Abstract
The spin-lattice relaxation rates at 293 K for three anionic semiquinones (2,5-di-t-butyl-1,4-benzosemiquinone, 2,6-di-t-butyl-1,4-benzosemiquinone, and 2,3,5,6-tetramethoxy-1,4-benzosemiquinone) were studied at up to 8 frequencies between 250 MHz and 34 GHz in ethanol or methanol solution containing high concentrations of OH-. The relaxation rates are about a factor of 2 faster at lower frequencies than at 9 or 34 GHz. However, in perdeuterated alcohols the relaxation rates exhibit little frequency dependence, which demonstrates that the dominant frequency-dependent contribution to relaxation is modulation of dipolar interactions with solvent nuclei. The relaxation rates were modeled as the sum of two frequency-independent contributions (spin rotation and a local mode) and two frequency-dependent contributions (modulation of dipolar interaction with solvent nuclei and a much smaller contribution from modulation of g anisotropy). The correlation time for modulation of the interaction with solvent nuclei is longer than the tumbling correlation time of the semiquinone and is consistent with hydrogen bonding of the alcohol to the oxygen atoms of the semiquinones.
Introduction
Semiquinones are ubiquitous in biology, from photosynthesis to environmental toxicology [1, 2]. They are involved in many electron transfer reactions [3, 4]. Semiquinones have been studied extensively by EPR [5], and their spin relaxation properties are important to understanding their EPR spectra. Semiquinones were selected for studies of mechanisms of electron spin relaxation because their g values are higher than for carbon-centered radicals, but their nuclear hyperfine couplings are smaller than for nitroxides.
Semiquinones were among the first species for which spin-lattice relaxation rates were measured directly by saturation recovery in fluid solution [6, 7]. Semiquinone relaxation rates have been studied extensively at X-band as a function of temperature and viscosity [7-12]. In alcohol solutions relaxation rates could be fit with the relationship shown in Eq. (1) [7-11].
| (1) |
where η is the viscosity of the solution, ΔE was found to be about 1 kcal/mole (ΔE/R = 500 K), A and B were determined experimentally and depend both on semiquinone and on solvent [7-11].
There is general agreement that the term in Eq. (1) in T/η is due to spin rotation, which is usually written as in Eq. (2) [13, 14].
| (2) |
where i = x,y,z and ge is 2.0023.
The origin of the second term in Eq. (1) was not well understood and has been attributed to hindered rotation [7, 11] and/or spin rotation [10]. A study at X- and Q-band of five semiquinones in glass-forming solvents between 25 and 295 K found that the dominant contributions to relaxation at 295 K were spin rotation and a local mode [15]. The energy for the local mode was 600 K, which is similar to the activation energy reported previously for the second contribution to semiquinone relaxation (Eq. 1). The full expression for a local mode is given by Eq. (3) [16].
| (3) |
where Δloc is the energy of the local mode in Kelvin and Clocal is determined experimentally. The local mode relaxation mechanism was initially proposed for defects in ionic lattices [16]. The temperature dependence of relaxation described by Eq. 3 has been observed for molecular species in glassy matrices, including organic radicals and transition metal complexes [17-19]. The magnitude of the contribution from the local mode is larger in soft matrices such as sucrose octaacetate than in harder ones such as sorbitol [18]. For a molecular species in a glass the local mode is interpreted as an intramolecular vibration that is impacted by interaction with the matrix. For nitroxides in highly viscous media [18, 19] and for trityl radicals in water:glycerol [20] the local mode dominates relaxation in the glassy state below the glass transition temperature. There is no change in slope for a plot of log(1/T1) vs. log(T) in the vinicity of the glass transition temperature, so it is proposed that the local mode relaxation mechanism persists in solution.
If Δloc ≫ T, Eq. (3) can be approximated as in Eq. (4), which has the same temperature dependence as the second term in Eq. (1).
| (4) |
These results indicate that the two dominant contributions to spin-lattice relaxation at X-band for semiquinones tumbling rapidly in solution are spin rotation and a local mode with an energy of 500 to 600 K. The contributions from these two relaxation mechanisms are independent of resonance frequency.
Studies of the frequency dependence of spin-lattice relaxation for carbon-centered trityl radicals [21] and nitrogen-centered nitroxide radicals [22-24] in fluid solution have demonstrated the significance of additional relaxation mechanisms. These frequency-dependent processes modulate anisotropic interactions – g anisotropy (Eq. 5) [25, 26], hyperfine (Ai) anisotropy (Eq. (7) [25-27], dipolar coupling to solvent nuclei (Eq. 8) [21], or involve a thermally-activated process (Eq. 9) [23].
| (5) |
where Δg = gzz − 0.5(gxx+gyy), δg = 0.5(gxx − gyy), μB is the electron Bohr magneton and J(ω) is the Bloembergen, Purcell, Pound (BPP) spectral density function (Eq. 6).
| (6) |
where τR is the tumbling correlation time of the semiquinone and ω is the resonance frequency.
| (7) |
where Ai is a component of the nitroxide nitrogen hyperfine coupling in angular frequency units, Ā is the average nitrogen hyperfine, and I is the nitrogen nuclear spin.
| (8) |
where τSolvent is the correlation time for motion of the solvent relative to the radical, and Csolvent is a function of the dipolar interaction with solvent nuclei.
| (9) |
where τtherm = τc0 exp(Ea/RT), Ea is the activation energy, τc0 is the pre-exponential factor, Ctherm is the coefficient for the contribution of the thermally-activated process, and ωref = 9.5 GHz. Variable temperature studies of semiquinone spin lattice relaxation did not find evidence of a thermally-activated process as described by Eq. (9) [7, 15] so this contribution was not included in the models used in this paper.
To determine the extent to which frequency-dependent processes (Eq. 5,7,8) contribute to the relaxation of semiquinones in alcohol solvents at 293 K, the three radicals shown in Figure 1 were studied at frequencies between 250 MHz and 34 GHz.
Figure 1.
Structures of the semiquinones studied.
2. Experimental
2.1 Semiquinone preparation
2,5-Di-t-butyl-1,4-benzosemiquinone (25DTBSQ) was prepared by mixing equal volumes of air-saturated ethanol solutions of 2,5-di-t-butyl hydroquinone (5 mM, Alfa Aesar, 98% purity) and KOH (5 mM) [28]. 2,6-Di-t-butyl-1,4-benzosemiquinone (26DTBSQ) was prepared from 2,6-di-t-butyl-1,4-benzoquinone (Aldrich, 98% purity) using the same procedure. 2,3,5,6-Tetramethoxy-1,4-benzosemiquinone (TMBSQ) was prepared by mixing a 2:1 volume ratio of 25 mM KOH and 5mM tetraflouro-1,4-benzoquinone (Sigma-Aldrich, 97% purity) in methanol. Formation of the radicals was confirmed by X-band CW EPR [28]. Concentrations of the semiquinones were determined by comparison of the double integrals of the EPR spectra with double integrals of signals for 0.20 mM 15N-CTPO (3-carbamoyl-2,2,5,5-tetramethyl-3-pyrroline-1-oxyl) in the same solvent. Concentrations were in the range of 0.2 to 0.5 mM. 1,4-benzosemiquinones form adducts with, and react with, O2 [29]. The EPR signals of the semiquinones in air-saturated solution are very broad and difficult to observe. Air-saturated solutions stored at 4°C for up to 30 days produced well-resolved semiquinone EPR signals after deoxygenation. In deoxygenated sealed tubes the EPR signal decays within hours to days.
2.2 Oxygen Removal
Samples were purged with N2 to remove O2. Relaxation time measurements were repeated, with continued N2 purging, until no further increase in relaxation time constant was observed. At Q-band the samples were in Teflon tubes with 0.30 mm ID and 0.66 mm OD that were placed inside a 1.6 mm OD quartz capillary tube that was open at both ends. The Bruker cryostat was purged with N2 throughout the measurements. At frequencies between 1 and 9 GHz samples were in 4 mm OD quartz tubes. The vapor space in the tube was initially purged vigorously with N2, after which N2 was gently bubbled into the solution through thin Teflon tubing. At X-band the resonator also was purged with N2. At L-, S-, and X-band samples were purged continuously with N2. At 600 MHz and 250 MHz 16 mm and 25 mm OD quartz tubes were used, respectively. The height of the solution in the tube was about 3 cm. To purge the large vapor space in these tubes the following protocol was developed: 15 min purging at the top of the tube, 15 min near the top, 15 min in the middle of the tube and 30 min gentle bubbling into the solution followed by constant gentle purging with N2 at the top of the tube during the relaxation time measurements.
2.3 Relaxation times measurements
Spin-lattice relaxation times, T1, were measured by inversion recovery at 250 MHz [30], 600 MHz [23], L-band (1.0, 1.5 GHz) [31], S-band (2.57, 3.0 GHz) [31], and X-band (9.5 GHz) [32] on locally designed spectrometers and at Q-band (34 GHz) on a Bruker E580 spectrometer. The experiments were performed at a resonator temperature of ∼293 K. Multifit, a locally written program based on the algorithms of Provencher, was used to determine T1 from the decay curves [33]. Single exponential fits were in good agreement with the data. Replicate measurements demonstrated that uncertainties were in the range of 5 to 15%, as shown Fig. 2 - 4. At lower frequencies the signal-to-noise was poorer than at higher frequencies, which resulted in greater uncertainty in T1.
Figure 2.
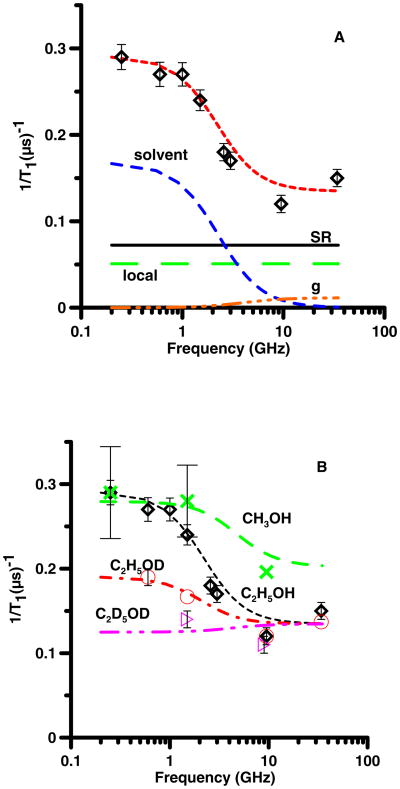
(A) Frequency dependence of 1/T1 ( ) for 25DTBSQ in ethanol at 293 K. The relaxation is modeled as the sum (- -, short dash) of contributions from spin rotation (SR) (—, solid) Eq. (2), a local mode (local) (— —, long dash) Eq. (3), modulation of g-anisotropy (g) (— - - -, dash dot) Eq. (5) and modulation of dipolar interactions with solvent nuclei (solvent) (– –, medium dash) Eq. (8). (B) Frequency dependence of 1/T1 in C2H5OH ( ), in CH3OH (x), in C2H5OD (○) and in C2D5OD (▷). The dashed lines through the data are the sums of contributions to the spin lattice relaxation as described in the text, calculated with Eq. 2, 3, 5, and 8.
Figure 4.
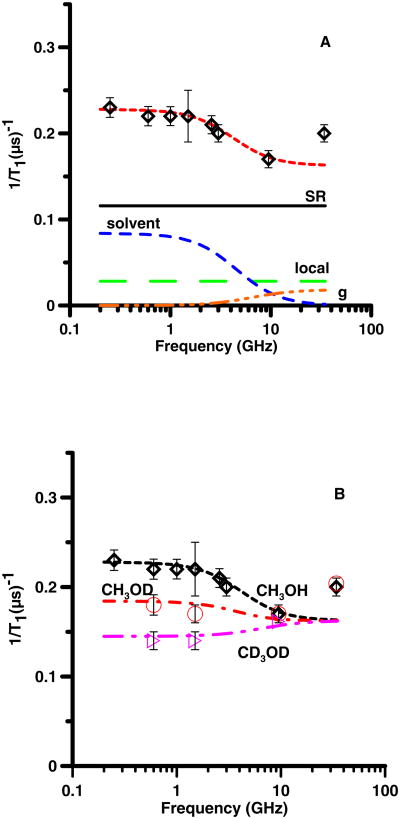
(A) Frequency dependence of 1/T1 ( ) for TMBSQ in methanol at 293 K. The relaxation is modeled as the sum (- -, short dash) of contributions from spin rotation (SR) (—, solid) Eq. (2), a local mode (local) (— —, long dash) Eq. (3), modulation of g-anisotropy (— - - -, dash dot) Eq. (5) and modulation of dipolar interactions with solvent nuclei (solvent) (– –, medium dash) Eq. (8). (B) Frequency dependence of 1/T1 in CH3OH ( ), in CH3OD (○), and in CD3OD (▷). The dashed lines through the data are the sums of contributions to the spin lattice relaxation as described in the text, calculated with Eq. 2, 3, 5, and 8.
3. Modeling the frequency dependence of 1/T1
3.1 Frequency-independent contributions to 1/T1
The two frequency-independent contributions to 1/T1 for molecules tumbling rapidly in fluid solution are spin rotation (Eq. 2) and the local mode (Eq. 3,4). The contribution 1/T1local was calculated using Eq. (3) with Δloc = 500 K to be consistent with ΔE = 1 kcal/mole from the extensive early work on semiquinones [7]. In Ref. [7] the value of B (Eq. 1) for 25DTBSQ in ethanol or methanol was 0.29×106 s-1. A value of Δloc = 500 K in Eq. (3) is not large enough to be fully in the limit of Δloc ≫ T at 293 K. To make the value of 1/T1local at 293 K calculated using Eq. (3) with Δloc = 500 K equal to that calculated with Eq. (1) and ΔE = 1 kcal/mole requires multiplication of B by about a factor of 0.7, which gives Clocal ∼ 0.19×106 s-1. In ref. [15] the contribution from the local mode for 25DTBSQ in 1:4 glycerol:ethanol or 1:2 glycerol:ethanol was calculated with Δloc = 600 K and Clocal = 0.18×106 s-1 or 0.13×106 s-1, respectively. To obtain the same magnitude of contribution at 293 K with Δloc = 500 K would require Clocal = 0.11×106 s-1 or 0.08×106 s-1 for the two solvent mixtures, respectively. Since there is a weak dependence of Clocal on solvent and semiquinone, and there are uncertainties in parameters obtained in prior experiments, the values of Clocal in the modeling of the frequency dependence of 1/T1 were constrained to be between 0.08×106 and 0.19×106 s-1.
Calculation of 1/T1SR requires g values and τR. The g values for 25DTBSQ, 26DTBSQ, and TMBSQ in hydrogen-bonding solvents are gx = 2.0065, gy = 2.0052, and gz = 2.0023 [15]. The viscosities at 298 K are 0.545 cP for CH3OH and 1.08 cP for C2H5OH [34]. Based on these viscosities, tumbling correlation times calculated using the Stokes-Einstein equation are 109 ps for 25DTBSQ or 26DTBSQ in C2H5OH and 57 ps for TMBSQ in CH3OH at 293 K. In the early studies of semiquinone relaxation the spin rotation term was expressed as AT/η (Eq. 1), which can be related to the expression in Eq. (2) by using τR = cslip V η/kT where V is the molecular volume and k is Boltzmann's constant [35, 36]. The adjustable parameter cslip ranges from 0 to 1.0 depending on the extent of solute-solvent interaction [35, 36]. When molecular volumes were calculated assuming a density of 0.90, the values of A (Eq. 1) in ref. [7] correspond to cslip ranging from 0.10 to 0.40 with an average of 0.24, including cslip = 0.40 and 0.25 for 25DTBSQ in CH3OH and C2H5OH, respectively, and cslip = 0.36 for TMBSQ in CH3OH. These values of cslip are larger than those for nitroxides [23] which are 0.11 for tempone (which has a keto group) in water, 0.15 for mHCTPO (which has a carboxamide group) in water, and 0.21 for proxyl (which has a carboxy group) in water. The larger values of cslip for the anionic semiquinones than for neutral nitroxides are consistent with the expectation of stronger interaction of the semiquinones with the H-bonding polar solvents. Values of cslip in water:glycerol mixtures for trityl radicals with three carboxy groups are about 0.65 [21]. Since the molar masses of the trityls are in the range of 975 to 1695 [21] and the Stokes-Einstein model results in cslip closer to 1 for larger molecules, the values of cslip for semiquinones with molar masses about 220 are expected to be smaller than for the trityls. In modeling the frequency dependence of 1/T1 the values of cslip were constrained to be between 0.24 and 0.50. It was assumed that cslip was similar in ethanol and methanol, unaffected by solvent deuteration, and similar for 25DTBSQ and 26DTBSQ. The resulting values of cslip were 0.37 for 25DTBSQ and 26DTBSQ and 0.44 for TMBSQ (Table 1). The higher value for TMBSQ may reflect additional solvent interaction with the methoxy oxygens.
Table 1. Contributions to Spin-Lattice Relaxation of Semiquinones at 293 K in Fluid Solution.
| Semiquinone | Solvent | τR (ps)a, (cslip) | Δloc (K)b | Cloc (s-1) | τsolvent (ps) | Csolvent (s-1) |
|---|---|---|---|---|---|---|
| 25DTBSQ | C2H5OH | 40 (0.37) | 500 | 0.18×106 | 70 | 2.4×1015 |
| C2H5OD | 40 (0.37) | 500 | 0.18×106 | 70 | 1.0×1015 | |
| C2D5OD | 40 (0.37) | 500 | 0.18×106 | 70 | <5×1013 | |
| CH3OH | 20 (0.36) | 500 | 0.18×106 | 35 | 2.4×1015 | |
| 26DTBSQ | C2H5OH | 40 (0.37) | 500 | 0.18×106 | 70 | 2.6×1015 |
| C2H5OD | 40 (0.37) | 500 | 0.18×106 | 70 | 1.2×1015 | |
| TMBSQ | CH3OH | 25 (0.44) | 500 | 0.10×106 | 35 | 2.4×1015 |
| CH3OD | 25 (0.44) | 500 | 0.10×106 | 35 | 1.2×1015 | |
| CD3OD | 25 (0.44) | 500 | 0.10×106 | 35 | <5×1013 |
3.2 Frequency-dependent contributions to 1/T1
The contribution from modulation of g anisotropy (Eq. 5) is calculated from the known g values and the value of τR that was determined by analysis of the contributions from frequency-independent terms at X-band.
Eq. (7), the contribution from modulation of anisotropic nuclear hyperfine interaction, was derived for a single nuclear spin. That contribution is proportional to the square of the hyperfine anisotropy. Analogous equations to treat the contributions to relaxation from couplings to the two semiquinone ring protons in 25DTBSQ or 26DTBSQ have not been derived. The anisotropic hyperfine couplings to the two ring protons are (1.92, 2.14, 2.35 G) and (0.92, 1.28, 1.44 G), respectively [15]. If it is assumed that the contributions from multiple protons are additive, the estimated contributions from modulation of hyperfine anisotropy for the two ring protons are about two orders of magnitude smaller than the observed frequency dependence of 1/T1 (Fig. 2-4). Although the assumption of additivity is over-simplified, more sophisticated analyses are unlikely to dramatically change the magnitude of the contribution, so modulation of the anisotropic ring proton hyperfine coupling was omitted from the analysis.
The parameters Csolvent and τsolvent were adjusted to match the experimental data. The inflection point in the plot of 1/T1solvent vs. frequency is strongly dependent on τsolvent so those values are tightly constrained. The value of Csolvent was adjusted to model the magnitude of the frequency-dependent contribution at low frequency.
4. Results and Discussion
The spin lattice relaxation rates for the three semiquinones were studied at 293 K at up to 8 frequencies between 250 MHz and 34 GHz. As reported previously [7] there was negligible concentration dependence of T1 for semiquinone concentrations of the order of 1 mM. This lack of concentration dependence has been attributed to the solvent cage around the radicals [7]. The negative charge on the anionic semiquinones also may decrease collision probabilities. In a comparable concentration range significant concentration dependence has been observed for nitroxide radicals [37].
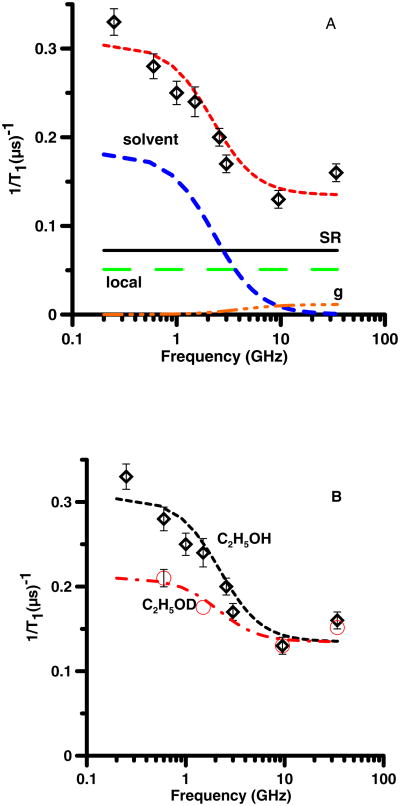
For 25DTBSQ in C2H5OH or CH3OH (Fig. 2), 26DTBSQ in C2H5OH (Fig. 3), and TMBSQ in CH3OH (Fig. 4) the spin lattice relaxation rates were about a factor of two faster at lower frequencies than at 9 GHz. The relaxation was modeled as the sum of contributions from two frequency-independent processes, (spin rotation (Eq. 2) and a local mode (Eq. 3)), and two frequency-dependent processes (modulation of dipolar interaction with solvent nuclei (Eq. 8) and a smaller contribution from modulation of g-anisotropy (Eq. 5)) (Table 1). Spin-lattice relaxation at X-band is dominated by the frequency-independent processes and is unchanged by solvent deuteration. Decreasing τR increases the contribution from spin rotation which can be compensated by increasing the coefficient Clocal. The range of acceptable values of τR is limited by the range of Clocal found in prior variable temperature experiments [7, 15]. At 295 K the viscosity of CH3OH (0.545 cP) is about half that of C2H5OH (1.08 cP) so τR decreases by about a factor of 2 when the solvent is changed from C2H5OH (Fig. 2A) to CH3OH (Fig. 2B). Comparison of relaxation rates for 25DTBSQ in C2H5OH and CH3OH permitted separation of the tumbling-dependent spin-rotation contribution from the tumbling-independent local mode contribution. These constraints permitted definition of the values of τR. Calculation of T1 at X-band for four semiquinones in ethanol at 293 K by substitution of reported values of A and B into Eq. (1) [7] shows that in this rapid tumbling regime the contribution from the first term (spin rotation) is 1.3 to 2.1 times larger than the contribution from the second term (the local mode). For 25DTBSQ in ethanol at X-band the ratio of the contributions from spin rotation and the local mode is 1.5, which is consistent with the earlier reports. Although the molar masses differ by only about 20%, τR for semiquinones (Table 1) in ethanol (η=1.05 cP) are longer than for neutral tempone (Table 2) in water (η= 1.0 cP), which is attributed to hydrogen bonding between the anionic semiquinones and the solvent [38-40].
Figure 3.
(A) Frequency dependence of 1/T1 ( ) for 26DTBSQ in ethanol at 293 K. The relaxation is modeled as the sum (- -, short dash) of contributions from spin rotation (SR) (—, solid) Eq. (2), a local mode (local) (— —, long dash) Eq. (3), modulation of g-anisotropy (— - - -, dash dot) Eq. (5), and modulation of dipolar interactions with solvent nuclei (solvent) (– –, medium dash) Eq. (8). (B) Frequency dependence of 1/T1 in C2H5OH ( ) and in C2H5OD (○). The dashed lines through the data are the sums of contributions to the spin lattice relaxation as described in the text and calculated with Eq. 2, 3, 5, and 8.
Table 2. Properties of Representative Trityl, Semiquinone, and Nitroxide Radicals.
| Radicalb | Solvent | g-values | Molar mass | τR (ps)a | Ref. |
|---|---|---|---|---|---|
|
| |||||
| Trityl-OX31 | H2O | 2.0030 | 1695 | 500 | [20, 21] |
| 2.0027 | |||||
| 2.0021 | |||||
|
| |||||
| Trityl-CH3 | H2O | 2.0030 | 975 | 300 | [20, 21] |
| 2.0027 | |||||
| 2.0021 | |||||
|
| |||||
| 25DTBSQ | C2H5OH | 2.0065 | 220 | 40 | This work |
| 2.0052 | |||||
| 2.0023 | |||||
|
| |||||
| 25DTBSQ | CH3OH | 2.0065 | 220 | 20 | This work |
| 2.0052 | |||||
| 2.0023 | |||||
|
| |||||
| Tempone-d16 | 69% glycerol | 2.0092 | 186 | 50 | [23] |
| 2.0061 | |||||
| 2.0022 | |||||
|
| |||||
| Tempone-d16 | H2O | 2.0092 | 186 | 9 | [23, 42] |
| 2.0061 | |||||
| 2.0022 | |||||
At 293 K
Abbreviations for radicals are defined in the papers cited.
The modulation of g anisotropy by molecular tumbling (Eq. 5) makes a relatively small contribution to 1/T1 and is significant for the semiquinones only at Q-band. For each of the semiquinones the relaxation rates at Q-band were faster than predicted by modeling with Eq. (2, 3, 5, and 8). It is therefore proposed that an additional process contributes at higher frequencies, which will require further study. Studies of spin lattice relaxation for nitroxides at Q-band and W-band also have found rates that are faster than at X-band, and the mechanism is not known [24, 41].
The dominant frequency-dependent contribution to 1/T1 was decreased by deuteration of the solvent (Fig. 2 – 4). For all three semiquinones replacement of solvent OH by OD decreased the contribution from the frequency-dependent contribution by about a factor of two (Table 1). Complete solvent deuteration eliminated this contribution, which demonstrated domination of the frequency dependent contribution to relaxation by interaction with solvent nuclei. The contributions calculated from Eq. (8) are strongly dependent on the value of τsolvent. The simulations required τsolvent about twice as long as τR The uncertainties for τsolvent are about ±10% and the uncertainties for τR are about 25%, so the difference of about a factor of 2 is larger than the uncertainties in the correlation times. The observation of τsolvent > τR indicates that the dynamic process that modulates the interaction between the unpaired electron and the solvent nuclei is not molecular tumbling. The OH proton is only one of 4 protons in CH3OH and one of 6 protons in C2H5OH, so the factor of two reduction in the frequency-dependent contribution to relaxation (Eq. 8) when only the OH proton is replaced by a deuteron is substantially larger than predicted if all of the protons contributed equally. Water and alcohols are strongly hydrogen bonded to semiquinones [38-40]. The observations that (i) the contribution from Eq. (8) decreased by about a factor of 2 when only the OH was replaced by OD (Fig. 2 – 4), (ii) the frequency dependent contribution to relaxation was eliminated by solvent perdeuteration, and (iii) values of τsolvent differ from τR, are consistent with assignment of fluctuations in hydrogen bonding as the process that drives τsolvent. Hydrogen bonding to the semiquinone oxygens is the most probable source of the frequency-dependent contribution to relaxation.
4.1 Comparison with relaxation rates and mechanisms for trityls and nitroxides
Differences in relaxation rates between trityls, semiquinones, and nitroxides illustrate factors that impact relaxation for organic radicals in liquid solution (Table 3). Carbon-centered trityl-CH3 and trityl-OX31 have relatively high molar masses, and therefore long τR, as well as g values close to 2, so spin rotation is not an effective spin lattice relaxation mechanism [21]. At X-band 1/T1 for trityls is dominated by a local mode [21]. At lower frequencies modulation of dipolar interactions with the many protons in the radicals by molecular tumbling causes 1/T1 to be larger than at X-band [21]. Relaxation rates for semiquinones at X-band are faster than for the trityls because tumbling correlation times are shorter and g values are further from g = 2 (Table 2), so spin rotation makes a substantial contribution, along with a local mode. The relative importance of spin rotation and the local mode depends on τR. At lower frequencies it is proposed that the relaxation rates for semiquinones are faster than at X-band because dipolar interactions with solvent protons are modulated by fluctuations in the hydrogen bonding between the solvent and the semiquinone oxygens. For nitroxides the contribution to relaxation from modulation of the large anisotropic nitrogen hyperfine coupling is strongly dependent on frequency and τR [23]. The contribution from this process is larger at lower frequency and for longer τR (Eq. 7). This process is not significant for trityls or semiquinones because these molecules do not have large anisotropic hyperfine couplings. The relaxation rates for all three classes of radicals are faster at lower frequencies than at X-band (Table 3). Although the mechanisms are different, in each case an additional contribution becomes significant as the frequency is decreased and as the rate of a dynamic processes become comparable to the resonance frequency, ie ω ∼ 1/τ. For the slowly tumbling trityls this occurs below about 1 GHz, and for the more rapidly tumbling semiquinones it occurs below about 5 GHz.
Table 3. Frequency Dependence of Relaxation Times and Mechanisms for Representative Trityl, Semiquinone, and Nitroxide Radicals.
| Radicala | Solvent | 9 GHz | 250 MHz | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| T1 (μs) | 1/T1 (μs)-1 | mechanism | T1 (μs) | 1/T1 (μs)-1 | mechanism | |||
| Trityl-OX31 | H2Ob | 14 | 0.071 | local mode | 5.9 | 0.17 | modulation of proton dipole interactions by trityl tumbling | [21] |
| Trityl-CH3 | H2Ob | 16 | 0.062 | local mode | 7.9 | 0.13 | [21] | |
| 25DTBSQ | C2H5OH | 7.8 | 0.13 | spin rotation + local mode | 3.4 | 0.29 | modulation of solvent proton interactions by solvent motion | This work |
| 25DTBSQ | CH3OH | 5.0 | 0.20 | spin rotation + local mode | 3.4 | 0.29 | This work | |
| Tempone-d16 | 69% glycerolb | 2.2 | 0.45 | modulation of Ad | 0.25e | 4.0e | modulation of Ad | [23] |
| Tempone-d16 | H2Oc | 0.58 | 1.7 | spin rotation + modulation of Ad | 0.60 | 1.7 | spin rotation + modulation of Ad | [22, 23] |
Abbreviations for radicals are defined in the papers cited
0.2 mM concentration
0.5 mM concentration
A refers to the nitrogen hyperfine coupling
Measured at 630 MHz
Research Highlights.
Spin lattice relaxation was measured for benzosemiquinones in alcohol + OH-.
Relaxation at 293 K is faster at 250 MHz than at 34 GHz.
In perdeutered solvent, frequency dependence of relaxation is dramatically reduced.
Enhanced relaxation at low frequency is attributed to modulation of hydrogen bonding.
Acknowledgments
Partial support of this work by NIH grant P41 EB002034 to GRE, Howard J. Halpern, PI, and by the University of Denver is gratefully acknowledged. HE was partially supported by a scholarship from the Libyan Ministry of Higher Education and Scientific Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Song Y, Buettner GR. Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Rad Biol Med. 2010;49:919–962. doi: 10.1016/j.freeradbiomed.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishra S, Malhotra P, Gupta AK, Singh PK, Javed S, Kumar R. Semiquinone glucoside derivative (SQGD) isolated from bacillus sp. INM-1 protects against gamma radiation-induced oxidative stress. Envir Toxicol Pharm. 2014;37:553–562. doi: 10.1016/j.etap.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Armendariz-Vidales G, Hermandez-Munoz LS, Gonzalez FJ, de Souza AA, de Abreu FC, Jardim GAM, da Silva EN, Gulart MO, Frontana C. Nature of electrogenerated intermediates in nitro-substituted nor B-lapachones: The structure of radical species during successive electron transfer in multiredox centers. J Org Chem. 2014;79:5201–5208. doi: 10.1021/jo500787q. [DOI] [PubMed] [Google Scholar]
- 4.Guin PS, Das S, Mandal PC. Int J Electrochem. 2011 [Google Scholar]
- 5.Pederson JA. CRC Handbook of EPR Spectra from Natural and Synthetic Quinones and Quinols. CRC Press; Boca Raton, FL: 1985. [Google Scholar]
- 6.Fessenden RW, Hornak JP, Venkataram B. Electron spin-lattice relaxation times of transient free radicals. J Chem Phys. 1981;74:3694–3704. [Google Scholar]
- 7.Rengan SK, Khakhar MP, Prabhananda BS, Venkataraman B. Study of molecular motions in liquids by electron spin-lattice relaxation measurements, I. Semiquinone ions in hydrogen bonding solvents. Pramana. 1974;3:95–121. [Google Scholar]
- 8.Rengan SK, Khakhar MP, Prabhananda BS, Venkataraman B. Electron Spin-Lattice Relaxation in Organic Free Radicals in Solutions. Pure Appl Chem. 1972;32:287–305. [Google Scholar]
- 9.Rengan SK, Khakhar MP, Prabhananda BS, Venkataraman B. Study of molecular motions in liquids by electron spin-lattice relaxation measurements, II. 2,5-Di-tert-butylsemiquinone Ions in Acetonitrile and Tetrahydrofuran. J Magn Reson. 1974;16:35–43. [Google Scholar]
- 10.Prabhananda BS, Hyde JS. Study of molecular motions in liquids by electron spin relaxation: halogenated p-semiquinone anions in alcohols. J Chem Phys. 1986;85:6705–6712. [Google Scholar]
- 11.Krishnamoorthy G, Prabhananda BS. Molecular motions in liquidlike pockets of frozen solutions: Electron spin relaxation study of semiquinones in DMSO and DMSO-ethanol mixtures. J Chem Phys. 1982;76:108–113. [Google Scholar]
- 12.Leniart DS, Connor HD, Freed JH. An ESR and ENDOR study of spin relaxation of semiquinone in liquid solution. J Chem Phys. 1975;63:165–199. [Google Scholar]
- 13.Atkins PW, Kivelson D. ESR Linewidths in Solution. II. Analysis of Spin-Rotational Relaxation Data. J Chem Phys. 1966;44:169–174. [Google Scholar]
- 14.Muus LT, Atkins PW. Electron Spin Relaxation in Liquids. Plenum Press; 1972. [Google Scholar]
- 15.Kathirvelu V, Sato H, Eaton SS, Eaton GR. Electron Spin Relaxation Rates for Semiquinones between 25 and 295 K in Glass-Forming Solvents. J Magn Reson. 2009;198:111–120. doi: 10.1016/j.jmr.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castle JG, Jr, Feldman DW. Temperature dependence of paramagnetic relaxation at point defects in vitreous silica. J Appl Phys. 1965;36:124–128. [Google Scholar]
- 17.Eaton SS, Eaton GR. Relaxation times of organic radicals and transition metal ions. Biol Magn Reson. 2000;19:29–154. [Google Scholar]
- 18.Sato H, Kathirvelu V, Fielding AJ, Bottle SE, Blinco JP, Micallef AS, Eaton SS, Eaton GR. Impact of molecular size on electron spin relaxation rates of nitroxyl radicals in glassy solvents between 100 and 300 K. Mol Phys. 2007;105:2137–2151. [Google Scholar]
- 19.Sato H, Bottle SE, Blinco JP, Micallef AS, Eaton GR, Eaton SS. Electron spin-lattice relaxation of nitroxyl radicals in temperature ranges that span glassy solutions to low-viscosity liquids. J Magn Reson. 2008;191:66–77. doi: 10.1016/j.jmr.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fielding AJ, Carl PJ, Eaton GR, Eaton SS. Multifrequency EPR of Four Triarylmethyl Radicals. Appl Magn Reson. 2005;28:239–249. [Google Scholar]
- 21.Owenius R, Eaton GR, Eaton SS. Frequency (250 MHz to 9.2 GHz) and Viscosity Dependence of Electron Spin Relaxation of Triarylmethyl Radicals at Room Temperature. J Magn Reson. 2005;172:168–175. doi: 10.1016/j.jmr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Biller JR, Meyer VM, Elajaili H, Rosen GM, Eaton SS, Eaton GR. Frequency Dependence of Electron Spin Relaxation Times in Aqueous Solution for a Nitronyl Nitroxide Radical and Per-deuterated-Tempone between 250 MHz and 34 GHz. J Magn Reson. 2012;225:52–57. doi: 10.1016/j.jmr.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biller JR, Elajaili H, Meyer V, Rosen GM, Eaton SS, Eaton GR. Electron Spin Lattice Relaxation Mechanisms of Rapidly-Tumbling Nitroxide Radicals. J Magn Reson. 2013;236:47–56. doi: 10.1016/j.jmr.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyde JS, Yin JJ, Subczynski WK, Camenisch TG, Ratke JJ, Froncisz W. Spin-labeled EPR T1 Values Using Saturation Recovery from 2 to 35 GHz. J Phys Chem B. 2004;108:9524–9529. [Google Scholar]
- 25.Robinson BH, Haas DA, Mailer C. Molecular dynamics in liquids: spin-lattice relaxation of nitroxide spin labels. Science. 1994;263:490–493. doi: 10.1126/science.8290958. [DOI] [PubMed] [Google Scholar]
- 26.Robinson BH, Mailer C, Reese AW. Linewidth analysis of spin labels in liquids. I. Theory and data analysis. J Magn Reson. 1999;138:199–209. doi: 10.1006/jmre.1999.1737. [DOI] [PubMed] [Google Scholar]
- 27.Mailer C, Nielsen RD, Robinson BH. Explanation of Spin-Lattice Relaxation Rates of Spin Labels Obtained with Multifrequency Saturation Recovery EPR. J Phys Chem A. 2005;109:4049–4061. doi: 10.1021/jp044671l. [DOI] [PubMed] [Google Scholar]
- 28.Kathirvelu V, Sato H, Quine RW, Rinard GA, Eaton SS, Eaton GR. EPR Free Induction Decay Coherence Observed after a Single-Pulse in Saturation Recovery Experiments for Samples with Resolved Multi-line CW Spectra. Appl Magn Reson. 2007;32:269–281. [Google Scholar]
- 29.Valgimigli L, Amorati R, Fumo MG, DiLabio GA, Pedulli GF, Ingold KU, Pratt DA. The Unusual Reaction of Semiquinone Radicals with Molecular Oxygen. J Org Chem. 2008;73:1830–1841. doi: 10.1021/jo7024543. [DOI] [PubMed] [Google Scholar]
- 30.Quine RW, Rinard GA, Eaton SS, Eaton GR. A pulsed and continuous wave 250 MHz electron paramagnetic resonance spectrometer. Magn Reson Engineer. 2002;15:59–91. [Google Scholar]
- 31.Quine RW, Rinard GA, Ghim BT, Eaton SS, Eaton GR. A 1-2 GHz pulsed and continuous wave electron paramagnetic resonance spectrometer. Rev Sci Instrum. 1996;67:2514–2527. [Google Scholar]
- 32.Quine RW, Eaton GR, Eaton SS. Pulsed EPR spectrometer. Rev Sci Instrum. 1987;58:1709–1723. [Google Scholar]
- 33.Provencher SW. An eigenfunction expansion method for the analysis of exponential decay curves. J Chem Phys. 1976;64:2772–2777. doi: 10.1016/S0006-3495(76)85660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon AJ, Ford RA. The Chemist's Companion: A Handbook of Practical Data, Techniques, and References. Wiley-Interscience Publication; New York: 1972. Properties of Solvents and Common Liquids; pp. 4–5. [Google Scholar]
- 35.McClung RED, Kivelson D. ESR Linewidths in Solution. V. Studies of Spin–Rotational Effects Not Described by Rotational Diffusion Theory. J Chem Phys. 1968;49:3380–3391. [Google Scholar]
- 36.Kivelson D, Madden P. Light scattering studies of molecular liquids. Annu Rev Phys Chem. 1980;31:523–558. [Google Scholar]
- 37.Biller JR, Meyer V, Elajaili H, Rosen GM, Kao JPY, Eaton SS, Eaton GR. Relaxation Times and Line Widths of Isotopically-Substituted Nitroxides in Aqueous Solution at X-band. J Magn Reson. 2011;212:370–377. doi: 10.1016/j.jmr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flores M, Isaacson RA, Calvo R, Feher G, Lubitz W. Probing Hydrogen Bonding to Quinone Anions Radicals by 1H and 2H ENDOR Spectroscopy at 35 GHz. Chem Phys. 2003;294:401–413. [Google Scholar]
- 39.Paddock ML, Flores M, Isaacson R, Shepherd JN, Okamura MY. EPR and ENDOR Investigation of Rhodosemiquinone in Bacterial Reaction Centers Formed by B-Branch Electron Transfer. Appl Magn Reson. 2010;37:39–48. doi: 10.1007/s00723-009-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ackermann P, Barbarin F, Germain JP, Fabre C. Resonance Paramagnetique Electronique. Influence de la solvation sur la relaxation electronique anisotrope de radicaus stable neutres ou charges Proceedings of the Colloquium Spectroscopicum Internationale. 1975;2:440–445. [Google Scholar]
- 41.Hofbauer W, Earle KA, Dunnam CR, Moscicki JK, Freed JH. High-power 95 GHz pulsed electron spin resonance spectrometer. Rev Sci Instrum. 2004;75:1194–1208. [Google Scholar]
- 42.Hwang JS, Mason RP, Hwang LP, Freed JH. Electron spin resonance studies of anisotropic rotational reorientation and slow tumbling in liquid and frozen media. III. Perdeuterated 2,2,6,6-tetramethyl-4-piperidone-N-oxide and an analysis of fluctuating torques. J Phys Chem. 1975;79:489–511. [Google Scholar]