exercise (Berzosa et al. 2011; Fisher-Wellman et al. 2009; Fisher-Wellman and Bloomer 2009; Terblanche 2000). One notable difference in methodology is that many of these studies found the increased activity immediately post-exercise where, in the present study, the sampling time points were 10- and 30-min post-exercise. The lower response in the older group is however in agreement with animal studies. Older adult mice have been shown to respond less efficiently to exercise, with impaired nuclear erythroid-2 like factor-2 (Nrf2) binding and subsequent lower antioxidant defenses, compared to young mice (Gounder et al. 2012).
It seems likely that repeated exercise stimuli may be necessary to increase resistance against oxidative stress in older adults. It is clear from the literature that older adults are still capable of responding to exercise. To this extent, mitochondrial biogenesis was shown to be stimulated through exercise in older sedentary adults, at levels similar to young (Cobley et al. 2012). Nevertheless, as shown in this study, the functional translation and/or upregulation of antioxidant enzymes in response to acute exercise is impaired in older adults compared to younger adults.
In summary, our findings demonstrate that a single bout of exercise confers increased resistance to a subsequent non-exercise oxidative challenge and that this protection is attenuated in older adults. In addition, our data suggest that a diminished SOD response to acute exercise in older adults preceding an oxidative challenge may partially explain this age-related impairment. While our small sample size and measurement of antioxidant capacity limited to one marker are limitations to our study, the careful control of confounding variables adds to its strength. Future studies should include a larger sample size and measurement of multiple markers of antioxidant capacity.
Acknowledgments
We would like to thank Dave Lang, M.D., for providing medical supervision, Dr. Matthew Gage for assistance with assays, and undergraduate students in the Traustadottir Lab who helped with data collection. We also thank our study volunteers for participating. This study was supported in part by the Northern Arizona University Faculty Grant Program to TT.
References
- Abete P, et al. Angina-induced protection against myocardial infarction in adult and elderly patients: a loss of preconditioning mechanism in the aging heart. J Am Coll Cardiol. 1997;30:947–954. doi: 10.1016/S0735-1097(97)00256-8. [DOI] [PubMed] [Google Scholar]
- Abete P, Testa G, Cacciatore F, Della-Morte D, Galizia G, Langellotto A, Rengo F. Ischemic preconditioning in the younger and aged heart. Aging Dis. 2011;2:138–148. [PMC free article] [PubMed] [Google Scholar]
- Alessio HM, Goldfarb AH, Cutler RG. MDA content increases in fast- and slow-twitch skeletal muscle with intensity of exercise in a rat. Am J Physiol. 1988;255:C874–877. doi: 10.1152/ajpcell.1988.255.6.C874. [DOI] [PubMed] [Google Scholar]
- Baar K, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- Berzosa C, et al. Acute exercise increases plasma total antioxidant status and antioxidant enzyme activities in untrained men. J Biomed Biotechnol. 2011;2011:540458. doi: 10.1155/2011/540458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobley JN, et al. PGC-1alpha transcriptional response and mitochondrial adaptation to acute exercise is maintained in skeletal muscle of sedentary elderly males. Biogerontology. 2012;13:621–631. doi: 10.1007/s10522-012-9408-1. [DOI] [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/S0006-291X(82)80124-1. [DOI] [PubMed] [Google Scholar]
- Davies SS, Traustadottir T, Stock AA, Ye F, Shyr Y, Harman SM, Roberts LJ., 2nd Ischemia/reperfusion unveils impaired capacity of older adults to restrain oxidative insult. Free Radic Biol Med. 2009;47:1014–1018. doi: 10.1016/j.freeradbiomed.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devan AE, et al. Endothelial ischemia-reperfusion injury in humans: association with age and habitual exercise. Am J Physiol Heart Circ Physiol. 2011;300:H813–819. doi: 10.1152/ajpheart.00845.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Abed K, et al. Antioxidant status and oxidative stress at rest and in response to acute exercise in judokas and sedentary men. J Strength Cond Res/Natl Strength Cond Assoc. 2011;25:2400–2409. doi: 10.1519/JSC.0b013e3181fc5c35. [DOI] [PubMed] [Google Scholar]
- Fatouros IG, Jamurtas AZ, Villiotou V, Pouliopoulou S, Fotinakis P, Taxildaris K, Deliconstantinos G. Oxidative stress responses in older men during endurance training and detraining. Med Sci Sports Exerc. 2004;36:2065–2072. doi: 10.1249/01.MSS.0000147632.17450.FF. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Dickson EW, Meyer TE, Dobson JG., Jr Aging reduces the cardioprotective effect of ischemic preconditioning in the rat heart. J Mol Cell Cardiol. 2000;32:1371–1375. doi: 10.1006/jmcc.2000.1189. [DOI] [PubMed] [Google Scholar]
- Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dyn Med : DM. 2009;8:1. doi: 10.1186/1476-5918-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Wellman K, Bell HK, Bloomer RJ. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxidative Med Cell Longev. 2009;2:43–51. doi: 10.4161/oxim.2.1.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S, et al. Regular exercise: an effective means to reduce oxidative stress in old rats. Annals N Y Acad Sci. 2004;1019:471–474. doi: 10.1196/annals.1297.085. [DOI] [PubMed] [Google Scholar]
- Gounder SS, et al. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS ONE. 2012;7:e45697. doi: 10.1371/journal.pone.0045697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru D, Borsa C, Ionescu C, Margina D. Advanced oxidative and glycoxidative protein damage markers in the elderly with type 2 diabetes. J Proteome. 2013;92:313–322. doi: 10.1016/j.jprot.2013.03.034. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Powers SK, Sugiura T, Kim S, Lennon S, Tumer N, Mehta JL. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am J Physiol Heart Circ Physiol. 2001;281:H1346–1352. doi: 10.1152/ajpheart.2001.281.3.H1346. [DOI] [PubMed] [Google Scholar]
- Hollander J, Fiebig R, Gore M, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Arch Eur J Physiol. 2001;442:426–434. doi: 10.1007/s004240100539. [DOI] [PubMed] [Google Scholar]
- Kloner RA, et al. Previous angina alters in-hospital outcome in TIMI 4. A clinical correlate to preconditioning. Circulation. 1995;91:37–45. doi: 10.1161/01.CIR.91.1.37. [DOI] [PubMed] [Google Scholar]
- Kohrt WM, et al. Effects of gender, age, and fitness level on response of VO2max to training in 60-71 yr olds. J Appl Physiol. 1991;71:2004–2011. doi: 10.1152/jappl.1991.71.5.2004. [DOI] [PubMed] [Google Scholar]
- Koltai E, Hart N, Taylor AW, Goto S, Ngo JK, Davies KJ, Radak Z (2012) Age-associated declines in mitochondrial biogenesis and protein quality control factors are minimized by exercise training. American journal of physiology Regulatory, integrative and comparative physiology 303:R127-134 doi:10.1152/ajpregu.00337.2011 [DOI] [PMC free article] [PubMed]
- Lennon SL, Quindry JC, Hamilton KL, French JP, Hughes J, Mehta JL, Powers SK. Elevated MnSOD is not required for exercise-induced cardioprotection against myocardial stunning. Am J Physiol Heart Circ Physiol. 2004;287:H975–980. doi: 10.1152/ajpheart.01208.2003. [DOI] [PubMed] [Google Scholar]
- Lynch SM, et al. Vascular superoxide dismutase deficiency impairs endothelial vasodilator function through direct inactivation of nitric oxide and increased lipid peroxidation. Arterioscler Thromb Vasc Biol. 1997;17:2975–2981. doi: 10.1161/01.ATV.17.11.2975. [DOI] [PubMed] [Google Scholar]
- McArdle A, Vasilaki A, Jackson M. Exercise and skeletal muscle ageing: cellular and molecular mechanisms. Aging Res Rev. 2002;1:79–93. doi: 10.1016/S0047-6374(01)00368-2. [DOI] [PubMed] [Google Scholar]
- McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A. 2004;101:8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann Neurol. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ., 2nd Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. doi: 10.1016/S0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- Muthusamy VR et al. (2012) Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free radical biology & medicine 52:366-376 doi:10.1016/j.freeradbiomed.2011.10.440 [DOI] [PMC free article] [PubMed]
- Nikolaidis MG, Kyparos A, Vrabas IS. F(2)-isoprostane formation, measurement and interpretation: the role of exercise. Prog Lipid Res. 2011;50:89–103. doi: 10.1016/j.plipres.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Razmara A, Duckles SP, Krause DN, Procaccio V. Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res. 2007;1176:71–81. doi: 10.1016/j.brainres.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev S, Davies KJ (2008) Production, detection, and adaptive responses to free radicals in exercise Free radical biology & medicine 44:215-223 doi:10.1016/j.freeradbiomed.2007.07.019 [DOI] [PubMed]
- Starnes JW, Taylor RP. Exercise-induced cardioprotection: endogenous mechanisms. Med Sci Sports Exerc. 2007;39:1537–1543. doi: 10.1249/mss.0b013e3180d099d4. [DOI] [PubMed] [Google Scholar]
- Strehlow K, et al. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93:170–177. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- Syu GD, Chen HI, Jen CJ. Severe exercise and exercise training exert opposite effects on human neutrophil apoptosis via altering the redox status. PLoS ONE. 2011;6:e24385. doi: 10.1371/journal.pone.0024385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terblanche SE. The effects of exhaustive exercise on the activity levels of catalase in various tissues of male and female rats. Cell Biol Int. 2000;23:749–753. doi: 10.1006/cbir.1999.0442. [DOI] [PubMed] [Google Scholar]
- Totter JR. Spontaneous cancer and its possible relationship to oxygen metabolism. Proc Natl Acad Sci U S A. 1980;77:1763–1767. doi: 10.1073/pnas.77.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traustadóttir T, Stock AA, Harman SM. High-dose statin use does not impair aerobic capacity or skeletal muscle function in older adults. Age (Dordr) 2008;30:283–291. doi: 10.1007/s11357-008-9070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traustadóttir T, Davies SS, Stock AA, Su Y, Heward CB, Roberts LJ, 2nd, Harman SM. Tart cherry juice decreases oxidative stress in healthy older men and women. J Nutr. 2009;139:1896–1900. doi: 10.3945/jn.109.111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traustadóttir T, Davies SS, Su Y, Choi L, Brown-Borg HM, Roberts LJ, 2nd, Harman SM. Oxidative stress in older adults: effects of physical fitness. Age (Dordr) 2012;34:969–982. doi: 10.1007/s11357-011-9277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Munckhof I, et al. Aging attenuates the protective effect of ischemic preconditioning against endothelial ischemia-reperfusion injury in humans. Am J Physiol Heart Circ Physiol. 2013;304:H1727–1732. doi: 10.1152/ajpheart.00054.2013. [DOI] [PubMed] [Google Scholar]
- Weiss EP, Arif H, Villareal DT, Marzetti E, Holloszy JO. Endothelial function after high-sugar-food ingestion improves with endurance exercise performed on the previous day. Am J Clin Nutr. 2008;88:51–57. doi: 10.1093/ajcn/88.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]