Abstract
Study Objectives:
In 2007 the American Academy of Sleep Medicine (AASM) published polysomnography (PSG) scoring guidelines, which were updated in 2012. A key change in terms of scoring respiratory events in children was the threshold for reduction in airflow (50% vs 30%) for the definition of hypopnea. This study aimed to determine the impact of different scoring rules on the assessment of severity of obstructive sleep apnea (OSA) in children.
Methods:
Forty-two children (mean age 4.3 y, 16 F) underwent PSG. An obstructive apnea-hypopnea index (OAHI) was determined using three scoring rules: (1) ATS 1996 rules with minor modifications (modified ATS 1996); (2) AASM 2007 rules (AASM 2007); and (3) AASM 2007 rules with respiratory event related arousals included in the OAHI (AASM+RERA).
Results:
The AASM 2007 OAHI (median 0.4 events/h, range 0, 14) was lower than the modified ATS 1996 OAHI (median 0.8 range 0, 26.1, p < 0.001), underestimating severity of disease in 24% of cases. The AASM+RERA OAHI (median 0.8, range 0, 19.1) was also lower than the modified ATS 1996 OAHI (p = 0.02), but the difference was not clinically significant except at very high OAHIs.
Conclusion:
The AASM 2007 rules lead to a lower OAHI and lesser OSA severity when compared to the previous standard. Inclusion of RERAs in the AASM 2007 OAHI leads to a comparable OAHI to the previous rules. Given that morbidity has been demonstrated even in mild OSA, these results support the inclusion of events with a reduction in airflow of less than 50% as included in the updated AASM rules in 2012.
Citation:
Nixon GM, Hyde M, Biggs SN, Walter LM, Horne RS, Davey MJ. The impact of recent changes to the respiratory scoring rules in pediatrics. J Clin Sleep Med 2014;10(11):1217-1221.
Keywords: polysomnography, scoring, apnea hypopnea index, obstructive sleep apnea
Clinical interpretation of polysomnography (PSG) and research studies investigating outcomes of obstructive sleep apnea (OSA) in childhood rely on the consistency and reliability of metrics scored and reported as part of PSG. While this is not a perfect “gold standard,” especially as morbidity is not clearly related to these metrics,1,2 they remain the most widely used way of expressing the severity of the disease. In particular, the obstructive apnea-hypopnea index (OAHI) is usually relied upon to define the difference between primary snoring and OSA,3 and to define the difference between mild and more severe disease4—distinctions which have implications for treatment including whether to proceed with surgical treatment. Clearly, the way individual events are identified, scored, and summarized in an index will affect these conclusions.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Recent changes in scoring systems for respiratory events during PSG in children have included significant changes in the threshold for scoring hypopnea. This study aimed to determine the magnitude of these changes and the impact on assessment of severity of OSA.
Study Impact: The findings of this study support inclusion of respiratory events with a fall in airflow of less than 50% in the obstructive apneahypopnea index, given the body of literature that exists to support the sequelae of even mild OSA in childhood.
In 2007 the American Academy of Sleep Medicine (AASM) published a manual on performance and interpretation of PSG,5 to both update previously relied upon references and to standardize methodology for characterizing respiratory events and the nature of sleep. Prior to that time, the principal guideline followed was the American Thoracic Society (ATS) standards for scoring of respiratory events.6 However leading pediatric sleep laboratories used variations on the ATS rules in many of the influential publications of the time, including those providing normal ranges and evidence for morbidity of OSA in childhood.7–9 These variations typically reduced the fall in airflow required to define a hypopnea (≥ 50% in the ATS standards) and/or lowered the extent of desaturation scored in association with a hypopnea (> 4% in the ATS standards). In the 2007 AASM guidelines for pediatric sleep medicine, the scoring rules for defining a hypopnea included a requirement for 50% reduction in airflow from baseline for ≥ 90% of the event, with either ≥ 3% oxygen desaturation or an arousal from sleep. Events with a lesser reduction in airflow were termed respiratory event related arousals (RERA) if associated with an arousal (omitted from the actual manual but corrected in subsequent online updates [http://www.aasmnet.org/scoring-manualfaq.aspx#80, last accessed February 28th 2014]). Events without an arousal that were associated with ≥ 3% desaturation were not included, but this modification was suggested by the Australasian Sleep Association and the Australasian Sleep Technologists Association in a guide published in 2011 [http://www.sleep.org.au/documents/item/218. Last accessed June 12, 2014]. At the time the AASM 2007 manual was published, our laboratory was following the American Thoracic Society standards for scoring of respiratory events,6 with three exceptions, typical of rules in other laboratories at the time: a “discernible decrease” in airflow was included in the definition of hypopnea (if associated with a desaturation and/or an arousal), rather than a strict requirement for a reduction in airflow of ≥ 50%; desaturation of ≥ 3% rather than > 4%, and; duration of an event > 2 respiratory cycles, rather than no duration criteria given in ATS 1996. Institution of the 2007 AASM rules was delayed in Australia awaiting publication of the findings of a consensus working group [http://www.sleep.org.au/documents/item/218. Last accessed June 12, 2014]. One year after the latter was adopted in our center, we aimed to better understand the effect of the various changes in scoring rules on the determination of severity of sleep disordered breathing (SDB) in children. Specifically, this study compares the OAHI determined using the modified ATS 1996 rules (modified ATS 1996) to the AASM 2007 rules (AASM 2007) and the 2007 AASM rules with the addition of RERAs (AASM+RERA).
METHODS
Patient Group
Subjects included in this report formed part of a previously published research project involving full overnight PSG between 2008-2011.10 All subjects who consented to return for repeat PSG 3 years after the original study (between July 2011 and April 2013) formed the study population, having their original study rescored as detailed below.
PSG Recording
Overnight attended PSG was carried out in a pediatric sleep laboratory using a commercially available sleep system (E Series, Compumedics, Melbourne, Australia). Electroencephalograms (EEG: C3/A2, C4/A1, O1/A2, O2/A1), electroculograms, submental electromyogram, electrocardiogram, left and right leg electromyogram, and body position were recorded. Oxygen saturation (SpO2) was measured by pulse oximetry measured using Masimo Radical SET (Masimo Corp., CA, USA) or the Bitmos GmbH, (Dusseldorf, Germany), both of which use Masimo signal extraction technology (SET) for signal processing and were set at a 2-s averaging time. Thoracic and abdominal breathing movements were recorded via uncalibrated respiratory inductance plethysmography (Pro-Tech zRIP Effort Sensor, Pro-Tech Services Inc., Mukilteo, WA, USA). Transcutaneous carbon dioxide was measured using a TCM4/40 or TINA TCM3 (Radiometer, Copenhagen, Denmark). Airflow was measured using nasal pressure (Salter Style, Salter Labs, Arvin, CA, USA) and oronasal airflow (Sandman BreathSensor, Child Airflow Thermistor, Tyco Healthcare, UK).
PSG Scoring
Scoring of the original PSG studies was conducted by trained pediatric sleep technicians who maintained a concordance rate > 85% for both sleep scoring and respiratory events. Each study was then rescored by a single scorer using the AASM 2007 rules. RERAs were scored and counted separately.
Three different summary metrics were generated for each subject as detailed below. The OAHI includes obstructive apneas and hypopneas and mixed apneas, unless otherwise specified (for AASM+RERA). Event duration was ≥ 2 missed breaths in all cases. A comparison of the key differences between the scoring systems compared in this study is shown in Table 1.
Table 1.
Key features differentiating the different scoring rules assessed in this study.

OAHI Using Previous Local Scoring Rules Based on ATS 19966 (modified ATS 1996)
An obstructive apnea was defined as a decrease in flow by ≥ 90% in the presence of continued or increased respiratory effort. An obstructive hypopnea was scored when a discernible decrease in flow signal from baseline occurred in the presence of respiratory effort (with paradox or phase shift) and was associated with snoring or noisy breathing at event termination in conjunction with an arousal, awakening, or ≥ 3% SpO2 desaturation.
OAHI Using AASM 2007 Rules5 (AASM 2007)
Obstructive apnea was defined as a > 90% fall in airflow for ≥ 90% of event duration, with continued or increased respiratory effort. Mixed apneas consisted of a central component followed by an obstructive component. A hypopnea was associated with ≥ 50% fall in airflow signal for ≥ 90% of event duration associated with an arousal, awakening, or ≥ 3% desaturation.
Summation of AASM 2007 OAHI and RERAs (AASM+RERA)
Scored as for AASM 2007, with the addition of RERAs into the OAHI. RERAs were scored as per AASM 2007 (flattening of the nasal pressure waveform, associated with snoring, noisy breathing, elevation of end-tidal or transcutaneous pCO2 and/or visual evidence of increased work of breathing, leading to an arousal from sleep) with the notable exception that desaturation as a consequence of the event was considered enough to score the event even if arousal was not present. An esophageal pressure sensor was not used.
Statistical Analysis
Due to the skewed nature of the OAHI data, respiratory indices (modified ATS 1996, AASM 2007, and AASM+RERA) were compared using the Wilcoxon signed-rank test. Analysis was performed in pairs to elucidate the differences between the individual systems rather than across 3 systems at once. OSA severity was classified using each index into: primary snoring (PS): ≤ 1 event/h; mild OSA: > 1- ≤ 5 events/h; moderate OSA: > 5 and ≤ 10 events/h; severe OSA: > 10 events/h.4 Proportions falling into each severity category under the different scoring systems were compared using the Fisher exact test. Differences between the indices derived using the different scoring rules are displayed using Bland Altman plots.
RESULTS
A single PSG on each of 42 children was included in the comparison. Children were aged 3-6 years (mean 4.3y), and 16 (38%) were female. Average total sleep time was 435 minutes (SD 47), with average sleep efficiency of 88% (SD 8%). REM sleep formed 20.3% (SD 3.7%) of total sleep time, and the mean total arousal index was 15.4/h (SD 6.3).
The AASM 2007 OAHI (median 0.4 events/h IQR 0,1.8 events/h) was significantly lower than the modified ATS 1996 OAHI (median 0.8 events/h IQR 0, 4.6; p < 0.001) (Figure 1A). The higher the OAHI, the more the AASM 2007 rules underestimated the OAHI when compared to the modified ATS 1996 scoring rules (Figure 2A). Using the AASM 2007 rules led to reclassification of OSA severity in comparison to the modified ATS 1996 rules in 10 subjects (24%), all to a less severe category of OSA: 3 mild to PS, 1 moderate to PS, 2 moderate to mild, 1 severe to mild, and 3 severe to moderate OSA (p < 0.001).
Figure 1. Scatter plot comparing individual obstructive indices using the various scoring systems.
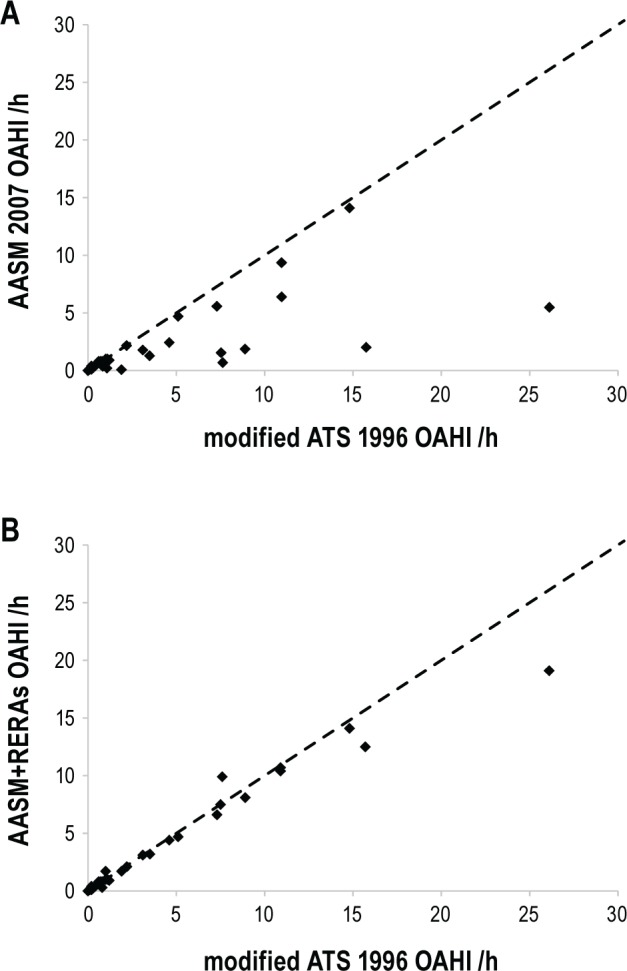
(A) AASM 20075 and modified ATS 19966; and (B) AASM+RERA and modified ATS 1996. The dotted line represents the line of equality.
Figure 2. Bland-Altman plot comparing OAHI using the various scoring systems.
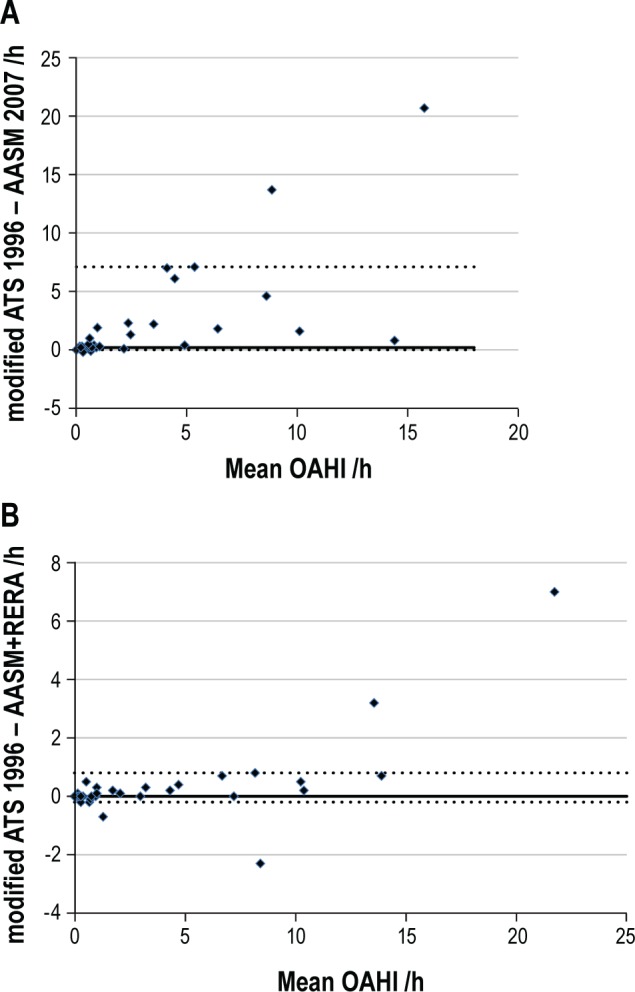
(A) AASM 20075 and modified ATS 19966; and (B) AASM+RERA and modified ATS 1996. Solid line represents the median difference. Dotted lines represent the 5th and 95th percentiles.
The AASM+RERA OAHI (median 0.8 events/h IQR 0, 4.4 events/h) was also significantly lower than the modified ATS 1996 OAHI (p = 0.02) (Figure 1B). In comparing these 2 indices however, it is apparent that the difference only becomes important at a high OAHI, with small and clinically unimportant differences < 10 events/h (Figure 2B). Using the AASM+RERA OAHI to define severity in comparison to the modified ATS 1996, only 4 children (10%) were reclassified, each due to values just on either side of the respective cut-off values: 2 from mild OSA to PS, 1 from PS to mild OSA and 1 from moderate to mild OSA.
DISCUSSION
Use of different scoring systems with relatively small differences in the definition of partial obstructive events leads to changes in severity of OSA as depicted by the OAHI, with potentially clinically significant impacts. The original AASM 2007 scoring system5 results in a significantly lower OAHI than previous rules based on the ATS 1996 scoring criteria, which at the time were modified in many laboratories including our own to be more sensitive in detecting events (smaller fall in airflow and less desaturation required to define a hypopnea). The AASM 2007 would result in re-classification of many cases into a lower severity category. The addition of RERAs to the total obstructive index (as reflected in the AASM+RERA OAHI in this study) results in an OAHI that is also marginally lower than the modified ATS 1996 scoring rules, but with little or no clinical significance. This highlights that requiring a 50% reduction in airflow for the definition of a hypopnea will lead to a significant number of events not being counted that are associated with arousal from sleep and/ or desaturation. This likelihood was mentioned in the most recent update to the AASM rules,11 with the return of events with > 30% reduction in flow being counted in the OAHI as hypopneas, rather than counted separately as RERAs. Our results suggest that a “discernible decrease” in flow (as in our modified ATS 1996 OAHI) equates to the typical threshold for scoring of a RERA (as in AASM+RERA) as long as desaturation is allowed as a consequence in addition to arousal. Given that the extent of reduction in flow is rarely if ever measured quantitatively, we suggest that the 2012 AASM updated rules are likely to result in a very similar OAHI to the modified ATS 1996 guidelines.
If one accepts that a “discernible decrease” in flow equates to > 30%, then the major difference between our previous rules (modified ATS 1996) and the AASM 2012 update is removal of subcortical arousals from the definition of hypopnea and RERA. Counting of subcortical arousals occurred in pediatric practice following reports that children often do not experience cortical arousals following obstructive events.12–14 However this literature is in infants or small numbers of children. As inclusion of subcortical arousals (modified ATS 1996 in this study) made negligible difference to the total OAHI compared to current rules, our results suggest that reexamination of the issue of arousals from obstructive events is needed to confirm whether subcortical arousals should be included or not.
This study does not address the clinical relevance of the differences in OSA severity that are determined using the different scoring systems. Most data in the literature, however, do not support a dose effect—in other words, outcome (cardiovascular, cognitive, or behavioral functioning in particular) is not usually related to severity of OSA as determined by OAHI, whether expressed as a categorical or continuous variable. Even children with primary snoring have been demonstrated in a number of studies to have deficits in cardiovascular and neurocognitive functioning in comparison to controls.15–17 Nonetheless, a universal “language” for clinicians and researchers in the field is important, and consistency of the metrics used is crucial for future studies related to morbidity and treatment effects for childhood OSA. It is also important clinically, with clinical guidelines supporting use of medical therapies for mild OSA and reserving surgical treatment for more severe cases.18 We submit that the 2007 AASM scoring rules are likely to have led to an underestimation of obstructive events in comparison to the body of literature produced prior to introduction of those rules, and herewith provide supportive evidence for adoption of the recent 2012 updated rules.11
DISCLOSURE STATEMENT
This research was supported by the National Health and Medical Research Council of Australia and the Victorian Government's Operational Infrastructure Support Program. The work contained in this manuscript was carried out in The Ritchie Centre, MIMR-PHI Institute of Medical Research, Melbourne, Australia. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Horne RS, Yang JS, Walter LM, et al. Elevated blood pressure during sleep and wake in children with sleep-disordered breathing. Pediatrics. 2011;128:e85–92. doi: 10.1542/peds.2010-3431. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien LM, Holbrook CR, Mervis CB, et al. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder.[see comment] Pediatrics. 2003;111:554–63. doi: 10.1542/peds.111.3.554. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146:1235–9. doi: 10.1164/ajrccm/146.5_Pt_1.1235. [DOI] [PubMed] [Google Scholar]
- 4.Katz ES, Greene MG, Carson KA, et al. Night-to-night variability of polysomnography in children with suspected obstructive sleep apnea. J Pediatr. 2002;140:589–94. doi: 10.1067/mpd.2002.123290. [DOI] [PubMed] [Google Scholar]
- 5.Iber C, Ancoli-Israel S, Chesson AL, Jr., Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events. [Google Scholar]
- 6.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 7.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117:e769–78. doi: 10.1542/peds.2005-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–53. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 9.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature.[see comment] Pediatr Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 10.Nisbet LC, Yiallourou SR, Biggs SN, et al. Preschool children with obstructive sleep apnea: the beginnings of elevated blood pressure? Sleep. 2013;36:1219–26. doi: 10.5665/sleep.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:682–6. doi: 10.1164/ajrccm.162.2.9908058. [DOI] [PubMed] [Google Scholar]
- 13.Katz ES, Lutz J, Black C, Marcus CL. Pulse transit time as a measure of arousal and respiratory effort in children with sleep-disordered breathing. Pediatr Res. 2003;53:580–8. doi: 10.1203/01.PDR.0000057206.14698.47. [DOI] [PubMed] [Google Scholar]
- 14.McNamara F, Issa FG, Sullivan CE. Arousal pattern following central and obstructive breathing abnormalties in infants and children. J Appl Physiol. 1996;81:2651–7. doi: 10.1152/jappl.1996.81.6.2651. [DOI] [PubMed] [Google Scholar]
- 15.Blunden S, Lushington K, Kennedy D, Martin J, Dawson D. Behavior and neurocognitive performance in children aged 5-10 years who snore compared to controls. J Clin Exp Neuropsychol. 2000;22:554–68. doi: 10.1076/1380-3395(200010)22:5;1-9;FT554. [DOI] [PubMed] [Google Scholar]
- 16.Jackman AR, Biggs SN, Walter LM, et al. Sleep-disordered breathing in preschool children is associated with behavioral, but not cognitive, impairments. Sleep Med. 2012;13:621–31. doi: 10.1016/j.sleep.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Walter LM, Yiallourou SR, Sands SA, et al. Impaired blood pressure control in children with obstructive sleep apnoea. Sleep Med. 2013;14:858–66. doi: 10.1016/j.sleep.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–84. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]


