Abstract
Purpose
Optimization of prostate biopsy requires addressing the shortcomings of standard systematic transrectal ultrasound guided biopsy, including false-negative rates, incorrect risk stratification, detection of clinically insignificant disease and the need for repeat biopsy. Magnetic resonance imaging is an evolving noninvasive imaging modality that increases the accurate localization of prostate cancer at the time of biopsy, and thereby enhances clinical risk assessment and improves the ability to appropriately counsel patients regarding therapy. In this review we 1) summarize the various sequences that comprise a prostate multiparametric magnetic resonance imaging examination along with its performance characteristics in cancer detection, localization and reporting standards; 2) evaluate potential applications of magnetic resonance imaging targeting in prostate biopsy among men with no previous biopsy, a negative previous biopsy and those with low stage cancer; and 3) describe the techniques of magnetic resonance imaging targeted biopsy and comparative study outcomes.
Materials and Methods
A bibliographic search covering the period up to October 2013 was conducted using MEDLINE®/PubMed®. Articles were reviewed and categorized based on which of the 3 objectives of this review was addressed. Data were extracted, analyzed and summarized.
Results
Multiparametric magnetic resonance imaging consists of anatomical T2-weighted imaging coupled with at least 2 functional imaging techniques. It has demonstrated improved prostate cancer detection sensitivity up to 80% in the peripheral zone and 81% in the transition zone. A prostate cancer magnetic resonance imaging suspicion score has been developed, and is depicted using the Likert or PI-RADS (Prostate Imaging Reporting and Data System) scale for better standardization of magnetic resonance imaging interpretation and reporting. Among men with no previous biopsy, magnetic resonance imaging increases the frequency of significant cancer detection to 50% in low risk and 71% in high risk patients. In low risk men the negative predictive value of a combination of negative magnetic resonance imaging with prostate volume parameters is nearly 98%, suggesting a potential role in avoiding biopsy and reducing over detection/overtreatment. Among men with a previous negative biopsy 72% to 87% of cancers detected by magnetic resonance imaging guidance are clinically significant. Among men with a known low risk cancer, repeat biopsy using magnetic resonance targeting demonstrates a high likelihood of confirming low risk disease in low suspicion score lesions and of upgrading in high suspicion score lesions. Techniques of magnetic resonance imaging targeted biopsy include visual estimation transrectal ultrasound guided biopsy; software co-registered magnetic resonance imaging-ultrasound, transrectal ultrasound guided biopsy; and in-bore magnetic resonance imaging guided biopsy. Although the improvement in accuracy and efficiency of visual estimation biopsy compared to systematic appears limited, co-registered magnetic resonance imaging-ultrasound biopsy as well as in-bore magnetic resonance imaging guided biopsy appear to increase cancer detection rates in conjunction with increasing suspicion score.
Conclusions
Use of magnetic resonance imaging for targeting prostate biopsies has the potential to reduce the sampling error associated with conventional biopsy by providing better disease localization and sampling. More accurate risk stratification through improved cancer sampling may impact therapeutic decision making. Optimal clinical application of magnetic resonance imaging targeted biopsy remains under investigation.
Keywords: prostate, image-guided biopsy, magnetic resonance imaging, prostatic neoplasms, risk assessment
Approximately 1 million prostate biopsies are performed annually in the United States. An increased PSA most frequently triggers an extended 12-core systematic TRUS guided biopsy, which is endorsed by the American Urological Association as the optimal biopsy method.1 As the designation of systematic sites on biopsy is largely operator dependent, this strategy relies on random sampling for cancer detection. This biopsy strategy is subject to sampling error and provides poor localization of disease. The primary limitations of the 12-core random systematic biopsy include failure to detect clinically significant cancer (according to Epstein criteria); imprecise tumor risk stratification (high risk cancers are improperly classified as low risk); and detection of small, low risk clinically insignificant cancers. This diagnostic uncertainty can lead to repeat biopsy, delayed detection of significant disease and disease overtreatment.
With the increasing challenge to preferentially detect higher grade PCa while avoiding lower grade tumors, noninvasive imaging may offer a means of selective disease localization. The use of MRI in evaluating the necessity of prostate biopsy and the guidance of biopsy location have gained considerable momentum due to improvements in the ability of multiparametric MRI to localize and noninvasively assess risk.2 The ability to improve the detection and localization of PCa using modern MRI techniques has prompted the development of MRI targeted biopsy strategies by visual estimation MRI targeting, in-bore MRI guidance and MRI-US fusion targeting.
MATERIALS AND METHODS
We searched MEDLINE/PubMed for English language articles published up to October 2013 using combinations of the terms MRI, multiparametric, MRI-guided, MRI-targeted, image-guided, MRI-ultrasound fusion, cognitive, prostate, prostate cancer, prostate neoplasm, biopsy, detection, localization, risk assessment, risk stratification, cancer detection and visual estimation. Supplemental articles were identified through hand searches. Non-English articles were excluded from analysis. Relevant studies were then screened by 3 authors (MAB, XM, JLN), and data were extracted, analyzed and summarized.
LIMITATIONS OF CONTEMPORARY SYSTEMATIC BIOPSY TECHNIQUE
False-Negative Biopsy (under sampling)
The contemporary, random, systematic biopsy strategy relies on sampling efficiency for cancer detection and, thus, is subject to sampling error (fig. 1). Under sampling occurs in up to 30% of cases with clinically significant tumors being missed on initial biopsy.3 Cancers are often small, intermingled with benign stroma and not uniformly distributed in the gland. As a result, clinically significant cancers frequently go undetected. Due to the random nature of systematic sampling, larger glands are subject to a greater risk of missed cancer.3 This risk is not greatly improved by increasing the core number to more than 12.1
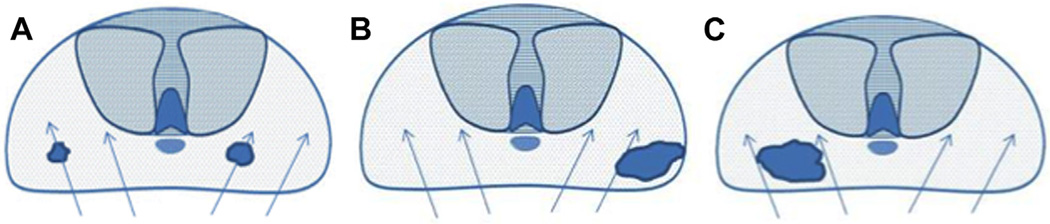
Figure 1.
Current limitations of prostate biopsy. A, clinically insignificant cancers are often identified by chance during systematic biopsy (over sampling). B, systematic biopsies may lead to incorrect risk stratification categorizing clinically significant tumors as low volume or low grade (under sampling). C, systematic deployment of needle biopsies may lead to clinically significant tumors being missed on initial biopsy (under sampling).
Incorrect Risk Stratification (under sampling)
Under sampling of the prostate during ultrasound guided biopsy also leads to incorrect risk stratification in a subset of men with a potential for categorization of clinically significant tumors (by Epstein criteria) as low volume or low grade (fig. 1). Random nontargeted prostate biopsies risk inadequate sampling of a cancer lesion, often at its periphery. This may reveal a small length of tumor in a core with a low Gleason score when, in fact, a clinically significant lesion may exist adjacent to the biopsy site. Mufarrij et al reported that 46% of cases considered low risk and candidates for active surveillance based on preoperative systematic biopsy had disease upgraded to a Gleason score of 7 or greater at final histopathology.4 Interestingly increasing the core number, as in saturation or repeat biopsy techniques, does not appear to greatly reduce the risk of under sampling and incorrect risk classification.1
Detection of Clinically Insignificant Disease (over sampling)
Approximately 30% to 50% of men older than age 50 harbor clinically insignificant PCa at autopsy. These clinically insignificant cancers are often identified by chance during a systematic biopsy approach, contributing, in part, to the problem of over detection and overtreatment of indolent PCa (fig. 1). In a study evaluating the impact of an extended biopsy pattern on the detection of clinically insignificant cancer Siu et al reported the detection of insignificant cancer in more than 17% of cases on initial biopsy.5 Repeat biopsy increases the detection of clinically insignificant PCa. The recent trend of overcoming sampling error by increasing core number or repeating biopsies further increases the risk of identifying small, indolent cancers which may have little to do with the PSA increase.1
Necessity of Repeat Biopsy (reduction of sampling error by increasing sampling)
Efforts to overcome sampling error include performing multiple repeat biopsies and increasing the core number, which have resulted in the over detection of indolent cancers, morbidity attributed to unnecessary biopsies and an increase in cost. Several studies have shown that when serial biopsies are indicated, most cancers that are detected are clinically insignificant6 and the rate of indolent cancer detection increases.5 Furthermore, increasing the number of cores beyond the extended systematic TRUS guided biopsy only results in a marginal increase in the overall detection rate and simply increases the rate of insignificant cancer detection.1
PROSTATE MP-MRI FOR PCa DETECTION AND LOCALIZATION
T2-Weighted Imaging
T2-weighted MR images, reflecting tissue water content, have high spatial resolution and clearly define the zonal anatomy of the prostate, distinguishing the peripheral zone (high signal intensity) from the central zone (surrounding the ejaculatory ducts in the posterior prostate base and exhibiting decreased T2 signal intensity) and transition zones (surrounding the urethra, extending anteriorly and superiorly from the level of the verumontanum, and exhibiting heterogeneous, often swirled, signal intensity) (fig. 2).7 In the peripheral zone PCa can appear as an area of low signal intensity. The degree of intensity decrease differs with Gleason score as higher Gleason score components show lower signal intensities.8
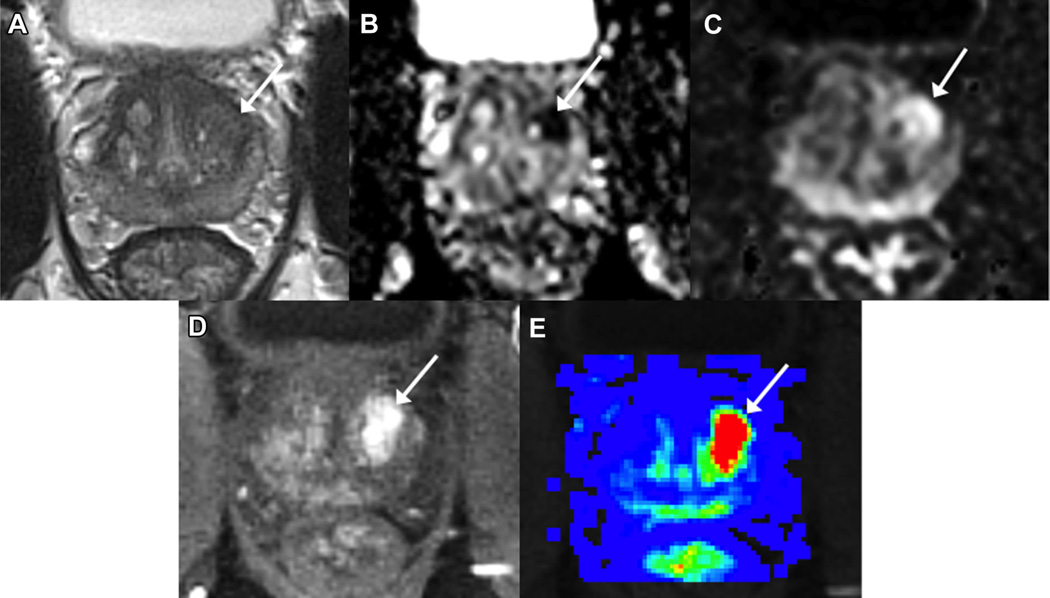
Figure 2.
Biopsy naïve man (68 years old) with PSA 5.1 ng/ml underwent mp-MRI demonstrating Likert scale suspicion score of 4/5 left anterolateral base-to-mid transition zone lesion on T2WI (A), ADC (B), DWI (b-value 1,500) (C), DCE (single post-contrast time point, D) and parametric perfusion map (E). Systematic 12-core biopsy revealed benign prostatic tissue while MRI targeted biopsy demonstrated GS 3 + 4 = 7 PCa in 4 of 4 cores involving up to 70% of core (longest cancer core 12 mm).
T2WI results in false-positive findings as low signal intensity can also be the consequence of benign abnormalities, including acute and chronic prostatitis, atrophy, scars, post-irradiation or hormonal treatment effects, hyperplasia and post-biopsy hemorrhage. Partly related to the heterogeneous appearance of BPH with areas of increased and decreased signal intensity, cancer in the transition zone may be more difficult to discern than in the peripheral zone, particularly for the less experienced radiologist. However, morphological features such as homogeneously low signal intensity, poorly defined irregular edges of the suspicious lesion, invasion into the urethra or the anterior fibromuscular stroma, and lenticular shape are helpful for the detection of transition zone tumor.9
Diffusion Weighted Imaging
Diffusion weighted MRI measures the random motion of water molecules. The strength of the gradient that determines the degree of diffusion weighting is reflected by the b-value of the sequence. By performing DWI with multiple b-values it is possible to calculate the apparent diffusion coefficient based on the signal intensity measured at each b-value image to quantify the restriction of water diffusion (fig. 2). Traditionally a maximal b-value of approximately 1,000 seconds per mm2 has been used. More recent data show that the use of higher b-values up to 2,000 seconds per mm2 helps eliminate background signal from the normal prostate, and may increase the accuracy of PCa detection10 in the peripheral and transition zones.11 However, modern MRI hardware and careful attention to sequence optimization are required to maintain image quality when using these high b-values.
On ADC maps PCa frequently shows a low apparent diffusion coefficient,12 and there is an inverse correlation between quantitative ADC values and Gleason score.13 While ADC does correlate with final Gleason score, the confidence intervals are overlapping, thus limiting the ability to use ADC as a surrogate for Gleason score. This is an area of ongoing investigation and technical optimization aimed at improving the predictive ability of ADC in the future.
Limitations of DWI include low signal-to-noise ratio and image distortion, both of which become more problematic at higher b-values. Nonetheless, DWI is a widely available technique with relatively straightforward acquisition and post-processing. Moreover, given its strong association with tumor aggressiveness, it may prove to be the primary sequence for tumor detection and characterization.14
Perfusion Imaging
Dynamic contrast enhanced MRI consists of a series of fast T1-weighted sequences covering the prostate before and after rapid injection (2 to 4 ml per second) of a bolus of a gadolinium chelate. Given the serial rapid imaging of the prostate, DCE-MRI allows the assessment of contrast kinetics in focal lesions (fig. 2). PCa typically enhances faster and to a greater extent than the surrounding prostate, and will also show more rapid washout of contrast in a fraction of cases. Although prostatitis related enhancement is usually diffuse and nonfocal in nature, and BPH related enhancement is often well encapsulated and spherical, the nonspecific nature of these patterns limits the usefulness of DCE findings in isolation, resulting in DCE often being applied largely as an adjunct to interpretations based primarily on T2WI and DWI findings.
A simple approach to evaluating DCE-MRI is through a subjective visual assessment of the raw dynamic images. Alternatively, semiquantitative parameters such as the time to peak, wash-in rate and washout rate may be calculated to allow pixel-wide construction of parametric perfusion maps. A compartment based model may also be performed to generate truly quantitative metrics. This has largely been performed using a Tofts model, which provides the parameter ktrans (transfer constant), reflecting the forward transfer rate constant between the plasma and extravascular extracellular space, which is increased in prostate cancer.15
One limitation of DCE-MR imaging relates to the overlap of cancer with prostatitis in the peripheral zone and the marked overlap with vascularized BPH nodules in the transition zone. Another limitation is the reduced spatial resolution due to fast imaging.
Accuracy in Detection/Performance Characteristics
While these individual sequences are useful in the detection of PCa, the results are optimized by mp-MRI as it combines all of the sequences in an integrated fashion (fig. 2). Mp-MRI offers superior diagnostic power for PCa detection, and can assist risk stratification based on lesion size, extent and ADC value.16 In one study mp-MRI sensitivity exceeded 80% for detecting 0.2 cm3 of Gleason 4 + 3 or greater and 0.5 cm3 of Gleason 3 + 4 or greater disease.17 In another study using a 3T magnet the addition of DCE and/or DW imaging to T2-weighted MRI significantly improved sensitivity from 63% to between 79% and 81% in the peripheral zone while maintaining a stable specificity.18
Yoshizako et al demonstrated that the combined use of DW, DCE and T2-weighted MRI increased accuracy in the detection of transition zone cancer compared to T2WI alone from 64% to 79%.19 Nevertheless, given their moderate specificity, mp-MRI findings require biopsy to confirm the presence of tumor and assess Gleason score.16 PCa MRI suspicion scores have been developed such as a 1 to 5-point Likert scale (based on reader’s gestalt impression) or the 1 to 5-point PI-RADS score (based on fixed criteria) for improved standardization of MRI interpretation and reporting.20, 21
Use of MRI before biopsy may not only alter the biopsy approach but also allow more accurate image interpretation. MRI studies after biopsy are confounded by artifacts such as hemorrhage, inflammation and hyperemia resulting from biopsy, which may persist for 2 to 6 months.22
Disease Localization and Correlation of MRI with Surgical Pathology
The use of mp-MRI in cancer detection and localization is supported by numerous studies. Rosenkrantz et al evaluated the usefulness of mp-MRI in the localization of the index lesion of PCa compared to prostatectomy specimens.23 The authors evaluated the likelihood of an exact match or an approximate match between the MRI and pathological findings in terms of assigned region. Based on 6 independent readers in exact match analysis the average sensitivity was 60.2% and the average positive predictive value was 65.3%, while in approximate match analysis the average sensitivity was 75.9% and the average positive predictive value was 82.6%.
In a study of 45 men (mean PSA 6.37 ng/ml) who had biopsy proven PCa and underwent mp-MRI before radical prostatectomy, Turkbey et al assessed the cancer detection rate using whole mount sectioning and a customized 3-dimensional mold to overcome the co-registration artifact between the specimen and MRI, and observed a high diagnostic accuracy.24 The sensitivity of MRI was higher for tumors larger than 5 mm in diameter as well as for those with higher Gleason scores (greater than 7, p <0.05). However, a correlation between histological lesions and MRI findings is difficult to determine, especially due to the variation of angles and section intervals of MR sections and prostatectomy slices, along with the shrinkage that occurs during histopathological processing of the specimens. Correction for this variability has been attempted using a shrinkage factor25 as well as different methods of co-registration between histology and imaging.26, 27
OUTCOMES OF MRI TARGETED BIOPSY IN CLINICAL PRACTICE
There are several potential benefits of MRI targeted biopsy reported in the literature. However, these benefits still need to be proven through further studies. In theory the accurate localization of significant cancer before biopsy may potentially correct the limitations of systematic biopsy. Accurate targeting of biopsy cores should reduce false-negative biopsies and improve accuracy in risk classification through better tumor sampling (table 1).18, 28–42 Secondarily a reduction in false-negative biopsies could reduce the necessity of repeat biopsies and, thus, decrease cost. Because targeted biopsy relies on image guidance, fewer cores would potentially be required, which would further decrease cost. Finally, if metrics can be established to demonstrate the lowest risk parameters for the detection of clinically significant disease, avoidance of biopsy in cases below that threshold may reduce the number of biopsies performed and, thus, reduce over detection. These principles remain to be proven fully but there is a growing body of evidence to support these assertions.
Table 1.
Summary of MRI targeted prostate biopsy studies
| % (No./total No.) |
% (No./total No.) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Indication for Biopsy |
Ca Detection Rates—Total |
Ca Detection Rates—Systematic Biopsy |
Ca Detection Rates—Targeted Biopsy |
Ca Core Length (mm) |
||||||||||
| References | No. | Biopsy Naïve |
Prior Neg Biopsy |
Prior Pos Biopsy |
All Ca | GS 7 or Greater, or Clinically Significant Ca |
Pos MRI Finding | Targeting Technique | All Ca | GS 7 or Greater, or Clinically Significant Ca |
All Ca | GS 7 or Greater, or Clinically Significant Ca |
Systematic Biopsy |
Targeted Biopsy |
| Haffner et al28 | 555 | 555 | 0 | 0 | 54 (302/555) | 45 (249/555) | 63 (351/555) | Visual estimation | 52 (290/555) | 43 (237/555) | 67 (236/351) | 67 (236/351) | 4.70 | 5.56 |
| Delongchamps et al18 | 391 | 127 | 0 | 0 | 46 (58/127) | 16 (20/127) | 43 (54/127) | Visual estimation | 43 (55/127) | 14 (18/127) | 31 (40/127) | 14 (18/127) | 0.68 | 3.8 |
| 131 | 0 | 0 | 60 (78/131) | 25 (33/131) | 60 (78/131) | Fusion (Esaote) | 46 (60/131) | 20 (26/131) | 49 (64/131) | 25 (33/131) | 0.65 | 4.49 | ||
| 133 | 0 | 0 | 53 (71/133) | 20 (27/133) | 62 (82/133) | Fusion (Koelis) | 33 (44/133) | 14 (19/133) | 47 (62/133) | 20 (27/133) | 1.35 | 3.72 | ||
| Watanabe et al29 | 1,448 | 1,448 | 0 | 0 | 48 (697/1,448) | Not reported | 61 (890/1,448) | Visual estimation | 13 (73/558) | Not reported | 70 (624/890)* | Not reported | Not reported | Not reported |
| Numao et al30 | 351 | 351 | 0 | 0 | 45 (158/351) | 34 (120/351) | 45 (157/351) | Visual estimation† | 45 (158/351) | 34 (120/351) | 64 (101/157)† | 54 (85/157)† | 5 | Not reported |
| Hoeks et al33 | 438 | 0 | 438 | 0 | 25 (108/438) | 21 (94/438) | 67 (294/438) | MRGB‡ | – | – | 41 (108/265) | 35 (94/265) | Not reported | Not reported |
| Vourganti et al34 | 195 | 0 | 195 | 0 | 37 (73/195) | 23 (45/195) | 100 (195/195) | Fusion (Philips) | 23 (45/195) | 11 (22/195) | 29 (56/195) | 21 (40/195) | Not reported | Not reported |
| Sonn et al35 | 105 | 0 | 105 | 0 | 34 (36/105) | 25 (26/105) | 96 (101/105) | Fusion (Eigen) | 27 (28/102) | 15 (15/102) | 24 (23/97) | 22 (21/97) | Not reported | Not reported |
| Labanaris et al36 | 260 | 0 | 260 | 0 | 55 (143/260) | Not reported | 65 (170/260) | Visual estimation | 18 (47/260) | Not reported | 56 (96/170) | Not reported | Not reported | Not reported |
| Vargas et al37 | 388 | 0 | 0 | 388 | 68 (264/388) | 20 (79/388) | Not reported | Visual estimation | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Margel et al38 | 60 | 0 | 0 | 60 | 71 (40/56) | 32 (18/56) | 62 (37/60) | Visual estimation | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Kasivisvanathan et al39 | 182 | 78 | 32 | 72 | 80 (145/182) | 71 (130/182) | 100 (182/182) | Visual estimation | 79 (144/182) | 62 (113/182) | 66 (120/182) | 57 (103/113) | Not reported | Not reported |
| Siddiqui et al40 | 582 | 262 | 320 | 0 | 54 (315/582) | 32 (184/582) | 100 (582/582) | Fusion (Philips) | 44 (255/582) | 10 (57/582) | 43 (253/582) | 15 (88/582) | Not reported | Not reported |
| Sonn et al32 | 171 | 0 | 65 | 106 | 53 (90/171) | 20 (34/171) | 89 (151/171) | Fusion (Eigen) | 44 (75/171) | 12 (21/171) | 35 (53/151) | 13 (20/151) | 3.3 | 5.1 |
| Kuru et al41 | 347 | 177 | 170 | 0 | 58 (200/347) | 42 (147/347) | 73 (253/347) | Fusion (MedCom) | 50 (175/347) | 38 (132/347) | 51 (128/253) | 41 (104/253) | Not reported | Not reported |
| Puech et al31 | 95 | 95 | 0 | 0 | 69 (66/95) | 67 (64/95) | 100 (95/95) | Fusion (Esaote) | 59 (56/95) | 52 (49/95) | 69 (66/95) | 67 (64/95) | 4.6 | 7.3 |
| Wysock et al42 | 125 | 67 | 34 | 24 | 65 (81/125) | 27 (34/125) | 100 (125/125) | Fusion (Eigen) | 57 (71/125) | 27 (34/125) | 65 (81/125) | 27 (34/125) | 4.2 | 6.7 |
CDR for positive MRI group included targeted and systematic biopsy as individual breakdown by biopsy method was not reported.
Only 50 patients had separate targeted cores in the lesion and the rest of the patients with a positive MRI had a systematic biopsy needle that passed through the area of suspicion. CDR for positive MRI group is a combination of targeted and systematic biopsy.
In-bore MRGB on sampled lesions of interest, systematic biopsy was not performed in men without MRI lesions. Of 294 patients with MRI lesions only 265 underwent MRGB.
Among Men with No Previous Biopsy
The use of MRI in men with no previous biopsy has been studied, but the cost-effectiveness and true benefit have yet to be determined through larger randomized studies and, as such, its use is currently investigational. Haffner et al recently reported a seminal series of 555 consecutive patients who underwent prebiopsy MRI followed by systematic biopsy and visual estimation biopsy of MRI abnormalities. 28 The overall CDR was 54% using extended systematic biopsy and 63% for the 351 cases with an abnormal MRI. Although systematic biopsy detected 66 more cases of cancer, 53 were deemed clinically insignificant. The MRI targeted approach detected more high grade cases and better quantified the cancer through increased cancer length per biopsy core.
Delongchamps et al also examined the use of prebiopsy mp-MRI in 391 consecutive patients, and reported a CDR of 45% using systematic biopsy and 47% using fusion targeted biopsy.43 Targeted biopsy was significantly better at detecting high Gleason score (greater than 3+3) cancer, missing only 2 of 63 (3%) high grade cancers detected by systematic biopsy while detecting an additional 17 high grade cancers missed by systematic biopsy. In a study of 1,448 men who underwent DW-MRI before initial biopsy Watanabe et al reported a CDR of 70.1% in 890 patients with MRI lesions who underwent targeted and systematic biopsy compared to only 13.1% in 558 patients with no MRI lesions who only underwent systematic biopsy.29 The CDR was 90.1% in 141 patients with anterior cancers found on MRI, an area easily missed with standard systematic biopsy.
In another prospective study of 351 consecutive patients with an increased PSA Numao et al reported a CDR of 43% to 50% with an abnormal MRI vs 9% to 13% with a negative MRI in the low risk group.30 In this low risk group (PSA less than 10 ng/ml and normal digital rectal examination) the negative predictive value of a negative MRI and prostate volume less than 33 ml was 95.1% to 97.5% for significant cancer on biopsy. Collectively the published literature suggests that overall cancer detection is decreased by MR targeted biopsy compared to systematic biopsy, but higher grade cancers are detected with fewer cores and insignificant cancers are detected less often.31, 32
Among Men with Previous Negative Biopsy
In a series of 438 consecutive patients with an increased PSA and at least 1 prior negative biopsy who underwent mp-MRI, Hoeks et al reported a CDR of 41% (108 of 265) using in-bore targeted biopsy, with 87% (94 of 108) of these cancers found to be clinically significant.33 Vourganti et al reported on 195 patients with a previous negative biopsy and suspicious mp-MRI, and found a CDR of 37% (73 of 195) using a combination of MRI-US fusion biopsy and systematic biopsy.34 In addition to detecting 9 additional high grade cancers missed by systematic biopsy, fusion biopsy led to pathological upgrading in 28 of 73 (38.4%) cases. Sonn et al found a CDR of 34% (36 of 105) in men with a previous negative biopsy and 72% (26 of 36) was clinically significant.35 MRI-US fusion biopsy detected clinically significant cancer in 21 of 23 (91%) men compared to only 15 of 28 (54%) with systematic biopsy. A highly suspicious MRI lesion was the most significant predictor of significant cancer on multivariate analysis. Even in patients with up to 4 prior negative biopsies, Labanaris et al found that among 170 of 260 (65%) patients with a suspicious MRI, PCa was detected on 96 of 170 (56%) targeted biopsies compared to only 30 of 170 (18%) systematic biopsies.36
Among Men with Low Risk Cancer
In men with a diagnosis of low risk PCa repeat biopsy yielded a wide range of upgrading depending on the method used (table 2).32, 37–39, 44, 45 In a study of 388 consecutive patients with low risk disease who underwent mp-MRI and confirmatory visual estimation co-registration biopsy, Vargas et al reported that 20% (79 of 388) of patients had disease upgraded on confirmatory biopsy.37 A 5-point MRI suspicion scale demonstrated excellent risk stratification with a high sensitivity for upgrading on confirmatory biopsy (0.87 to 0.98) for a score of 5/5. In a similar study Margel et al found that on confirmatory biopsy in 60 active surveillance cases 32.1% were reclassified as no longer fulfilling surveillance criteria.38 Only 2 of 23 (8.6%) with a normal MRI were reclassified compared to 10 of 13 (76.9%) with a MRI lesion larger than 1 cm.
Table 2.
Frequency of upgrading in patients on active surveillance using MRI and biopsy
| % (No./total No.) |
|||
|---|---|---|---|
| References | No. | Pos MRI | Frequency of Upgrading |
| Vargas et al37 | 388 | Not reported | 20 (79/388) |
| Margel et al38 | 56 | 62 (37/60) | 32 (18/56) |
| Park et al44 | 298 | 88 (263/288) | 47 (136/288) |
| Kasivisvanathan et al39 | 72 | 81 (58/72) | 72 (52/72) |
| Sonn et al32 | 106 | Not reported | 17 (18/106) |
| Stamatakis et al45 | 85 | Not reported | 29 (25/85) |
While subject to selection bias, in a retrospective series of 298 patients Park et al found a suggestion of cancer in 88.3% on preoperative mp-MRI before radical prostatectomy.44 Patients with cancer suspected on imaging had a higher likelihood of upgrading at radical prostatectomy compared to those with no suspicion on MRI (49.8% vs 14.3%). In a similar study Turkbey et al retrospectively analyzed 133 patients who underwent mp-MRI before radical prostatectomy.46 Mp-MRI had a 93% sensitivity, 57% positive predictive value and 92% overall accuracy for predicting the appropriate selection of surveillance candidates.
Limitations of MRI Targeted Biopsy
While MRI targeted biopsy has the potential to overcome the limitations of standard TRUS guided biopsy, it is not without several limitations. MRI targeted biopsy incurs additional cost that is not yet justified. Targeting methods are not purely defined and may still miss cancer. This targeting strategy may result in additional biopsies due to a false-positive MRI. Lastly, MRI targeted biopsy may overestimate cancer risk and further studies are needed to define the pathology of the targeted biopsy.
TECHNIQUE OF MRI TARGETED BIOPSY
Visual Estimation MR Targeted TRUS Biopsy
Visual estimation allows the adaptation of MRI targeted biopsy in clinical practice without significant up-front cost, but carries a significant learning curve and lacks real-time feedback regarding accuracy. The effectiveness of visual estimation targeted biopsy in detecting PCa varies among studies, likely reflecting inconsistencies in targeting precision. In a series of 351 of 555 (63%) patients with a positive MRI Haffner et al detected clinically significant PCa in 45% (248 of 555) by systematic biopsy compared to 43% (236 of 555) by visual estimation biopsy, but 53 of 66 cancers missed by targeted biopsy were clinically insignificant.28 In addition, Kasivisvanathan et al reported a lower CDR using visual estimation MRI targeted transperineal biopsy (57%) compared to transperineal template guided biopsy (62%) in 182 patients with suspicious lesions on mp-MRI.39 In contrast, Labanaris et al reported a CDR of 56% by targeted visual estimation MRI targeted biopsy alone but only 18% by systematic biopsy alone in 170 of 260 (65%) patients with a positive MRI.36 Collectively the currently published studies suggest improved accuracy and efficiency compared to systematic biopsy, but also demonstrate that experience with visual estimation biopsy varies by investigator experience and likely, in part, due to variable practices in imaging approach.
Software Co-Registered MRI Targeted TRUS Biopsy
Software co-registration potentially overcomes the limitation of cognitive fusion through reproducible methods of identifying MRI lesions on ultrasound. A number of commercial platforms have become available. These applications vary by method of co-registration (mechanical, electromagnetic or real-time) and use a different hardware platform to align the biopsy with the co-registered image. MRIUS fusion biopsy potentially has greater reproducibility because it involves less operator dependence and provides real-time feedback of actual biopsied locations. Disadvantages include a high up-front cost for the software/device, dependence on the software for accuracy, and the associated learning curve and operator training.
Siddiqui et al recently reported that the combination of extended systematic and targeted biopsy using the Philips/PercuNav device resulted in a CDR of 54% (315 of 582), with targeted biopsy identifying higher Gleason scores in 32% of patients and GS 7 or greater cancer in 17 patients that would have been missed with systematic biopsy.40 Sonn et al reported similar positive results using the Artemis device (Eigen, Grass Valley, California), finding a CDR of 53% (90 of 171 with a higher percentage of positive cores (21% vs 7%) and greater detection of GS 7 or greater cancer (38% vs 31%) using targeted biopsy.32 Patients with highly suspicious MRI lesions (5/5 grade) had a 94% rate of cancer diagnosis compared to only 43% in those with low suspicion lesions (2/5 grade). High detection rates have also been shown with transperineal MRI-US fusion biopsy. Kuru et al reported a CDR of 58% (200 of 347) using the MedCom/BiopSee® device, with a CDR of 82.6% (86 of 104) in patients with highly suspicious lesions compared to only 15% (14 of 94) in those with a normal mp-MRI.41
In-Bore MRI Guided Biopsy
Hoeks et al reported on 265 patients with suspicious lesions on mp-MRI with prior negative TRUS biopsies who underwent transrectal in-bore MRGB, resulting in a CDR of 41% with 87% of these detected cancers found to be clinically significant.33 Multiple studies have corroborated this result, demonstrating that in-bore MRGB is a feasible diagnostic technique in patients with prior negative biopsy with a median detection rate of 42%, significantly higher than reported detection rates for repeat systematic biopsy.47 This in-bore biopsy strategy has the advantages of real-time feedback of needle placement, fewer sampled cores and a low likelihood of missed target. However, it has the disadvantage of increased cost, use of scanner time (opportunity cost) and an inability to routinely sample the remaining gland. Additionally, with in-bore MRI guided biopsy urologists are largely removed from the diagnostic pathway with concerning implications for the ultimate management of the disease.
Comparative Studies
While many studies compare targeted to systematic biopsy, few have evaluated the CDR across different targeted techniques (table 1). Puech et al reported that software co-registration using MyLab® Navigator (MedCom, Darmstadt, Germany) had a slight advantage (53% vs 47% CDR) compared to cognitive fusion targeted biopsy of 79 identified MRI lesions.31 However, in this study a small subset of men underwent biopsy using both techniques. Wysock et al prospectively compared MRI-US fusion biopsy using the Eigen Artemis system vs visual estimation targeting in 125 consecutive men with suspicious regions on prebiopsy mp-MRI.42 They found that fusion targeting improved accuracy for smaller MRI lesions and trended toward increased detection compared to visual targeting for all cancer (32.0% vs 26.7%) as well as GS 7 or greater cancer (20.3% vs 15.1%) (fig. 3). Delongchamps et al reported that cognitive fusion was not significantly better than systematic random biopsies, while both software co-registration devices tested (MyLab®Twice, Esaote, Indianapolis, Indiana and Urostation, Koelis, LaTronche, France) significantly increased the CDR compared to systematic biopsies using conditional logistic regression analysis in a cohort of 391 patients.43
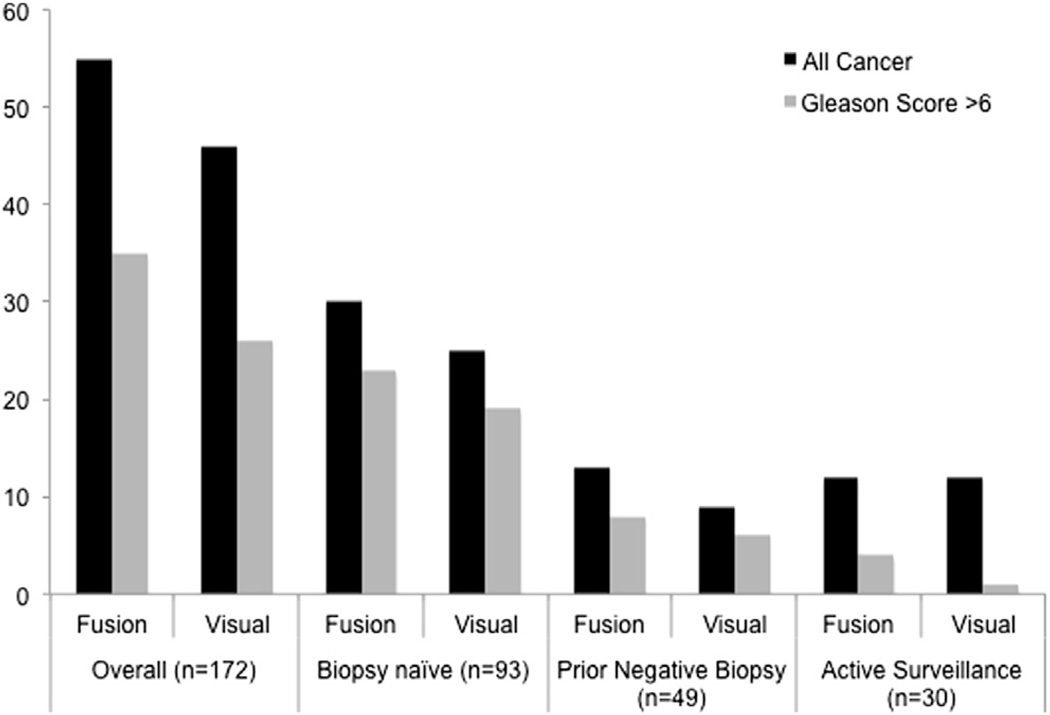
Figure 3.
Comparison of MRI-US fusion and visual estimation for detection of all PCa and GS greater than 6. Trend toward increased cancer detection with fusion biopsy was observed but did not reach statistical significance.42
Yet to be explored is the relationship of clinical factors such as prostate size, PSA and MRI lesion location to the accuracy of targeting with a cognitive or co-registered approach. While more comparative studies examining the efficacy of various techniques are needed, it is possible that the decision of an institution or practice to use a particular type of MRI targeted biopsy will be largely influenced by local factors such as cost, space and operator experience with MRI interpretation. Guidelines regarding conduct and standards in reporting MRI targeted biopsy studies were recently published through a consensus meeting.48
CONCLUSIONS
Mp-MRI represents a potential tool to address many of the limitations of contemporary systematic biopsy. Among men with no previous biopsy its role is poorly defined, but it has the potential to reduce false-negatives, improve risk classification, and contribute to the reduction of repeat biopsies and over detection. Among men with a previous negative biopsy but persistent suspicion, it has the potential to increase cancer detection and reduce further repeat biopsy. Among men with cancer who are contemplating surveillance, MR targeted biopsy potentially improves risk stratification and reduces the need for repeat biopsy. The optimal method for MR targeted biopsy has not yet been established, but emerging methods of co-registration may offer wider accessibility to the approach. Further comparative studies with standard of practice and evaluation of cost-effectiveness are warranted before consideration of wide adoption.
Acknowledgments
Supported by Grant UL1 TR000038 from the National Center for Advancing Translational Sciences, National Institutes of Health (MAB, JSW). Supported by the Joseph and Diane Steinberg Charitable Trust (MAB, JLN, JSW, ABR, SST).
Abbreviations and Acronyms
- ADC
apparent diffusion coefficient
- BPH
benign prostatic hyperplasia
- CDR
cancer detection rate
- DCE
dynamic contrast enhanced
- DW
diffusion weighted
- DWI
diffusion weighted imaging
- GS
Gleason score
- mp
multiparametric
- MR
magnetic resonance
- MRGB
magnetic resonance guided biopsy
- MRI
magnetic resonance imaging
- PCa
prostate cancer
- PSA
prostate specific antigen
- T2WI
T2-weighted imaging
- TRUS
transrectal ultrasound
- US
ultrasound
Footnotes
Nothing to disclose.
Financial interest and/or other relationship with MedReviews, Watson, Serenity, TheraCoat, Amgen and SonaCare Medical.
Financial interest and/or other relationship with Hitachi, Janssen and Elsevier.
REFERENCES
- 1.Bjurlin MA, Carter HB, Schellhammer P, et al. Optimization of initial prostate biopsy in clinical practice: sampling, labeling and specimen processing. J Urol. 2013;189:2039. doi: 10.1016/j.juro.2013.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore CM, Robertson NL, Arsanious N, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol. 2013;63:125. doi: 10.1016/j.eururo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Serefoglu EC, Altinova S, Ugras NS, et al. How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer? Can Urol Assoc J. 2012;1 doi: 10.5489/cuaj.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mufarrij P, Sankin A, Godoy G, et al. Pathologic outcomes of candidates for active surveillance undergoing radical prostatectomy. Urology. 2010;76:689. doi: 10.1016/j.urology.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 5.Siu W, Dunn RL, Shah RB, et al. Use of extended pattern technique for initial prostate biopsy. J Urol. 2005;174:505. doi: 10.1097/01.ju.0000165385.53652.7a. [DOI] [PubMed] [Google Scholar]
- 6.Zaytoun OM, Stephenson AJ, Fareed K, et al. When serial prostate biopsy is recommended: most cancers detected are clinically insignificant. BJU Int. 2012;110:987. doi: 10.1111/j.1464-410X.2012.10958.x. [DOI] [PubMed] [Google Scholar]
- 7.Hricak H, Dooms GC, McNeal JE, et al. MR imaging of the prostate gland: normal anatomy. AJR Am J Roentgenol. 1987;148:51. doi: 10.2214/ajr.148.1.51. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Mazaheri Y, Zhang J, et al. Assessment of biologic aggressiveness of prostate cancer: correlation of MR signal intensity with Gleason grade after radical prostatectomy. Radiology. 2008;246:168. doi: 10.1148/radiol.2461070057. [DOI] [PubMed] [Google Scholar]
- 9.Akin O, Sala E, Moskowitz CS, et al. Transition zone prostate cancers: features, detection, localization, and staging at endorectal MR imaging. Radiology. 2006;239:784. doi: 10.1148/radiol.2392050949. [DOI] [PubMed] [Google Scholar]
- 10.Kim CK, Park BK, Kim B. High-b-value diffusion-weighted imaging at 3 T to detect prostate cancer: comparisons between b values of 1,000 and 2,000 s/mm2. AJR Am J Roentgenol. 2010;194:W33. doi: 10.2214/AJR.09.3004. [DOI] [PubMed] [Google Scholar]
- 11.Katahira K, Takahara T, Kwee TC, et al. Ultra-high- b-value diffusion-weighted MR imaging for the detection of prostate cancer: evaluation in 201 cases with histopathological correlation. Eur Radiol. 2011;21:188. doi: 10.1007/s00330-010-1883-7. [DOI] [PubMed] [Google Scholar]
- 12.Mazaheri Y, Vargas HA, Akin O, et al. Reducing the influence of b-value selection on diffusion-weighted imaging of the prostate: evaluation of a revised monoexponential model within a clinical setting. J Magn Reson Imaging. 2012;35:660. doi: 10.1002/jmri.22888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol. 2012;61:177. doi: 10.1016/j.eururo.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 14.Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258:488. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker GJ, Tofts PS. Pharmacokinetic analysis of neoplasms using contrast-enhanced dynamic magnetic resonance imaging. Top Magn Reson Imaging. 1999;10:130. doi: 10.1097/00002142-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Turkbey B, Choyke PL. Multiparametric MRI and prostate cancer diagnosis and risk stratification. Curr Opin Urol. 2012;22:310. doi: 10.1097/MOU.0b013e32835481c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villers A, Puech P, Mouton D, et al. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol. 2006;176:2432. doi: 10.1016/j.juro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Delongchamps NB, Rouanne M, Flam T, et al. Multiparametric magnetic resonance imaging for the detection and localization of prostate cancer: combination of T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging. BJU Int. 2011;107:1411. doi: 10.1111/j.1464-410X.2010.09808.x. [DOI] [PubMed] [Google Scholar]
- 19.Yoshizako T, Wada A, Hayashi T, et al. Usefulness of diffusion-weighted imaging and dynamic contrast-enhanced magnetic resonance imaging in the diagnosis of prostate transition-zone cancer. Acta Radiol. 2008;49:1207. doi: 10.1080/02841850802508959. [DOI] [PubMed] [Google Scholar]
- 20.Jung JA, Coakley FV, Vigneron DB, et al. Prostate depiction at endorectal MR spectroscopic imaging: investigation of a standardized evaluation system. Radiology. 2004;233:701. doi: 10.1148/radiol.2333030672. [DOI] [PubMed] [Google Scholar]
- 21.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenkrantz AB, Kopec M, Kong X, et al. Prostate cancer vs. post-biopsy hemorrhage: diagnosis with T2- and diffusion-weighted imaging. J Magn Reson Imaging. 2010;31:1387. doi: 10.1002/jmri.22172. [DOI] [PubMed] [Google Scholar]
- 23.Rosenkrantz AB, Deng FM, Kim S, et al. Prostate cancer: multiparametric MRI for index lesion localization—a multiple-reader study. AJR Am J Roentgenol. 2012;199:830. doi: 10.2214/AJR.11.8446. [DOI] [PubMed] [Google Scholar]
- 24.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186:1818. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haider MA, van der Kwast TH, Tanguay J, et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol. 2007;189:323. doi: 10.2214/AJR.07.2211. [DOI] [PubMed] [Google Scholar]
- 26.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection—histopathologic correlation. Radiology. 2010;255:89. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orczyk C, Rusinek H, Rosenkrantz AB, et al. Preliminary experience with a novel method of three-dimensional co-registration of prostate cancer digital histology and in vivo multiparametric MRI. Clin Radiol. 2013;68:e652. doi: 10.1016/j.crad.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;108:E171. doi: 10.1111/j.1464-410X.2011.10112.x. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe Y, Terai A, Araki T, et al. Detection and localization of prostate cancer with the targeted biopsy strategy based on ADC map: a prospective large-scale cohort study. J Magn Reson Imaging. 2012;35:1414. doi: 10.1002/jmri.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Numao N, Yoshida S, Komai Y, et al. Usefulness of pre-biopsy multiparametric magnetic resonance imaging and clinical variables to reduce initial prostate biopsy in men with suspected clinically localized prostate cancer. J Urol. 2013;190:502. doi: 10.1016/j.juro.2013.02.3197. [DOI] [PubMed] [Google Scholar]
- 31.Puech P, Rouvièere O, Renard-Penna R, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy—prospective multicenter study. Radiology. 2013;268:461. doi: 10.1148/radiol.13121501. [DOI] [PubMed] [Google Scholar]
- 32.Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189:86. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoeks CM, Schouten MG, Bomers JG, et al. Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol. 2012;62:902. doi: 10.1016/j.eururo.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 34.Vourganti S, Rastinehad A, Yerram NK, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol. 2012;188:2152. doi: 10.1016/j.juro.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labanaris AP, Engelhard K, Zugor V, et al. Prostate cancer detection using an extended prostate biopsy schema in combination with additional targeted cores from suspicious images in conventional and functional endorectal magnetic resonance imaging of the prostate. Prostate Cancer Prostatic Dis. 2010;13:65. doi: 10.1038/pcan.2009.41. [DOI] [PubMed] [Google Scholar]
- 37.Vargas HA, Akin O, Afaq A, et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol. 2012;188:1732. doi: 10.1016/j.juro.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margel D, Yap SA, Lawrentschuk N, et al. Impact of multiparametric endorectal coil prostate magnetic resonance imaging on disease reclassification among active surveillance candidates: a prospective cohort study. J Urol. 2012;187:1247. doi: 10.1016/j.juro.2011.11.112. [DOI] [PubMed] [Google Scholar]
- 39.Kasivisvanathan V, Dufour R, Moore CM, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol. 2013;189:860. doi: 10.1016/j.juro.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64:713. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380. doi: 10.1016/j.juro.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 42.Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MRtargeted prostate biopsy: the PROFUS trial. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.10.048. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Delongchamps NB, Peyromaure M, Schull A, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol. 2013;189:493. doi: 10.1016/j.juro.2012.08.195. [DOI] [PubMed] [Google Scholar]
- 44.Park BH, Jeon HG, Choo SH, et al. Role of multiparametric 3.0 tesla magnetic resonance imaging in prostate cancer patients eligible for active surveillance. BJU Int. 2013 doi: 10.1111/bju.12423. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Stamatakis L, Siddiqui MM, Nix JW, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013;119:3359. doi: 10.1002/cncr.28216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turkbey B, Mani H, Aras O, et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology. 2013;268:144. doi: 10.1148/radiol.13121325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Overduin CG, Futterer JJ, Barentsz JO. MRI-guided biopsy for prostate cancer detection: a systematic review of current clinical results. Curr Urol Rep. 2013;14:209. doi: 10.1007/s11934-013-0323-z. [DOI] [PubMed] [Google Scholar]
- 48.Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013;64:544. doi: 10.1016/j.eururo.2013.03.030. [DOI] [PubMed] [Google Scholar]