Abstract
Study Objectives:
To determine if weight loss and/or changes in apnea-hypopnea index (AHI) improve sleep architecture in overweight/obese adults with type 2 diabetes (T2D) and obstructive sleep apnea (OSA).
Methods:
This was a randomized controlled trial including 264 overweight/obese adults with T2D and OSA. Participants were randomized to an intensive lifestyle intervention (ILI) or a diabetes and support education (DSE) control group. Measures included anthropometry, AHI, and sleep at baseline and year-1, year-2, and year-4 follow-ups.
Results:
Changes in sleep duration (total sleep time [TST]), continuity [wake after sleep onset (WASO)], and architecture stage 1, stage 2, slow wave sleep, and REM sleep) from baseline to year 1, 2, and 4 did not differ between ILI and DSE. Repeated-measure mixed-model analyses including data from baseline through year-4 for all participants demonstrated a significant positive association between AHI and stage 1 sleep (p < 0.001), and a significant negative association between AHI and stage 2 (p = 0.01) and REM sleep (p < 0.001), whereas changes in body weight had no relation to any sleep stages or TST. WASO had a significant positive association with change in body weight (p = 0.009).
Conclusions:
Compared to control, the ILI did not induce significant changes in sleep across the 4-year follow-up. In participants overall, reduced AHI in overweight/obese adults with T2D and OSA was associated with decreased stage 1, and increased stage 2 and REM sleep. These sleep architecture changes are more strongly related to reductions in AHI than body weight, whereas WASO may be more influenced by weight than AHI.
Clinical Trial Registration:
ClinicalTrials.gov identifier: NCT00194259
Citation:
Shechter A, St-Onge MP, Kuna ST, Zammit G, RoyChoudhury A, Newman AB, Millman RP, Reboussin DM, Wadden TA, Jakicic JM, Pi-Sunyer FX, Wing RR, Foster GD, Sleep AHEAD Research Group of the Look AHEAD Research Group. Sleep architecture following a weight loss intervention in overweight and obese patients with obstructive sleep apnea and type 2 diabetes: relationship to apnea-hypopnea index. J Clin Sleep Med 2014;10(11):1205-1211.
Keywords: sleep architecture, obstructive sleep apnea, obesity, type 2 diabetes
Obesity, type 2 diabetes (T2D), and obstructive sleep apnea (OSA) are three related disorders which have been demonstrated to co-occur in patients. Indeed, obesity is established as one of the leading risk factors for the development of both T2D and OSA, and a high prevalence (∼86.6%) of undiagnosed OSA was found in obese patients with T2D.1 Deficits in sleep duration and/or quality may be a common link among these three factors. A growing body of both epidemiological and laboratory-based studies supports a role of short sleep duration in the development of obesity.2–4 Sleep curtailment is also associated with increased incidence of T2D as well as deficits in glucose regulation.5,6 Experimental sleep fragmentation studies have demonstrated that in addition to duration, the quality and architecture of sleep episodes as determined by polysomnographic (PSG) recordings may influence metabolism and body weight regulation. Specifically, selectively suppressing slow wave sleep (SWS) without affecting total sleep time (TST) results in reduced glucose tolerance and insulin sensitivity.7 Simultaneous reduction of both SWS and REM sleep stages without affecting TST also reduces insulin sensitivity and glucose effectiveness.8 In the latter study, the reduction in SWS and REM sleep was compensated for by an increase in stage 1 sleep. In terms of factors influencing obesity, sleep fragmentation intervention that reduced stage 2 and REM sleep but not TST resulted in decreased next-day levels of glucagon-like peptide-1 (a hormonal satiety signal) and feelings of fullness.9
BRIEF SUMMARY
Current Knowledge/Study Rationale: A growing body of evidence supports a relationship between sleep and obesity. A long-term weight loss intervention (up to 4 years) has previously been shown to improve the severity of sleep disordered breathing in a group of over-weight and obese patients with obstructive sleep apnea (OSA) and type 2 diabetes, although the effects of the structured weight loss program on nocturnal sleep duration and architecture have not yet been explored.
Study Impact: Although the weight loss intervention was effective in reducing the severity of OSA over a 4-year period, it did not lead to changes in the various polysomnographic sleep measures, indicating that the ILI did not induce changes in sleep architecture, duration, or continuity. However, overall reductions in apnea-hypopnea index over the 4-year follow-up were found to be associated with increased REM sleep and stage 2 sleep, and decreased stage 1 sleep, which suggests that reducing OSA severity may induce improvements in nocturnal sleep architecture.
These sleep fragmentation studies may be particularly relevant for patients with OSA since the intermittent hypoxia associated with the disorder results in frequent nocturnal arousals and sleep architecture disruption. Compared to non-apneic individuals, OSA patients were shown to have significantly increased percentage of stage 1 sleep, as well as significantly decreased percentage of stage 2, REM sleep, and SWS.10 Moreover, obesity, even in the absence of OSA, has been shown to have deleterious effects on sleep architecture. Compared to controls, non-apneic obese individuals were shown to have increased percentage of stage 1 sleep and decreased percentage of REM sleep.10,11 Interestingly, results from a small weight loss trial found that compared to controls, individuals in a weight loss group experienced decreased stage 1 sleep and increased stage 2 sleep.12 Consistent with this, patients were observed to have decreased expression of stage 1 sleep and increased expression of stage 2 and REM sleep after bariatric surgery.13–15 Together, these findings indicate the importance of studying the effects of an intensive lifestyle intervention on sleep architecture.
Weight loss is frequently recommended as a treatment for OSA symptoms in obese individuals, and reductions in OSA severity and concomitant improvements in nocturnal sleep quality have been observed in response to surgical16 or diet-induced17 weight reduction. Results from the Sleep AHEAD (Action for Health in Diabetes) study previously showed that compared to a diabetes support and education (DSE) control group, an active weight loss intervention was effective in reducing body weight and OSA severity in obese patients with T2D at a 1-year follow-up.18 These improvements in OSA in the treatment group persisted over a 4-year period despite a ∼50% weight regain.19 The changes in sleep architecture across the 4-year follow-up period in response to the weight loss intervention have not yet been described. The current study aimed to determine whether the benefits of a structured weight loss intervention program on OSA severity also improved nocturnal sleep duration and architecture in these overweight and obese T2D patients who were found to have OSA at baseline. We hypothesized that the weight loss intervention and its subsequent reductions in OSA severity would be associated with improvements in nocturnal sleep architecture. Moreover, considering that OSA severity in the ILI group remained improved across the 4-year follow-up despite a ∼50% weight regain,19 we hypothesized that reduced AHI would be more strongly related to improved sleep architecture than changes in body weight.
METHODS
Participants
The present manuscript focuses on the 264 participants who had OSA at baseline. These included participants were enrolled in the Sleep AHEAD study, which is an ancillary study of the Look AHEAD trial. Look AHEAD is a 16-center randomized clinical trial investigating the long-term effects of weight loss on cardiovascular morbidity and mortality in overweight and obese T2D patients.20,21 In the Look AHEAD trial, primary inclusion criteria were age 45-76 years, body mass index (BMI) ≥ 25 kg/m2 or ≥ 27 kg/m2 if taking insulin, physician-verified presence of T2D, hemoglobin A1C < 11%, and blood pressure < 160/100 mm Hg. An exclusion criterion for initial participation in the Sleep AHEAD study was previous surgical or current medical treatment for OSA, although patients with a previous diagnosis of OSA who were not treated were eligible to participate.18 Experimental procedures, including administering the interventions and obtaining measures, were approved by the institutional review board of each of the participating Sleep AHEAD sites (University of Pennsylvania; University of Pittsburgh; St. Luke's-Roosevelt Hospital/Columbia University; and The Miriam Hospital). All participants provided written informed consent.
Intervention
Participants in the Look AHEAD trial were randomized to one of 2 groups: intensive lifestyle intervention (ILI) or DSE. Sleep technicians and PSG scorers were blinded to the randomization of participants.
Intensive Lifestyle Intervention
Individuals in the ILI group participated in a group behavioral weight loss program specifically developed for obese T2D patients.19,21 Participation in this program included a reduced daily caloric intake of 1200-1500 kcal for individuals < 113.6 kg, and 1500-1800 kcal for those with body weight > 113.6 kg. To promote adherence to these calorie goals, participants were provided with meal replacement products. The physical activity component included the prescription of 175 min/week of moderate intensity activity. During years 2-4 of the ILI, the intervention was a combination of both monthly individual sessions and group sessions.
Diabetes Support and Education
Participants in the DSE group were provided with 3 group sessions each year, which focused on diet, physical activity, and social support. Participants were not weighed at these group sessions, and there was no discussion of specific behavior change strategies.
Measures
Polysomnography
Sleep was recorded at baseline, and at year 1, year 2, and year 4 with an unattended overnight PSG at the participant's home using a portable system. Recordings at baseline, year 1 and year 2 were done using the Compumedics PS2 monitor (Compumedics Sleep, Abbotsville, Australia), whereas recordings at year 4 were done using the Compumedics Safiro monitor. A comparison of the PS2 and Safiro devices indicated that measures of sleep stages and respiratory outcomes were not different between the 2 machines.19 Measured signals in the recordings included electroencephalogram (C3-A2 and C4-A1), bilateral electrooculogram, chin electromyogram, electrocardiogram, rib cage and abdominal excursion, nasal airflow via oronasal thermistry as well as nasal pressure cannula, and heart rate and oxygen saturation via pulse oximetry. PSGs were scored manually using recommended criteria22 at a single reading laboratory. TST was the sum of the duration of stage 1 sleep, stage 2 sleep, SWS, and REM sleep. Sleep stages were expressed as percentage of TST. Apnea was defined as the cessation of airflow ≥ 10 sec with concomitant respiratory-related chest wall movement for obstructive apnea, or in the absence of respiratory-related chest wall movement for central apnea. Hypopnea was defined as ≥ 30% reduction in airflow or thoraco-abdominal movement for ≥ 10 sec with ≥ 4% oxygen desaturation. Apnea-hypopnea index (AHI) was the sum of the number of apneas and hypopneas per hour of sleep. Throughout the 4-year follow-up period, none of the participants received surgical or dental treatment for OSA. Participants who were receiving continuous positive airway pressure (CPAP) treatment for OSA during the follow-up period were asked not to use the treatment for 3 nights prior to experimental PSG recording. A recent report indicates that 3 nights is a sufficient interval to reverse any treatment-related effect on AHI.23
Intrascorer reliability across the 4 years was assessed by having the masked scorer rescore a subset of 92 randomly selected PSGs. This included 47 from baseline, 20 from year 1, 8 from year 2, and 17 from year 4. The original and rescored values were significantly related: TST (r = 0.96, p < 0.0001), wake after sleep onset (WASO; r = 0.96, p < 0.0001), stage 1 sleep (r = 0.66, p < 0.0001), stage 2 sleep (r = 0.83, p < 0.0001), SWS (r = 0.66, p < 0.0001), and REM sleep (r = 0.87, p < 0.0001).
Anthropometry
Body weight (measured in kg) was assessed at the annual clinic visit by blind assessors, and was done within 1 week of PSG recordings.
Statistical Analyses
Adjusted change from baseline was calculated for all sleep architecture parameters at year 1, year 2, and year 4. Changes from baseline for these parameters were adjusted for sex, age, race, and treatment site. Repeated-measure linear mixed-model analysis was used to compare the between-group differences in adjusted change-from-baseline at year 1, year 2, and year 4 for each sleep parameter. Repeated-measure mixed-model analysis was used to assess the relationship between sleep parameters and various predictor variables at baseline, year 1, year 2, and year 4 when data from all participants were pooled together. For the mixed-model analyses, individuals with baseline and ≥ 1 additional year of data were included. Dependent variables were measures of TST, sleep continuity (WASO), and sleep architecture (percentage of stage 1 sleep, stage 2 sleep, SWS, and REM sleep) at baseline, year 1, year 2, and year 4. Predictor variables for the models were treatment group (ILI vs. DSE), year, AHI, and body weight at baseline, year 1, year 2, and year 4. A treatment group × year interaction term was also included in the models. For these repeated-measure analyses, changes in variables over time were considered in the model. Year was treated as a continuous variable in the mixed-model analyses. Analyses were adjusted for sex, age, race, and treatment site. A p-value < 0.05 was used to define statistical significance in these analyses.
RESULTS
Participants
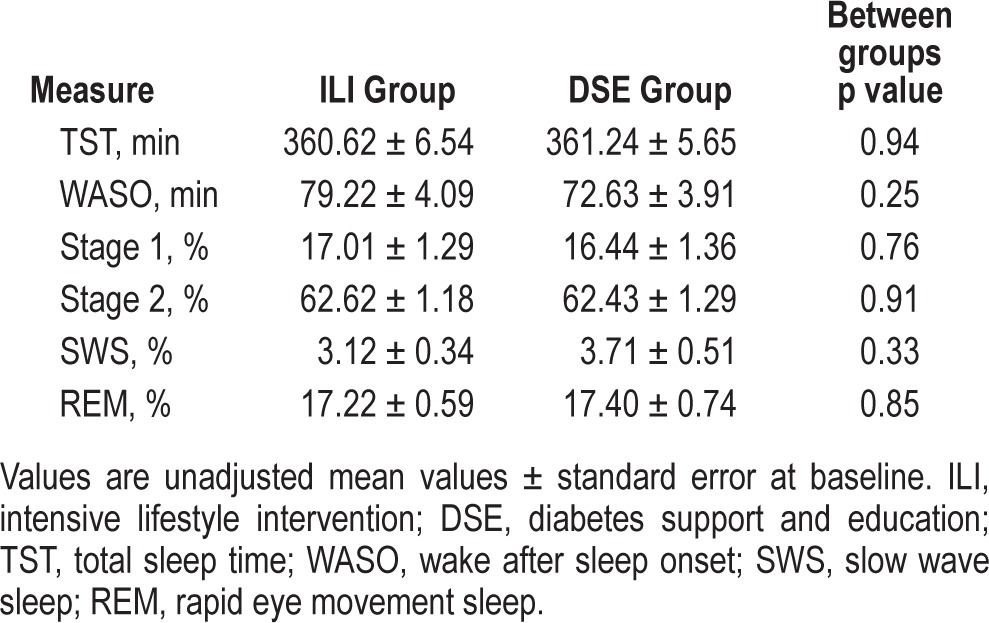
A summary of the number of participants who were initially enrolled in the Sleep AHEAD trial and who completed assessments at 1-, 2- and 4-year follow-up visits was previously described.19 A total of 305 participants underwent baseline PSG, and of these, 41 did not have OSA and were therefore excluded from the present analyses. In the remaining 264 participants, 125 were enrolled in the ILI group and 139 were enrolled in the DSE group. For ILI, 82% (n = 103) returned for follow-up at year 1, 79% (n = 99) returned for follow-up at year 2, and 66% (n = 82) returned at year 4. For DSE, 83% (n = 116) returned for follow-up at year 1, 80% (n = 111) returned for follow-up at year 2, and 60% (n = 83) returned for year 4.19 None of the baseline participant characteristics (including age, sex and race distribution, BMI, height, weight, waist and neck circumference, AHI, oxygen desaturation index > 4%, fasting glucose, and hemoglobin A1C) differed between ILI and DSE groups. Baseline sleep parameters did not differ between groups, and are summarized in Table 1. Effects of the ILI on body weight and AHI throughout the 4-year follow-up period have been previously described in these participants. Briefly, compared to DSE, participants in ILI had significantly greater reductions in body weight and AHI at years 1-4.19
Table 1.
Sleep duration and architecture measures at baseline.
Effects of the Intensive Lifestyle Intervention on PSG Sleep Measures
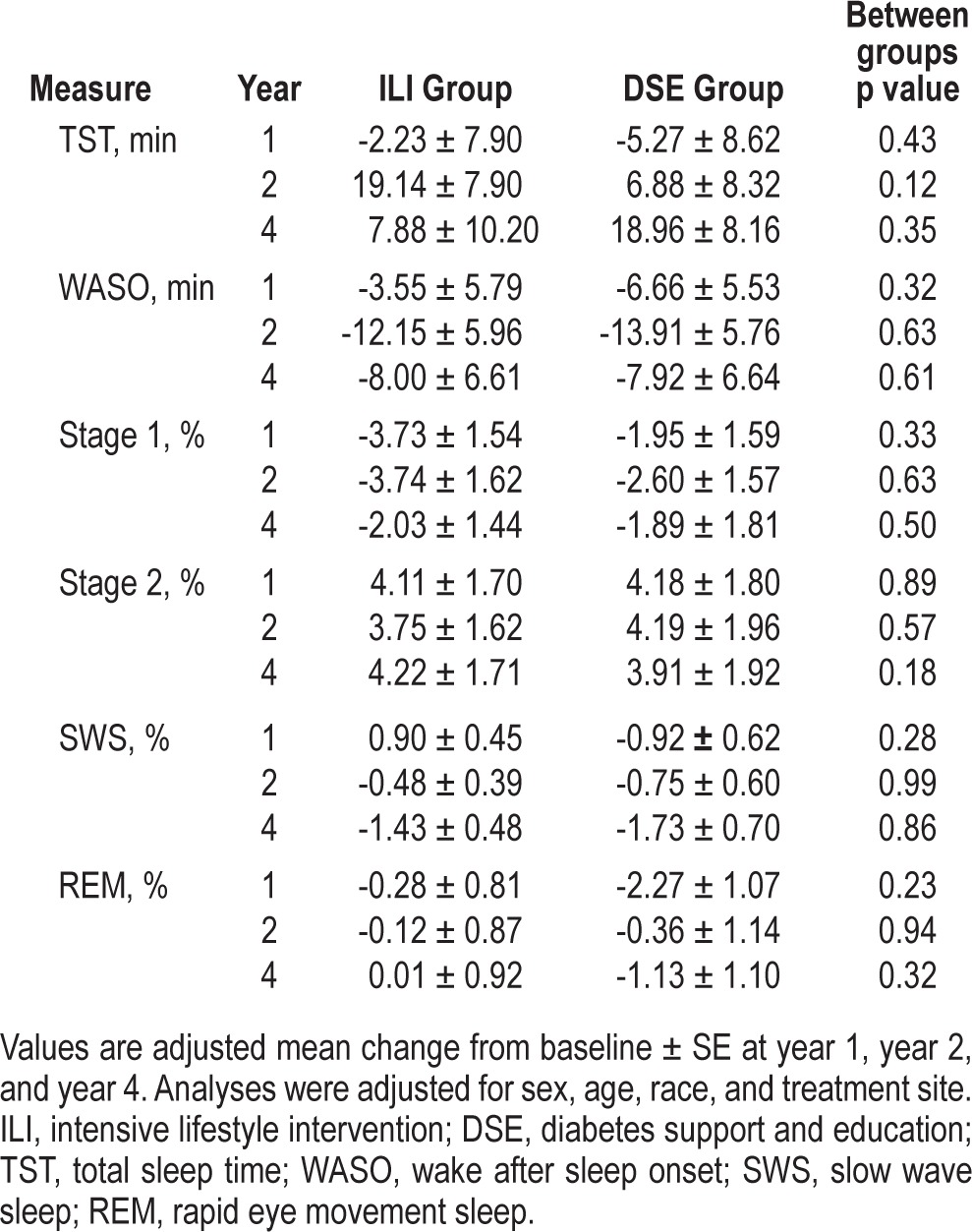
Table 2 shows the adjusted mean change from baseline in sleep duration, continuity, and architecture measures at year 1, year 2, and year 4 for ILI and DSE groups. Comparisons between ILI and DSE demonstrated that there were no between-group differences in change-from-baseline for TST, WASO, and percent TST spent in stage 1, stage 2, SWS, or REM sleep at year 1, year 2, or year 4 (Table 2).
Table 2.
Estimated adjusted mean (± standard error) changes in sleep measures from baseline at year 1, year 2, and year 4.
Role of OSA and Body Weight in Influencing Sleep Architecture: Repeated-Measure Mixed-Model Analyses
Repeated-measure mixed-model analyses were used to determine how various factors, including intervention group, time (year of assessment), and changes in AHI and body weight affect sleep duration, continuity, and architecture throughout the 4-year follow-up period for all participants (Table 3). There were no significant group × year interactions that contributed to the changes in sleep variables (Table 3). None of the predictor variables, including treatment group, year, AHI, and changes in body weight had an effect on TST (Table 3). In terms of sleep continuity, a significant positive association was seen between change in body weight and WASO (coefficient = 0.34, p = 0.009; Table 3).
Table 3.
Repeated measure mixed-model analysis with sleep measures over baseline, year 1, year 2, and year 4 as dependent variable.
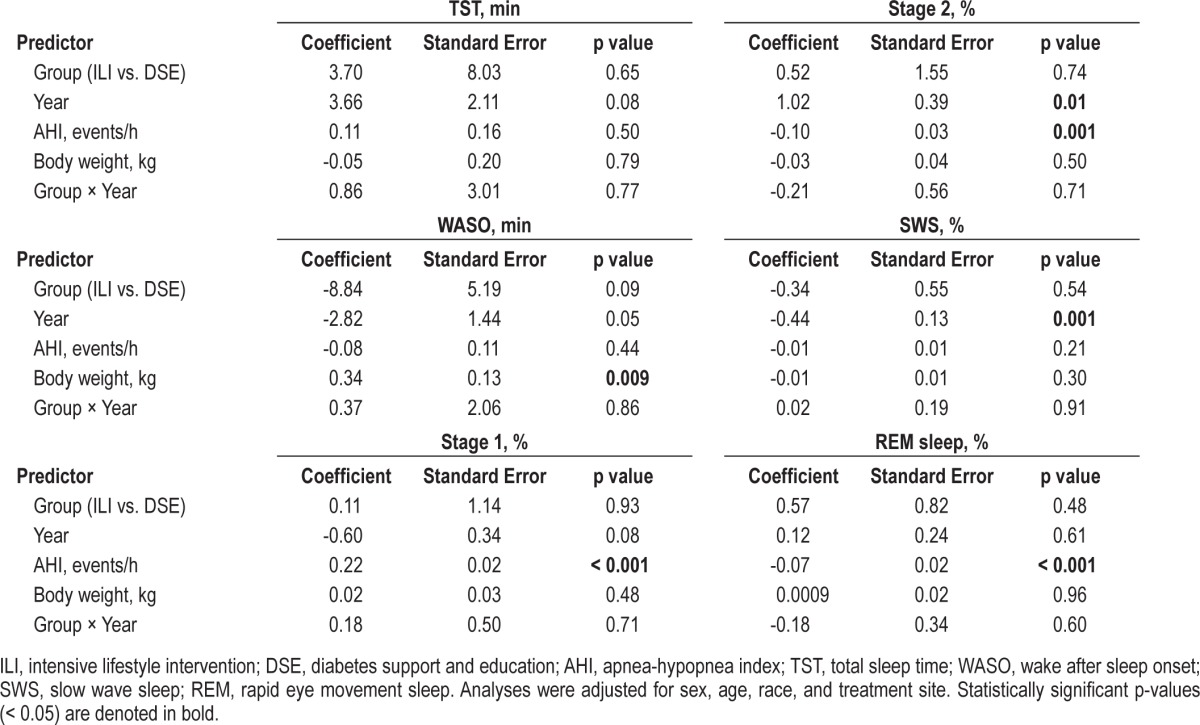
Treatment group and change in body weight were not found to have significant relationships with any of the sleep architecture measures (stage 1, stage 2, SWS, and REM sleep). Significant effects of year were seen for percentage of TST spent in stage 2 sleep (coefficient = 1.02, p = 0.01) and SWS (coefficient = -0.44, p = 0.001). These time effects indicate that for both groups overall, expression of stage 2 sleep was above baseline and expression of SWS was below baseline values across the year 1, year 2, and year 4 follow-ups. A significant positive association was seen between change in AHI and stage 1 % (coefficient = 0.22, p < 0.001; Table 3). For stage 2 %, a significant negative association was also seen with AHI (coefficient = -0.10, p = 0.001), and AHI also had a significant independent negative relationship with REM sleep % (coefficient = -0.07, p < 0.001; Table 3).
DISCUSSION
To our knowledge, this is the first report detailing the changes in nocturnal sleep duration and architecture in a long-term weight loss intervention with behavioral and diet recommendations. Although the intervention was effective in reducing the severity of OSA over a 4-year period, it did not lead to changes in the various PSG measures, suggesting that the ILI did not induce changes in sleep architecture, duration, or continuity across the 4-year follow-up. Thus, the main findings of this secondary investigation of the Sleep AHEAD outcomes are negative. However, when results were further analyzed as a longitudinal pooled observation study in which participants from the two groups were combined, we found that changes in sleep architecture (i.e., reduced stage 1 sleep and increased stage 2 and REM sleep) were significantly related to changes in AHI, but not to changes in body weight. Thus, sleep architecture changes can be considered to be more strongly associated with OSA severity than body weight. Interestingly, however, body weight was positively associated with disrupted sleep continuity (i.e., increased WASO).
Our results are somewhat in line with an earlier study by Smith et al. on the effects of weight loss on sleep disordered breathing in a small number of obese patients with OSA.12 In that study, patients were assigned to control (n = 8) or weight loss (n = 15) groups.12 The weight loss group was instructed to reduce caloric intake but was not prescribed specific diet or behavioral modification, and the control group was given no instructions to alter their caloric intake. In follow-up assessments ∼8 mo after baseline for control and ∼5 mo after baseline for weight loss, participants in the treatment group showed improved OSA, and also decreased percent time in stage 1 sleep, and increased percent time spent in stage 2 sleep compared to control.12 Whereas we report a similar association between reduced OSA severity and improved sleep architecture (i.e., increased stage 2 and decreased stage 1 sleep), we observed that the DSE control and ILI treatment groups did not differ in their expression of stage 1 and 2 sleep. In our study, participants in the DSE group were provided with education sessions focusing on diet, physical activity, and social support, which may have reduced some of the observed differences in sleep between the two groups. It remains unclear if aspects of these education and support sessions may have accounted for the lack of clear between group effects on the various sleep architecture parameters. Related to this, on an individual level, participants in the DSE group are likely to have experienced some weight loss,19 whereas those in the control group of Smith et al. were studied specifically after they had been weight-stable for a 1-month period. This is supported by the finding of a significant effect of year on stage 2 sleep, indicating that the expression of stage 2 sleep was above baseline values across the year 1, year 2, and year 4 follow-ups for both groups.
Since then, a series of more structured, supervised lifestyle intervention studies have been conducted.1,24,25 Of these, the Sleep AHEAD trial is the largest study to investigate the effects of a lifestyle intervention on OSA severity in a randomized controlled design.19 The other studies used a similar design involving a lifestyle intervention consisting of either prescribed low-calorie diet alone24 or low-calorie diet together with recommendations for increased physical activity25 and found reduced OSA severity in the treatment group compared to the control. Similar to what was observed in Sleep AHEAD,19 longer-term follow-up assessments demonstrated that the intervention was associated with a sustained reduction in OSA severity, despite moderate weight regain over time.26,27 However, PSG measures of sleep architecture in response to the intervention were not described in either of the structured, supervised intensive lifestyle intervention studies by Johansson et al. and Tuomilehto et al., or their follow-up reports.24–28
In addition to lifestyle interventions encouraging reduced caloric intake and increased physical activity, bariatric surgery is an effective method for inducing weight loss within obese patients, with sustained effects seen in a long-term follow-up.29,30 Results of a meta-analysis indicate that bariatric surgery is effective in resolving OSA in ∼80% of patients,29 and this surgical procedure was also shown to significantly improve subjective measures of sleep duration and quality.16 In addition to consistently lowering AHI, weight loss following surgery has been reported to improve nocturnal sleep architecture.13–15 This includes decreasing stage 1 sleep, and increasing the expression of stage 2 sleep, REM sleep, and SWS.13–15 Since in each case, body weight and AHI were concomitantly reduced after surgery, it is unclear which is driving these favorable changes in sleep: the weight loss or the improved AHI. Current results indicate that the improvements in stage 1, stage 2, and REM sleep, are more likely to be influenced by the reduction in OSA severity than by the loss of body weight per se.
Although the results of a large randomized sham-controlled trial indicate that it may actually induce modest weight gain,31 CPAP therapy, considered the gold-standard in OSA treatment, improves nocturnal sleep quality and architecture. In addition to relieving OSA symptoms, CPAP use has been found to decrease stage 1 sleep and increase SWS and REM sleep.32,33 Thus, CPAP should not only be considered as a means to relieve respiratory disturbances associated with OSA, but also as a tool to improve sleep architecture in these patients, particularly when daytime hypersomnolence is a problem.34 Prior studies demonstrating a link between sleep architecture and various cardiovascular and body weight outcomes imply that these induced changes in sleep architecture may be relevant. Specifically, REM sleep expression was found to be inversely related to central obesity, and SWS expression was also found to be inversely related to BMI and incident hypertension.35–37
PSG measures are important to document in obesity and OSA studies, as work done by our lab38 and others39 indicates a role of sleep architecture in influencing metabolism and body weight regulation. The percentage of SWS and REM sleep was negatively correlated with next-day intakes of fat and carbohydrates when participants were given an ad libitum eating opportunity.38 Stage 2 sleep appears particularly critical in the maintenance of energy balance, as a consistent pattern of lower resting metabolic rate, increased subjective appetite for sweet and salty foods, and increased intake of calories and fat were all seen in association with reduced stage 2 sleep.38 This is relevant since, as observed in the mixed-model analysis in the current study, alleviation of OSA severity is associated with increases in stage 2 sleep and REM sleep. In a study exploring the association between dietary patterns and sleep disordered breathing, Vasquez et al. observed that worsened OSA severity is associated with consumption of a diet high in total fat, total saturated fatty acids, cholesterol, and protein.40 More work, possibly including studies which selectively suppress particular sleep stages and monitor subsequent food intake, is required to determine if this is a true cause-effect relationship. Taken together, this may imply that methods to reduce AHI and improve sleep architecture should be encouraged for obese OSA patients to improve dietary patterns and metabolic health.
Considering that experimental manipulation studies have demonstrated an important role of SWS in influencing glucose metabolism,7,41 it is surprising that the ILI in these obese patients with T2D did not induce significant increases in SWS, and that this sleep stage was not associated with decreased body weight or OSA severity. In the total sample of Look AHEAD participants (n = 5,145; ILI: n = 2,570, DSE: n = 2,575); however, ILI was effective in significantly improving glycemic control over the 4 years of follow-up, and a significantly higher proportion of participants in the ILI group vs. the DSE group achieved the American Diabetes Association treatment goals (hemoglobin A1C < 7%) during year 1, year 2, and year 4.42
Some limitations were present in the current study. Not all patients returned for follow-up PSG assessments, and missing data could have confounded the results. No relationships were seen between the patterns of missing PSG and AHI or other covariates.19 Sample size remained large at follow-up assessments, and this is still the largest study to investigate the long-term effects of weight loss intervention and OSA improvement on sleep architecture in a randomized crossover design. An acclimatization night was not included in the current protocol, and PSG measures at baseline and at years 1, 2, and 4 were based on a single overnight recording. Thus, the possibility that a first night effect influenced the observed sleep architecture changes should be considered. Finally, it should be pointed out that results of the current study may not necessarily be generalized to patients without T2D, or patients with less severe OSA.
In conclusion, we observed no significant differences between ILI and DSE on changes in sleep architecture. However, overall reductions in AHI over the 4-year follow-up were found to be associated with increased REM sleep and stage 2 sleep, and decreased stage 1 sleep. Current findings further expose the important links that exist between obesity, T2D, sleep disordered breathing, and sleep, and indicate that reducing OSA severity can be associated with improvements in nocturnal sleep architecture.
DISCLOSURE STATEMENT
This was not an industry supported study. The study was supported by the National Institutes of Health NHLBI grant HL070301 and NIDDK grants DK60426, DK56992, DK057135, and DK007559. Dr. Kuna has received research support from Philips Respironics. Dr. Zammit is a consultant for Actelion, Alexza, Arena, Aventis, Biovail, Boehringer-Ingelheim, Cephalon, Elan, Eli Lilly, Evotec, Forest, Glaxo Smith Kline, Jazz, King Pharmaceuticals, Ligand, McNeil, Merck, Neurocrine Biosciences, Organon, Phizer, Renovis, Sanofi-Aventis, Select Comfort, Sepracor, Shire, Somnus, Takeda Pharmaceuticals, Vela, Wyeth-Ayerst Research; provides expert testimony for Acorda; has grants or grants pending from Abbott, Actelion, Ancile, Apnex, Arena, Aventis, Cephalon Inc, CHDI, Elan, Epis, Evotec, Forest, Galderma, Glaxo Smith Kline, H. Lundbeck A/S, King, Merck and Co., National Institute of Health (NIH), Neurim, Neurocrine Biosciences, Neurogen, Organon, Orphan Medical, Otsuka, Pfizer, Predix, Respironics, Sanofi-Aventis, Sanofi-Synthelabo, Schering-Plough, Sepracor, Shire, Samaxon, Takeda Pharmaceuticals North America, Targacept, Thymon, Transcept, UCB Pharma, Predix, Vanda, Wyeth-Ayerst Research. And has received payment for lectures from Neurocrine Biosciences, King Pharmaceuticals, McNeil, Sanofi-Aventis, Sanofi-Synthelabo, Sepracor, Takeda Pharmaceuticals, Vela Pharmaceuticals, Wyeth-Ayerst Research. Dr. Wadden serves on advisory boards for Orexigen Therapeutics and Novo Nordisk and has received grant support from Novo Nordisk and NutriSystem. Dr. Jakicic is a member of Free & Clear Scientific Advisory Board, has received grants or has grants pending from BodyMedia, Inc., and receives payment for lectures by JennyCraig. Dr. Foster serves on the Scientific Advisory Board of Con Agra Foods, Nutrisystem, Amylin, GI Dynamics, and United Health Group. He has received grants from Coca Cola Company and Orexigen. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Members of the Sleep AHEAD research group at each site consisted of the following individuals: St.Luke's-Roosevelt Hospital/Clinilabs: Jon Freeman, PPSGT, Ph.D., Jennifer Patricio; University of Pennsylvania: Brian McGuckin, Stephanie Krauthamer-Ewing, Allan Pack, M.B., Ch.B., Ph.D., Richard Schwab, M.D., Mary Jones-Parker, RPSGT, Matthew Anastasi, RPSGT, Beth Staley, RPSGT, Liz Roben; Brown University: Marie Kearns, Caitlin Egan; Temple University: Nida Cassim, Valerie Darcey, Sakhena Hin, Stephanie Vander Veur. A detailed list of the Look AHEAD Research Group is provided in reference 20. Members of the Observational Safety and Management Board were: Kingman P. Strohl, M.D. (chair), Donald L. Bliwise, Ph.D., and Helaine E. Resnick, Ph.D.
REFERENCES
- 1.Foster GD, Sanders MH, Millman R, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–9. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 7.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr. 2013;109:748–56. doi: 10.1017/S0007114512001894. [DOI] [PubMed] [Google Scholar]
- 10.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154:1705–11. [PubMed] [Google Scholar]
- 11.Vgontzas AN, Bixler EO, Tan TL, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med. 1998;158:1333–7. doi: 10.1001/archinte.158.12.1333. [DOI] [PubMed] [Google Scholar]
- 12.Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103:850–5. doi: 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- 13.Kalra M, Mannaa M, Fitz K, et al. Effect of surgical weight loss on sleep architecture in adolescents with severe obesity. Obes Surg. 2008;18:675–9. doi: 10.1007/s11695-008-9472-4. [DOI] [PubMed] [Google Scholar]
- 14.Lettieri CJ, Eliasson AH, Greenburg DL. Persistence of obstructive sleep apnea after surgical weight loss. J Clin Sleep Med. 2008;4:333–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon JB, Schachter LM, O'Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond) 2005;29:1048–54. doi: 10.1038/sj.ijo.0802960. [DOI] [PubMed] [Google Scholar]
- 16.Toor P, Kim K, Buffington CK. Sleep quality and duration before and after bariatric surgery. Obes Surg. 2011;22:890–5. doi: 10.1007/s11695-011-0541-8. [DOI] [PubMed] [Google Scholar]
- 17.Sampol G, Munoz X, Sagales MT, et al. Long-term efficacy of dietary weight loss in sleep apnoea/hypopnoea syndrome. Eur Respir J. 1998;12:1156–9. doi: 10.1183/09031936.98.12051156. [DOI] [PubMed] [Google Scholar]
- 18.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–26. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuna ST, Reboussin DM, Borradaile KE, et al. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep. 2013;36:641–9. doi: 10.5665/sleep.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bray G, Gregg E, Haffner S, et al. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res. 2006;3:202–15. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 22.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 23.Kohler M, Stoewhas AC, Ayers L, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184:1192–9. doi: 10.1164/rccm.201106-0964OC. [DOI] [PubMed] [Google Scholar]
- 24.Johansson K, Neovius M, Lagerros YT, et al. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. BMJ. 2009;339:b4609. doi: 10.1136/bmj.b4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuomilehto HP, Seppa JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:320–7. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 26.Johansson K, Hemmingsson E, Harlid R, et al. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: prospective observational follow-up study. BMJ. 2011;342:d3017. doi: 10.1136/bmj.d3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuomilehto H, Gylling H, Peltonen M, et al. Sustained improvement in mild obstructive sleep apnea after a diet- and physical activity-based lifestyle intervention: postinterventional follow-up. Am J Clin Nutr. 2010;92:688–96. doi: 10.3945/ajcn.2010.29485. [DOI] [PubMed] [Google Scholar]
- 28.Tuomilehto H, Seppa J, Uusitupa M, Tuomilehto J, Gylling H. Weight reduction and increased physical activity to prevent the progression of obstructive sleep apnea: a 4-year observational postintervention follow-up of a randomized clinical trial. [corrected] JAMA Intern Med. 2013;173:929–30. doi: 10.1001/jamainternmed.2013.389. [DOI] [PubMed] [Google Scholar]
- 29.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 30.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 31.Quan SF, Budhiraja R, Clarke DP, et al. Impact of treatment with continuous positive airway pressure (CPAP) on weight in obstructive sleep apnea. J Clin Sleep Med. 2013;9:989–93. doi: 10.5664/jcsm.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sforza E, Lugaresi E. Daytime sleepiness and nasal continuous positive airway pressure therapy in obstructive sleep apnea syndrome patients: effects of chronic treatment and 1-night therapy withdrawal. Sleep. 1995;18:195–201. [PubMed] [Google Scholar]
- 33.Valencia-Flores M, Bliwise DL, Guilleminault C, Cilveti R, Clerk A. Cognitive function in patients with sleep apnea after acute nocturnal nasal continuous positive airway pressure (CPAP) treatment: sleepiness and hypoxemia effects. J Clin Exp Neuropsychol. 1996;18:197–210. doi: 10.1080/01688639608408275. [DOI] [PubMed] [Google Scholar]
- 34.Rajagopal KR, Bennett LL, Dillard TA, Tellis CJ, Tenholder MF. Overnight nasal CPAP improves hypersomnolence in sleep apnea. Chest. 1986;90:172–6. doi: 10.1378/chest.90.2.172. [DOI] [PubMed] [Google Scholar]
- 35.Theorell-Haglow J, Berne C, Janson C, Sahlin C, Lindberg E. Associations between short sleep duration and central obesity in women. Sleep. 2010;33:593–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Rao MN, Blackwell T, Redline S, Stefanick ML, Ancoli-Israel S, Stone KL. Association between sleep architecture and measures of body composition. Sleep. 2009;32:483–90. doi: 10.1093/sleep/32.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fung MM, Peters K, Redline S, et al. Decreased slow wave sleep increases risk of developing hypertension in elderly men. Hypertension. 2011;58:596–603. doi: 10.1161/HYPERTENSIONAHA.111.174409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shechter A, O'Keeffe M, Roberts AL, Zammit GK, RoyChoudhury A, St-Onge MP. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am J Physiol Regul Integr Comp Physiol. 2012;303:R883–9. doi: 10.1152/ajpregu.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutters F, Gonnissen HK, Hursel R, Lemmens SG, Martens EA, Westerterp-Plantenga MS. Distinct associations between energy balance and the sleep characteristics slow wave sleep and rapid eye movement sleep. Int J Obes (Lond) 2012;36:1346–52. doi: 10.1038/ijo.2011.250. [DOI] [PubMed] [Google Scholar]
- 40.Vasquez MM, Goodwin JL, Drescher AA, Smith TW, Quan SF. Associations of dietary intake and physical activity with sleep disordered breathing in the Apnea Positive Pressure Long-Term Efficacy Study (APPLES) J Clin Sleep Med. 2008;4:411–8. [PMC free article] [PubMed] [Google Scholar]
- 41.Herzog N, Jauch-Chara K, Hyzy F, et al. Selective slow wave sleep but not rapid eye movement sleep suppression impairs morning glucose tolerance in healthy men. Psychoneuroendocrinology. 2013;38:2075–82. doi: 10.1016/j.psyneuen.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–75. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]


