Abstract
As the population of breast cancer survivors grows, it has become evident that chemotherapy has significant cardiotoxic side effects. Echocardiography is a noninvasive, cost-effective, and widely available imaging tool that is well-positioned to serve as a primary modality for monitoring chemotherapy-induced cardiotoxicity. Although left ventricular ejection fraction is a standard measurement by which to monitor chemotherapy-induced cardiotoxicity, its predictive value in identifying subsequent cardiotoxicity is limited.
More sophisticated echocardiography modalities may offer improved sensitivity and specificity for detecting chemotherapy-induced cardiotoxicity. These include tissue Doppler imaging measures, newer techniques based upon two- and three-dimensional strain and torsion analysis, and three-dimensional measures of cardiac size. While these modalities are not all currently part of clinical practice, a body of data supporting their use is steadily building. More research remains to be performed, and non-invasively detecting cancer therapy-induced cardiac dysfunction at the earliest stages is of increasing interest.
Keywords: breast cancer, chemotherapy, cardiotoxicity, echocardiography, strain
Introduction
Due to advances in screening and treatment strategies, the population of breast cancer survivors in the United States has steadily grown over the last three decades. Overall 5-year survival improved from 63% in the 1960s to 90% in 2012, and recent data indicate that there are 2.9 million women living in the US with a history of invasive breast cancer [1]. As a result, there is a growing prevalence of cancer therapy side effects, notably cardiovascular toxicity.
Doxorubicin and trastuzumab are commonly used therapies for breast cancer that have been widely recognized as contributors to asymptomatic and symptomatic declines in left ventricular ejection fraction (LVEF), cardiomyopathy, and heart failure (HF) [2]. Anthracycline-mediated cardiomyopathy is characterized by a dose-dependent decrease in left ventricular (LV) systolic function mediated by reactive oxygen species (ROS) that is typically irreversible [3,4]. Trastuzumab-related cardiotoxicity similarly presents with a decrease in LVEF but, in contrast to anthracycline cardiotoxicity, is dose independent and commonly reversible with discontinuation of treatment and institution of cardiac medications. As monotherapy, anthracyclines have been associated with a 5-10% incidence of cardiomyopathy or HF [5-7]. The risk of cardiomyopathy and HF with trastuzumab monotherapy has been estimated at 3-12% [7,8]. Patients receiving concurrent anthracycline and trastuzumab therapy are at highest risk of cardiotoxicity, with a reported 42% incidence of cardiomyopathy and HF in large retrospective analyses [2,7].
Because of the substantial cardiovascular toxicity of these treatment regimens, there is significant clinical interest in early detection of cardiac dysfunction. In theory, this could allow for modification of dosing and administration schedules, alteration of chemotherapeutic drug combinations, or the addition of prophylactic cardioprotective therapy to mitigate myocardial damage. For this purpose, the American Heart Association recommends close monitoring of cardiac function while receiving cardiotoxic chemotherapy. However, methods and thresholds that should be used to define cardiac dysfunction remain unspecified [9].
Echocardiography is a noninvasive, cost-effective, and widely available cardiac imaging tool that is well-positioned to serve as the primary screening modality for chemotherapy-induced cardiotoxicity. While LVEF is currently the most widely used index to quantify cardiac function in cardio-oncology, there are a number of emerging indices such as Doppler derived indices, speckle-tracking derived measures of strain and strain rate, and three-dimensional echocardiography derived parameters of myocardial motion that may offer additional sensitivity and prognostic value (Table 1).
Table 1.
Advantages and disadvantages of different echocardiographic modalities.
| Advantages | Disadvantages | |
|---|---|---|
| LVEF | -widely accessible - low LVEF may be associated with the development of clinical heart failure - 3-dimensional LVEF may have improved reproducibility |
- normal LVEF is insensitive for predicting subsequent cardiotoxicity - decreased LVEF may be a late finding - subject to effects of tethering - limited assessment in patients with poor acoustic windows |
| Tissue Doppler Imaging (S and E') | - can also measure diastolic performance | - angle-dependent - subject to effects of tethering - small shifts in sample volume can result in large changes in results |
| 2-Dimensional Strain | - more sensitive than LVEF in identifying subclinical cardiotoxicity - less influenced by tethering effects |
- requires specialized software for analysis - results may not be reproducible across analysis platforms - measures myocardial shortening in only one direction at a time |
| 3-Dimensional Strain | - measures myocardial shortening in multiple directions simultaneously | - requires specialized software for analysis - limited data exists to assess validity |
LVEF, left ventricular ejection fraction
Left ventricular ejection fraction
LVEF is a widely used measure that is indicative of cardiac systolic function and predictive of clinical outcomes in patients with systolic HF [10]. In the chemotherapy cardiotoxicity literature, a change in LVEF is commonly used to diagnose cardiotoxicity, although various studies have used different thresholds to define a clinically significant decline [11]. For example, the diagnostic criteria for cardiac dysfunction as defined by the Cardiac Review and Evaluation Committee (CREC) have been used by many and are defined as: 1) cardiomyopathy characterized by a global decrease in LVEF; 2) signs or symptoms of HF; or 3) decline in LVEF of at least 5% to less than 55% with signs or symptoms of HF or decline in LVEF of at least 10% to less than 55% without signs or symptoms of HF [8]. In contrast, the HERA study used a decline in LVEF of greater than or equal to 10% to less than 50% [12,13]. Others have also evaluated absolute quantitative change in LVEF over time [14].
Although pre-treatment LVEF has been shown to be associated with subsequent development of HF symptoms, the optimal threshold for prediction remains unclear. In a study of 120 women undergoing epirubicin therapy, baseline LVEF derived from multi-gated acquisition scans (MUGA) was significantly lower among patients who later developed symptomatic HF (median 53% versus median 61%), but it is important to note that these LVEF values were still well within the normal range [15]. Furthermore, there was no clear association between changes in LVEF during treatment and the subsequent development of HF. In a larger study of 1664 patients undergoing sequential doxorubicin and trastuzumab therapy, both pre-doxorubicin and pre-trastuzumab LVEF, again defined by MUGA, were associated with subsequent development of New York Heart Association (NYHA) class III or IV HF in univariate models. Patients with a borderline normal LVEF of 50-54% had a greater incidence of HF compared to those with an LVEF of 55-64% or ≥65% [16]. However, subsequent HF was still observed in patients who had a baseline normal LVEF. The HERA study also demonstrated that an LVEF of 55-60% was associated with cardiac events, compared to patients with an LVEF ≥60%, and similarly, for an LVEF of 60-65% compared to an LVEF ≥65% [17]. The robustness of these cutpoints–still well within the normal range for LVEF–along with their sensitivity, specificity, and positive and negative predictive value remain unknown. In another smaller prospective study of patients receiving doxorubicin followed by trastuzumab, post-anthracycline LVEF was not associated with risk of subsequent cardiotoxicity [18]. Finally, it is clear that LVEF is not a sensitive method to detect histological evidence of cardiomyocyte damage [19].
Overall, the accuracy of LVEF is limited by image quality, loading conditions, the presence of regional wall motion abnormalities, and the estimation inherent in its calculation [20,21]. In both systolic and diastolic HF, LVEF has demonstrated limited prognostic value in the low-normal range or higher [22], and is viewed as an insensitive marker for functional LV impairment, as myocardial compensatory mechanisms allow for adequate ventricular output even in the presence of cardiomyocyte dysfunction [23,24].
From a technical standpoint, contrast echocardiography and three-dimensional (3D) echocardiography may improve the acquisition and measurement of traditional LVEF. Contrast echocardiography enhances visualization of the endocardial border, thereby enabling LVEF measurements from previously non-diagnostic studies and reducing intra-observer and inter-observer variability of LVEF measurements, even in studies with optimal image quality [25,26]. 3D echocardiography has been shown to be more accurate than two-dimensional (2D) echocardiography for the measurement of ventricular volume and LVEF [27,28]. In fact, Jenkins, et al. suggest that contrast-enhanced 2D echocardiography and non-contrast enhanced 3D echocardiography have similar accuracy for LVEF when compared with cardiac MRI [28]. Specifically in patients receiving anthracyclines and trastuzumab, 3D echocardiography appears to be more reproducible than both 2DE and contrast-enhanced 2DE for serial examinations [29]. However, Hare, et al. failed to show a change in LVEF with trastuzumab therapy on 2D or 3D echocardiography [30]. Additional studies with clinically relevant endpoints are needed to determine the incremental value of contrast echocardiography and 3D echocardiography for the prediction of cardiotoxicity after chemotherapy.
Doppler-Derived Measures
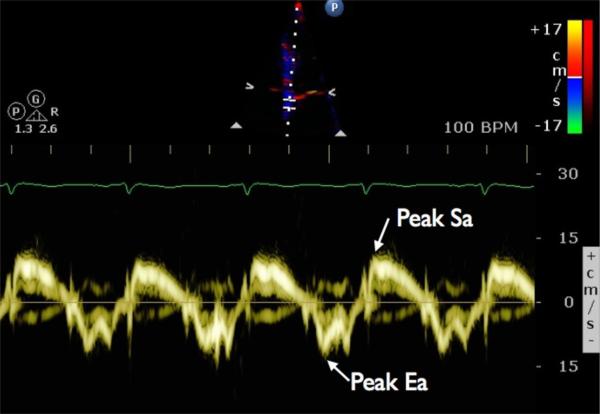
Tissue Doppler imaging (TDI) uses Doppler echocardiography to measure the velocity of myocardial tissue, allowing for quantitative and reproducible assessment of global and regional cardiac function. Commonly measured TDI indices include peak systolic myocardial velocity (Sm), peak systolic mitral annular velocity (Sa), peak early diastolic myocardial velocity (Em), and peak early mitral annular diastolic velocity (Ea) (Fig. 1). Many studies have demonstrated impairments in peak systolic or diastolic velocities despite normal LVEF, mostly in diastolic heart failure [31], but also in diabetic patients without overt heart failure [32]. Both systolic and diastolic tissue velocities have been shown to be strong predictors of outcome in several conditions including systolic HF, valvular heart disease, and left ventricular hypertrophy [33-35]. In these populations, peak early diastolic velocity appears to be the strongest predictor of outcomes [35-37].
Figure 1.
Tissue Doppler velocities of the mitral annulus are shown for a sample patient. The sample volume is positioned over the septal annulus of the mitral valve. Peak systolic annular velocity (Sa) and early diastolic annular velocity (Ea) are indicated. Similarly, values for peak systolic myocardial velocity (Sm) and peak early diastolic myocardial velocity (Em) can be obtained by positioning the sample volume over a basal segment of the left ventricular wall
Among patients treated with anthracyclines and trastuzumab, TDI measurements appear to be sensitive indicators of LV dysfunction. In cross-sectional analysis, Ho, et al. showed that peak systolic velocity and early diastolic annular velocity were significantly lower in patients receiving anthracyclines as compared with chemotherapy-naïve controls, as well as in trastuzumab-treated patients as compared with trastuzumab-naïve patients, despite absence of significant change in LVEF with anthracycline or trastuzumab therapy [38]. A small study of 20 patients receiving anthracycline therapy showed a significant change in peak early diastolic velocity within 1 to 3 months of chemotherapy, whereas changes in peak systolic velocity and LVEF were not detected until 3 years after cessation of chemotherapy [39].
TDI parameters may also be predictors of outcome in some high risk patients treated by anthracyclines and trastuzumab. Fallah-Rad, et al. prospectively followed 42 women undergoing adjuvant trastuzumab therapy, of which a high percentage (24%) developed echocardiographic and clinical symptoms of LV dysfunction [40]. Three months after receiving trastuzumab, there was a significant decrease in peak systolic annular velocity in all 10 patients that later developed HF, with no false positives in the normal cohort. In comparison, differences in LVEF were not detected until 6 months after receiving trastuzumab. Interestingly, there were no differences in peak early diastolic annular velocity or late diastolic velocity between the normal and cardiomyopathy cohorts at any time point.
There are important limitations to the use of TDI technology. As with all Doppler techniques, TDI measures only the vector of motion that is parallel to the direction of the ultrasound beam, resulting in significant angle-dependence and signal noise in the data obtained. In addition, TDI measures absolute tissue motion and is unable to distinguish between passive motion (related to translation or tethering) and active motion (fiber shortening or lengthening). Finally, ensuring reproducibility of TDI data is challenging, as small shifts in sample regions can yield significant differences in measurements.
The Tei index is a Doppler-derived parameter proposed by Tei, et al. in 1995 that is considered to be a global index of cardiac function as it includes elements of diastolic and systolic function [41]. It is defined as the sum of isovolumetric contraction and relaxation time divided by the ejection time, and has been shown to correlate well with other invasive and noninvasive measures of LV function [42]. It is a sensitive indicator of overall cardiac dysfunction in a variety of disease states [41,43,44], and has also demonstrated prognostic significance in dilated cardiomyopathy, primary pulmonary hypertension, and amyloid cardiomyopathy [45,46].
In the setting of anthracycline use, the Tei index does appear to correlate with impaired cardiac function. In 67 patients receiving doxorubicin therapy, Belham, et al. showed that an increase in Tei index, suggestive of worsening systolic and diastolic function, occurred earlier than changes in LVEF and peak systolic and diastolic velocities [47]. In a separate population of 100 patients receiving anthracyclines, 17.7% had a significant decline in LVEF whereas 78.8% had a significant increase in Tei index; moreover, the change in Tei index was detected just 1 month after receiving chemotherapy [48]. However, it remains unclear whether impairment in Tei index is also associated with worse clinical outcomes. Additional evaluation with clinically relevant endpoints is required in order to determine the clinical utility and prognostic significant of the Tei index in this population.
Strain and Torsion
While LV systolic function has been classically assessed by LVEF, as measured by 2D echo, this modality is limited by high inter-observer variability, dependence on loading conditions, and tethering of wall segments. This final factor is particularly important, as it can hinder the identification of subtle regional variations LV function and contractility.
This problem is addressed by methods that can quantify intrinsic myocardial deformation, such as strain. Strain is a mechanical property of any material, defined as the distance a material deforms relative to its length at rest. Initially, myocardial strain rate (and its integral over a cardiac cycle, strain) was measured with TDI, by comparing the velocities of adjacent areas of myocardium divided by the distance between these areas during the cardiac cycle [49]. However, this method suffers from the same limitations that all Doppler-derived measurements suffer from, namely angle-dependence and aliasing [50]. A newer technology, speckle-tracking, identifies unique echocardiographic signals (speckles) corresponding to a specific location along the myocardium, and tracks hundreds of pairs of these speckles during the cardiac cycle to measure the distance between them and calculate the strain in various parts of the myocardium [51]. Strain echocardiography has been used to predict outcomes after acute MI [52-54], infiltrative cardiomyopathy, and clinical outcomes in systolic heart failure [55-58].
After its development, strain analysis quickly provided utility in identifying chemotherapy-induced cardiotoxicity earlier than conventional echocardiographic methods. Early murine data showed that TDI-based strain rate imaging could detect decreases in contractility 1 day after a dose of doxorubicin, while decreases in LVEF and fractional shortening were not evident until day 5 [59]. An early human study showed that reductions in strain rate could be detected with cumulative doses of anthracycline as low as 200mg/m2, and that these changes corresponded to increases in levels of ROS, the purported mechanism of anthracycline-induced cardiotoxicity [60]. LVEF and measures of diastolic function have been measured after similar doses and have been found to be unchanged [60,61]. Similar results documenting early decreases in strain following chemotherapy have been reported when analyzing data from patients treated with anthracyclines and trastuzumab in patients with breast cancer [38,62], and in patients with other malignancies treated with anthracyclines alone [63,64].
Strain echocardiography has also been shown to predict subsequent consequences of anthracycline chemotherapy. In the murine study of Neilan, et. al, early changes in strain rate predicted later mortality when doxorubicin was administered for 5 weeks [59]. In several studies of patients with breast cancer treated with anthracyclines with or without trastuzumab, decreases in longitudinal strain detectable at 3-6 months of therapy predicted identification of cardiotoxicity at 6 to 12 months of therapy [65,66]. Unfortunately, studies following patients for longer periods of time after chemotherapy have not yet been published, so the natural history of strain abnormalities in chemotherapy-induced cardiotoxicity are unknown.
In addition to measuring linear deformation in multiple dimensions, speckle-tracking imaging can also track rotational movement of myocardium. From this, one can derive torsion, or the difference in twisting angle between the base and apex, and torsion velocity, or the rate of twisting. In 25 adult patients receiving anthracyclines, both torsion and torsion velocity were significantly decreased 1 month after starting chemotherapy, while LVEF and Doppler-derived parameters, including the Tei index, were unchanged [67]. Limited data suggest that these decreases in torsion may persist. A recent case-control study analyzed 36 children treated with anthracyclines and compared them to 20 healthy controls. Cases were analyzed a median of 7 years after completion of chemotherapy and showed significant reductions in torsion and torsion velocity relative to controls [68]. In this study however, patients also had significantly reduced LVEF relative to controls. Patients were not analyzed immediately after completion of chemotherapy, so the ability of torsion and torsion velocity to predict later decreases in LVEF was not able to be assessed.
While strain echocardiography represents a promising advance in detecting chemotherapy-induced cardiotoxicity, it has important limitations. First, analysis software requires high-quality images in order to track each myocardial segment accurately, which may be difficult to obtain in all patients. Second, the reproducibility of measurements needs to be improved. Both intra- and inter-observer variability have been noted to be high, especially for circumferential and radial strain measurements, whereas longitudinal strain has been noted to be most reproducible [69,70]. Variability has also been observed when comparing different analysis software packages, most likely due to the use of unique algorithms for calculating strain [71]. Third, as with any derived or calculated measurement, it is important to ensure that the results actually represent physiologic phenomena. The arrangement of myocardial fibers is complex, with longitudinal, circumferential, and obliquely oriented fibers, and how the individual strain components represent the independent function of these muscle fibers remains unknown [72,73]. Additionally, if myocardial fibers are assumed to be incompressible, longitudinal or circumferential fiber shortening will invariably result in fiber thickening, which will in turn result in wall thickening. As a result, while this wall thickening will be measured as radial strain, it may not represent intrinsic radial systolic function of the myocardium. Indeed, more recent studies have begun to limit reporting of radial strain, focusing more on longitudinal and circumferential strain [74]. Finally, long term data using strain, as well as verification of these findings in multiple patient cohort studies with definition of optimal cutpoints, are lacking. Further research with validation in large cohort studies is necessary before strain echocardiography can be relied upon to diagnose and monitor chemotherapy-induced cardiotoxicity.
Future Directions
There are a number of additional methods to identify chemotherapy-induced cardiotoxicity currently being investigated. Each method described below attempts to improve upon or overcome limitations of existing methods, but only limited data exist describing their utility.
It may be possible to increase the sensitivity of detecting chemotherapy-induced cardiotoxicity by combining strain echocardiography with the measurement of circulating biomarkers. Both strain and serum troponin I measurements may be abnormal early in the course of anthracycline chemotherapy, and both are predictive of development of future cardiotoxicity [65]. Furthermore, the combination of longitudinal strain < 19% and troponin I > 30pg/mL increased the positive predictive value to 67% from 53% when considering longitudinal strain alone. The absence of either abnormality had a 91% negative predictive value [18]. Similar increases in sensitivity and specificity have been obtained using troponin T in combination with longitudinal strain [75]. However, it is not clear that the optimal combination of measurements has been established, and the clinical significance of this strategy has not yet been determined.
Recently, 3D strain echocardiography has been developed as a way to further increase the sensitivity for detection of chemotherapy-induced cardiotoxicity. Major advantages of this method are its ability to track myocardial deformation in multiple dimensions simultaneously and its ability to quantify LV torsion. While there have been little data published using this technique, early work is promising. For example, in one study comparing 53 children treated with anthracyclines to 38 healthy controls, using 3D strain parameters in combination to characterize global LV performance had greater than 80% sensitivity and specificity in separating the groups [76]. In a study of 50 adults treated with anthracyclines, global area strain, a measure of the fractional reduction in LV endocardial area during systole, was independently associated with the total cumulative doxorubicin dose in patients with preserved ejection fraction after chemotherapy [77]. Clearly, more and larger studies are needed to validate these findings.
Another area of investigation involves the right ventricle (RV). While the vast majority of studies focus on LV systolic function, RV function can also be impaired. One such study assessed RV performance in patients with breast cancer after receiving 3 cycles of anthracycline chemotherapy. Their results show that while LVEF, LV end diastolic diameter, and LV end systolic diameter were unchanged, RV fractional area change, tricuspid annular plane systolic excursion, and Doppler measurements of RV diastolic function were all significantly decreased with anthracycline chemotherapy, and that the effect increased with the number of chemotherapy cycles received [78]. Another study followed patients for 12 months after chemotherapy and found that one-third of their patients developed RV dysfunction, defined as a depressed RVEF seen on cardiac MRI [79]. As these results have not yet been replicated by echocardiography, it remains to be seen whether echocardiography is a viable modality to follow RV function in these patients.
Conclusion
Given the increased survival rates of patients with breast cancer, long term cardiovascular care is becoming increasingly important. In the case of cancer therapy-induced cardiotoxicity, both identifying affected patients early and monitoring their response to therapy may have important clinical implications. Reproducible and broadly accessible imaging modalities such as echocardiography will likely have an important role in clinical practice and care of these patients. Additional research is critical to conclusively define the value of both conventional and novel parameters, such as Doppler derived measures and 2D and 3D strain echocardiography, in the identification and prediction of cancer therapy-induced cardiotoxicity.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Gaurav Gulati, Kathleen W. Zhang, Marielle Scherrer-Crosbie, and Bonnie Ky declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.American Cancer Society . Cancer Treatment and Survivorship Facts & Figures 2012-2013. American Cancer Society; Atlanta: 2012. [Google Scholar]
- 2.Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. J Am Coll Cardiol. Vol. 60. Elsevier Inc; 2012. Incidence of Heart Failure or CardiomyopathyAfter Adjuvant Trastuzumab Therapy for Breast Cancer. pp. 2504–12. [DOI] [PubMed] [Google Scholar]
- 3.Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB. Anthracycline Cardiotoxicity: From Bench to Bedside. Journal of Clinical Oncology. 2008;26:3777–84. doi: 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rickard J, Kumbhani DJ, Baranowski B, Martin DO, Tang WH, Wilkoff BL. Usefulness of cardiac resynchronization therapy in patients with Adriamycin-induced cardiomyopathy. The American Journal of Cardiology. 2010;105:522–6. doi: 10.1016/j.amjcard.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J. Clin. Oncol. 2005;23:8597–605. doi: 10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- 6.Du XL, Xia R, Liu C-C, Cormier JN, Xing Y, Hardy D, et al. Cardiac toxicity associated with anthracycline-containing chemotherapy in older women with breast cancer. Cancer. 2009;115:5296–308. doi: 10.1002/cncr.24621. [DOI] [PubMed] [Google Scholar]
- 7.Bowles EJA, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J. Natl. Cancer Inst. 2012;104:1293–305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J. Clin. Oncol. 2002;20:1215–21. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 9.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 10.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–42. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 11.Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, et al. Trastuzumab-Associated Cardiac Adverse Effects in the Herceptin Adjuvant Trial. Journal of Clinical Oncology. 2007;25:3859–65. doi: 10.1200/JCO.2006.09.1611. [DOI] [PubMed] [Google Scholar]
- 13.Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, et al. Longer-Term Assessment of Trastuzumab-Related Cardiac Adverse Events in the Herceptin Adjuvant (HERA) Trial. Journal of Clinical Oncology. 2010;28:3422–8. doi: 10.1200/JCO.2009.26.0463. [DOI] [PubMed] [Google Scholar]
- 14.Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, et al. Protective Effects of Carvedilol Against Anthracycline-Induced Cardiomyopathy. J Am Coll Cardiol. 2006;48:2258–62. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 15.Jensen BV, Skovsgaard T, Nielsen SL. Functional monitoring of anthracycline cardiotoxicity: a prospective, blinded, long-term observational study of outcome in 120 patients. Ann. Oncol. 2002;13:699–709. doi: 10.1093/annonc/mdf132. [DOI] [PubMed] [Google Scholar]
- 16.Tan-Chiu E, Yothers G, Romond E, Geyer CE, Ewer M, Keefe D, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2–overexpressing breast cancer: NSABP B-31. J. Clin. Oncol. American Society of Clinical Oncology. 2005;23:7811–9. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 17*.de Azambuja E, Procter MJ, van Veldhuisen DJ, Agbor-Tarh D, Metzger-Filho O, Steinseifer J, et al. Trastuzumab-Associated Cardiac Events at 8 Years of Median Follow-Up in the Herceptin Adjuvant Trial (BIG 1-01). Journal of Clinical Oncology. 2014 doi: 10.1200/JCO.2013.53.9288. in press. [The HERA trial is a large, randomized controlled trial studying the use of trastuzumab in 5102 patients with HER2 positive breast cancer. Unlike other pivotal adjuvant trastuzumab trials, HERA included patients receiving trastuzumab for both 1 year and 2 years. This study includes data from 8 years of follow-up.] [DOI] [PubMed] [Google Scholar]
- 18*.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circulation: Cardiovascular Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [This study demonstrates a role for longitudinal strain and biomarkers in the identification of patients at risk for subsequent cardiotoxicity with doxorubicin and trastuzumab therapy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ewer MS, Ali MK, Mackay B, Wallace S, Valdivieso M, Legha SS, et al. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving Adriamycin. J. Clin. Oncol. 1984;2:112–7. doi: 10.1200/JCO.1984.2.2.112. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Shah AM, Solomon SD. Myocardial deformation imaging: current status and future directions. Circulation. 2012;125:e244–8. doi: 10.1161/CIRCULATIONAHA.111.086348. [DOI] [PubMed] [Google Scholar]
- 22.Solomon SD, Anavekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–44. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 23.Ewer MS, Lenihan DJ. Left ventricular ejection fraction and cardiotoxicity: is our ear really to the ground? J. Clin. Oncol. 2008;26:1201–3. doi: 10.1200/JCO.2007.14.8742. [DOI] [PubMed] [Google Scholar]
- 24.Altena R, Perik PJ, van Veldhuisen DJ, de Vries EGE, Gietema JA. Lancet Oncology. Vol. 10. Elsevier Ltd; 2009. Cardiovascular toxicity caused by cancer treatment: strategies for early detection. pp. 391–9. [DOI] [PubMed] [Google Scholar]
- 25.Cheng S-S, Dy TC, Feinstein SB. Contrast echocardiography: review and future directions. AJC. 1998;81:41G–48G. doi: 10.1016/s0002-9149(98)00053-8. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann R, Bardeleben von S, Cate ten F, Borges AC, Kasprzak J, Firschke C, et al. Assessment of systolic left ventricular function: a multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography. European Heart Journal. 2005;26:607–16. doi: 10.1093/eurheartj/ehi083. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins C, Chan J, Hanekom L, Marwick TH. Accuracy and feasibility of online 3-dimensional echocardiography for measurement of left ventricular parameters. J Am Soc Echocardiogr. 2006;19:1119–28. doi: 10.1016/j.echo.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins C, Moir S, Chan J, Rakhit D, Haluska B, Marwick TH. European Heart Journal. Vol. 30. Eur Soc Cardiology; 2009. Left ventricular volume measurement with echocardiography: a comparison of left ventricular opacification, three-dimensional echocardiography, or both with magnetic resonance imaging. pp. 98–106. [DOI] [PubMed] [Google Scholar]
- 29.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 30.Hare JL, Brown JK, Leano R, Jenkins C, Woodward N, Marwick TH. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancertreatment with trastuzumab. American Heart Journal. 2009;158:294–301. doi: 10.1016/j.ahj.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 31.Sanderson JE. Heart failure with a normal ejection fraction. Heart. 2007;93:155–8. doi: 10.1136/hrt.2005.074187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang ZY, Leano R, Marwick TH. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin. Sci. 2004;106:53–60. doi: 10.1042/CS20030153. [DOI] [PubMed] [Google Scholar]
- 33.Agricola E, Galderisi M, Oppizzi M, Schinkel AFL, Maisano F, De Bonis M, et al. Pulsed tissue Doppler imaging detects early myocardial dysfunction in asymptomatic patients with severe mitral regurgitation. Heart. 2004;90:406–10. doi: 10.1136/hrt.2002.009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M, Yip GWK, Wang AYM, Zhang Y, Ho PY, Tse MK, et al. Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value. J Am Coll Cardiol. 2003;41:820–6. doi: 10.1016/s0735-1097(02)02921-2. [DOI] [PubMed] [Google Scholar]
- 35.Wang M, Yip G, Yu C-M, Zhang Q, Zhang Y, Tse D, et al. Independent and incremental prognostic value of early mitral annulus velocity in patients with impaired left ventricular systolic function. J Am Coll Cardiol. 2005;45:272–7. doi: 10.1016/j.jacc.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 36.Richartz BM, Werner GS, Ferrari M, Figulla HR. Comparison of left ventricular systolic and diastolic function in patients with idiopathic dilated cardiomyopathy and mild heart failure versus those with severe heart failure. AJC. 2002;90:390–4. doi: 10.1016/s0002-9149(02)02495-5. [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Yip GW, Wang AY, Zhang Y, Ho PY, Tse MK, et al. Tissue Doppler imaging provides incremental prognostic value in patients with systemic hypertension and left ventricular hypertrophy. J. Hypertens. 2005;23:183–91. doi: 10.1097/00004872-200501000-00029. [DOI] [PubMed] [Google Scholar]
- 38.Ho E, Brown A, Barrett P, Morgan RB, King G, Kennedy MJ, et al. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: a speckle tracking echocardiographic study. Heart. 2010;96:701–7. doi: 10.1136/hrt.2009.173997. [DOI] [PubMed] [Google Scholar]
- 39.Tassan-Mangina S, Codorean D, Metivier M, Costa B, Himberlin C, Jouannaud C, et al. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: early and late alterations of left ventricular function during a prospective study. Eur J Echocardiogr. 2006;7:141–6. doi: 10.1016/j.euje.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–70. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 41.Tei C. New non-invasive index for combined systolic and diastolic ventricular function. J Cardiol. 1995;26:135–6. [PubMed] [Google Scholar]
- 42.Tei C, Nishimura RA, Seward JB, Tajik AJ. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10:169–78. doi: 10.1016/s0894-7317(97)70090-7. [DOI] [PubMed] [Google Scholar]
- 43.Tei C, Dujardin KS, Hodge DO, Kyle RA, Tajik AJ, Seward JB. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol. 1996;28:658–64. doi: 10.1016/0735-1097(96)00202-1. [DOI] [PubMed] [Google Scholar]
- 44.Bruch C, Schmermund A, Marin D, Katz M, Bartel T, Schaar J, et al. Tei-index in patients with mild-to-moderate congestive heart failure. European Heart Journal. 2000;21:1888–95. doi: 10.1053/euhj.2000.2246. [DOI] [PubMed] [Google Scholar]
- 45.Dujardin KS, Tei C, Yeo TC, Hodge DO, Rossi A, Seward J. Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic-dilated cardiomyopathy. AJC. 1998;82:1071–6. doi: 10.1016/s0002-9149(98)00559-1. [DOI] [PubMed] [Google Scholar]
- 46.Yeo TC, Dujardin KS, Tei C, Mahoney DW, McGoon MD, Seward J. Value of a Doppler-derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. AJC. 1998;81:1157–61. doi: 10.1016/s0002-9149(98)00140-4. [DOI] [PubMed] [Google Scholar]
- 47.Belham M, Kruger A, Mepham S, Faganello G, Pritchard C. Monitoring left ventricular function in adults receiving anthracycline-containing chemotherapy. Eur J Heart Fail. 2007;9:409–14. doi: 10.1016/j.ejheart.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Dodos F, Halbsguth T, Erdmann E, Hoppe UC. Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin Res Cardiol. 2008;97:318–26. doi: 10.1007/s00392-007-0633-6. [DOI] [PubMed] [Google Scholar]
- 49.Edvardsen T, Gerber BL, Garot J, Bluemke DA, Lima JAC, Smiseth OA. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation. 2002;106:50–6. doi: 10.1161/01.cir.0000019907.77526.75. [DOI] [PubMed] [Google Scholar]
- 50.Marwick TH. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol. 2006;47:1313–27. doi: 10.1016/j.jacc.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 51.Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–9. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 52.Antoni ML, Mollema SA, Delgado V, Atary JZ, Borleffs CJW, Boersma E, et al. Prognostic importance of strain and strain rate after acute myocardial infarction. European Heart Journal. 2010;31:1640–7. doi: 10.1093/eurheartj/ehq105. [DOI] [PubMed] [Google Scholar]
- 53.Hung CL, Verma A, Uno H, Shin SH, Bourgoun M, Hassanein AH, et al. J Am Coll Cardiol. Vol. 56. Elsevier Inc; 2010. Longitudinal and CircumferentialStrain Rate, Left Ventricular Remodeling, and Prognosis After Myocardial Infarction. pp. 1812–22. [DOI] [PubMed] [Google Scholar]
- 54.Ersbøll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ, et al. Prediction of All-Cause Mortality and Heart Failure Admissions From Global Left Ventricular Longitudinal Strain in Patients With Acute Myocardial Infarction and Preserved Left Ventricular Ejection Fraction. J Am Coll Cardiol. 2013;61:2365–73. doi: 10.1016/j.jacc.2013.02.061. [DOI] [PubMed] [Google Scholar]
- 55.Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ. J Am Coll Cardiol. Vol. 54. American College of Cardiology Foundation; 2009. Global 2-Dimensional Strain as a New Prognosticator in Patients With Heart Failure. pp. 618–24. [DOI] [PubMed] [Google Scholar]
- 56.Mignot A, Donal E, Zaroui A, Reant P, Salem A, Hamon C, et al. Journal of the American Society of Echocardiography. Vol. 23. Elsevier Inc; 2010. Global Longitudinal Strain as a Major Predictor of Cardiac Events in Patients with Depressed Left Ventricular Function: A Multicenter Study. pp. 1019–24. [DOI] [PubMed] [Google Scholar]
- 57.Motoki H, Borowski AG, Shrestha K, Troughton RW, Tang WHW, Thomas JD, et al. J Am Coll Cardiol. Vol. 60. Elsevier Inc; 2012. Incremental Prognostic Value ofAssessing Left Ventricular Myocardial Mechanics in Patients With Chronic Systolic Heart Failure. pp. 2074–81. [DOI] [PubMed] [Google Scholar]
- 58.Zhang KW, French B, May Khan A, Plappert T, Fang JC, Sweitzer NK, et al. Strain Improves Risk Prediction Beyond Ejection Fraction in Chronic Systolic Heart Failure. Journal of the American Heart Association. 2013 doi: 10.1161/JAHA.113.000550. doi:10.1161/JAHA.113.000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neilan TG, Jassal DS, Perez-Sanz TM, Raher MJ, Pradhan AD, Buys ES, et al. Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury. European Heart Journal. 2006;27:1868–75. doi: 10.1093/eurheartj/ehl013. [DOI] [PubMed] [Google Scholar]
- 60.Mercuro G, Cadeddu C, Piras A, Dessi M, Madeddu C, Deidda M, et al. Early Epirubicin-Induced Myocardial Dysfunction Revealed by Serial Tissue Doppler Echocardiography: Correlation with Inflammatory and Oxidative Stress Markers. The Oncologist. 2007;12:1124–33. doi: 10.1634/theoncologist.12-9-1124. [DOI] [PubMed] [Google Scholar]
- 61.Appel JM, Sogaard P, Mortensen CE, Skagen K, Nielsen DL. Journal of the American Society of Echocardiography. Vol. 24. Elsevier Inc; 2011. Tissue-Doppler Assessment of Cardiac Left Ventricular Function during Short-Term Adjuvant Epirubicin Therapy for Breast Cancer. pp. 200–6. [DOI] [PubMed] [Google Scholar]
- 62.Stoodley PW, Richards DAB, Boyd A, Hui R, Harnett PR, Meikle SR, et al. Left ventricular systolic function in HER2/neu negative breast cancer patients treated with anthracycline chemotherapy: a comparative analysis of left ventricular ejection fraction and myocardial strain imaging over 12 months. Eur. J. Cancer. 2013;49:3396–403. doi: 10.1016/j.ejca.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 63.Kang Y, Cheng L, Li L, Chen H, Sun M, Wei Z, et al. Early detection of anthracycline-induced cardiotoxicity using two-dimensional speckle tracking echocardiography. Cardiol J. 2013;20:592–9. doi: 10.5603/CJ.2013.0158. [DOI] [PubMed] [Google Scholar]
- 64.Mavinkurve-Groothuis AMC, Marcus KA, Pourier M, Loonen J, Feuth T, Hoogerbrugge PM, et al. Myocardial 2D strain echocardiography and cardiac biomarkers in children during and shortly after anthracycline therapy for acute lymphoblastic leukaemia (ALL): a prospective study. Eur Heart J Cardiovasc Imaging. 2013;14:562–9. doi: 10.1093/ehjci/jes217. [DOI] [PubMed] [Google Scholar]
- 65.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. The American Journal of Cardiology. 2011;107:1375–80. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66*.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Journal of the American Society of Echocardiography. Vol. 26. Elsevier Inc; 2013. Independent and Incremental Value of Deformation Indices for Prediction of Trastuzumab-Induced Cardiotoxicity. pp. 493–8. [This study compares different echocardiograpic modalities, including LVEF, Sa, Ea, and strain, over time in a population of breast cancer patients. It also attempts to identify optimal cutpoints for maximal sensitivity and specificity for detecting cardiotoxicity.] [DOI] [PubMed] [Google Scholar]
- 67.Motoki H, Koyama J, Nakazawa H, Aizawa K, Kasai H, Izawa A, et al. Torsion analysis in the early detection of anthracycline-mediated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2012;13:95–103. doi: 10.1093/ejechocard/jer172. [DOI] [PubMed] [Google Scholar]
- 68.Cheung Y-F, Li S-N, Chan GCF, Wong SJ, Ha S-Y. Left Ventricular Twisting and Untwisting Motion in Childhood Cancer Survivors. Echocardiography. 2011;28:738–45. doi: 10.1111/j.1540-8175.2011.01429.x. [DOI] [PubMed] [Google Scholar]
- 69.Koopman LP, Slorach C, Manlhiot C, McCrindle BW, Jaeggi ET, Mertens L, et al. J Am Soc Echocardiogr. Vol. 24. Elsevier Inc; 2011. Assessment of Myocardial Deformation in Children Using Digital Imaging and Communications in Medicine (DICOM) Data and Vendor Independent Speckle Tracking Software. pp. 37–44. [DOI] [PubMed] [Google Scholar]
- 70.Risum N, Ali S, Olsen NT, Jons C, Khouri MG, Lauridsen TK, et al. J Am Soc Echocardiogr. Vol. 25. Elsevier Inc; 2012. Variability of Global Left Ventricular Deformation Analysis Using Vendor Dependent and Independent Two-Dimensional Speckle-Tracking Software in Adults. pp. 1195–203. [DOI] [PubMed] [Google Scholar]
- 71.Marwick TH. Consistency of myocardial deformation imaging between vendors. European Journal of Echocardiography. 2010;11:414–6. doi: 10.1093/ejechocard/jeq006. [DOI] [PubMed] [Google Scholar]
- 72.Bovendeerd PHM, Kroon W, Delhaas T. Determinants of left ventricular shear strain. AJP: Heart and Circulatory Physiology. 2009;297:H1058–68. doi: 10.1152/ajpheart.01334.2008. [DOI] [PubMed] [Google Scholar]
- 73.Deng D, Jiao P, Ye X, Xia L. An Image-Based Model of the Whole Human Heart with Detailed Anatomical Structure and Fiber Orientation. Computational and Mathematical Methods in Medicine. 2012;2012:1–16. doi: 10.1155/2012/891070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan TC, Scherrer-Crosbie M. Cardiac complications of chemotherapy: role of imaging. Curr Treat Options Cardiovasc Med. 2014;16:296. doi: 10.1007/s11936-014-0296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang Y, Xu X, Cheng L, Li L, Sun M, Chen H, et al. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur J Heart Fail. 2014;16:300–8. doi: 10.1002/ejhf.8. [DOI] [PubMed] [Google Scholar]
- 76.Yu H-K, Yu W, Cheuk DKL, Wong SJ, Chan GCF, Cheung Y-F. Journal of the American Society of Echocardiography. Vol. 26. Elsevier Inc; 2013. New Three-Dimensional Speckle-Tracking Echocardiography Identifies Global Impairment of Left Ventricular Mechanics with a High Sensitivity in Childhood Cancer Survivors. pp. 846–52. [DOI] [PubMed] [Google Scholar]
- 77.Miyoshi T, Tanaka H, Kaneko A, Tatsumi K, Matsumoto K, Minami H, et al. Left Ventricular Endocardial Dysfunction in Patients with Preserved Ejection Fraction after Receiving Anthracycline. Echocardiography. 2013 doi: 10.1111/echo.12473. doi:10.1111/echo.12473. [DOI] [PubMed] [Google Scholar]
- 78.Tanindi A, Demirci U, Tacoy G, Buyukberber S, Alsancak Y, Coskun U, et al. Assessment of right ventricular functions during cancer chemotherapy. Eur J Echocardiogr. 2011;12:834–40. doi: 10.1093/ejechocard/jer142. [DOI] [PubMed] [Google Scholar]
- 79.Grover S, Leong DP, Chakrabarty A, Joerg L, Kotasek D, Cheong K, et al. Left and right ventricular effects of anthracycline and trastuzumab chemotherapy: a prospective study using novel cardiac imaging and biochemical markers. Int J Cardiol. 2013;168:5465–7. doi: 10.1016/j.ijcard.2013.07.246. [DOI] [PubMed] [Google Scholar]