Abstract
N Acylethanolamine acid amidase (NAAA) is a cysteine hydrolase that catalyzes the hydrolysis of endogenous lipid mediators such as palmitoylethanolamide (PEA). PEA has been shown to exert anti–inflammatory and antinociceptive effects in animals by engaging peroxisome proliferator–activated receptor–α (PPAR–α). Thus preventing PEA degradation by inhibiting NAAA may provide a novel approach for the treatment of pain and inflammatory states. Recently, 3–aminooxetan–2–one compounds were identified as a class of highly potent NAAA inhibitors. The utility of these compounds is limited, however, by their low chemical and plasma stabilities. In the present study, we synthesized and tested a series of N–(2–oxoazetidin–3–yl)amides as a novel class of NAAA inhibitors with good potency and improved physicochemical properties, suitable for systemic administration. Moreover, we elucidated the main structural features of 3–aminoazetidin–2–one derivatives that are critical for NAAA inhibition.
Keywords: β-lactams, NAAA, inhibitors, stability, structure-activity relationship
Introduction
Fatty acid ethanolamides (FAEs) are a family of lipid–derived messengers that play important roles in the control of pain, inflammation and energy balance.[1] Two structurally and functionally distinct classes of FAEs have been described: polyunsaturated FAEs, such as arachidonoylethanolamide (anandamide), which are endogenous ligands for cannabinoid receptors and participate, among other functions, in the control of stress–coping responses and pain initiation;[2] and monounsaturated and saturated FAEs, such as oleoylethanolamide (OEA) and palmitoylethanolamide (PEA), which are endogenous ligands for peroxisome proliferator–activated receptor–α (PPAR–α) and are involved in energy balance, pain and inflammation.[1a]
Within the latter class, PEA, the endogenous amide of palmitic acid and ethanolamine, has been shown to inhibit peripheral inflammation and mast cell degranulation[3] and to exhibit antinociceptive properties in rat and mouse models of acute and chronic pain.[3b, 4] Those properties depend on PPAR–α activation because they are absent in PPAR–α-null mice, are mimicked by synthetic PPAR–α agonists and are blocked by PPAR–α antagonists.[5] Furthermore, PEA has been reported to suppress pain behaviors induced by tissue injury, nerve damage or inflammation in mice[5c] and to attenuate skin inflammation and neuropathic pain in humans.[6]
Tissue FAE levels are regulated at the level of biosynthesis and degradation.[7] A molecularly unique phospholipase D releases FAEs from precursor N–acylphosphatidylethanolamines (NAPEs) present in cell membrane.[8] FAEs degradation to the corresponding free fatty acids and ethanolamine is carried out by either fatty acid amide hydrolase (FAAH) or N–acylethanolamine acid amidase (NAAA).[9] These two intracellular lipid amidases show different cellular localization and substrate selectivity with NAAA preferentially catalyzing the hydrolysis of PEA and OEA in innate immune cells.[4b]
NAAA[10] is a cysteine amidase that belongs to the N–terminal nucleophile (Ntn) family of enzymes,[11] and is characterized by a conserved amino acid catalytic triad Cys, Arg and Asp. The primary structure of NAAA has no homology with that of FAAH,[11] while it shares 33–34% identity and 70% similarity to acid ceramidase (AC), a cysteine amidase involved in the hydrolysis of the sphingolipid messenger ceramide. NAAA is thought to be localized to lysosomes[12] and shows common features with other lysosomal hydrolases, i.e. N–glycosylation, autoproteolytic cleavage[13] and activation at acidic pH (around 4.5–5.0).[9a] After its maturation, the N–terminal cysteine residue (Cys–131 in rodents; Cys–126 in humans) is responsible for the catalytic cleavage of nonpeptide C–N bonds in linear amides;[14] for this reason, NAAA is also linked to the choloylglycine hydrolase superfamily.[11] Site–directed mutagenesis[15] and mass spectrometry studies[16] have unambiguously identified human Cys–126 as the nucleophile responsible for both autoproteolysis and activity.
Recently, inhibition of NAAA activity has attracted increasing interest as a strategy to sustain endogenous PEA and OEA levels, and the enzyme has been envisaged as a new potential therapeutic target for inflammation.[5a]
So far, only a limited number of natural[17] or synthetic[18] NAAA inhibitors have been reported in the literature. Among them, α–amino–β–lactone (3–aminooxetan–2–one) derivatives have shown considerable promise because of their high inhibitory potency and target selectivity.[19] In particular, in vitro and in vivo studies have demonstrated that (S)–N–(2–oxo–3–oxetanyl)–3–phenylpropionamide ((S)–OOPP, 1, Figure 1), which inhibits rat-NAAA (r-NAAA) with a median inhibitory concentration (IC50) of 0.42 μM [19a, 19f], blocks FAE hydrolysis in activated inflammatory cells and dampens tissue reactions to various pro–inflammatory stimuli.[19b] More recently, 5–phenylpentyl N–[(2S,3R)–2–methyl–4–oxo–oxetan–3–yl]carbamate (ARN077, 2, Figure 1, r-NAAA IC50 = 0.050 μM),[19c, 19d, 19f] a new potent and selective β–lactone NAAA inhibitor, was shown to elevate PEA and OEA levels in mouse skin and sciatic nerve tissues, and to attenuate nociception in mice and rats following topical administration.[20]
Figure 1.

Structures of (S)–OOPP (1) and ARN077 (2)
Despite their high potency and efficacy, β–lactone–based NAAA inhibitors suffer from limited chemical and plasma stabilities[19c, 19e] due to the facile ring–opening to the corresponding β–hydroxyacid. Their low chemical stability makes these derivatives suitable for topical applications,[19c, 19e] but prevents their use as systemic agents.
To further explore the therapeutic utility of NAAA inhibitors, we started a program aimed at identifying new chemical series that might be suitable for systemic administration. Within this program, we investigated functionalized α–amino-β–lactam (3–amidoazetidin–2–one) derivatives, a chemo–type structurally similar to the 3–aminooxetan–2–one, to test whether the replacement of the β–lactone core with a β–lactam retained inhibitory activity towards NAAA, while increasing chemical and plasma stabilities.
In the present work, we report the discovery of 3–aminoazetidin–2–ones as a new class of NAAA inhibitors endowed with good potency and promising chemical and plasma stabilities, which may make these derivatives suitable for systemic administration. In particular, we prepared a small series of β–lactam analogues and evaluated their inhibitory activity on human NAAA (h–NAAA). This study allowed us to highlight key structural features for NAAA inhibition and derive an initial structure–activity relationship (SAR).
Chemistry
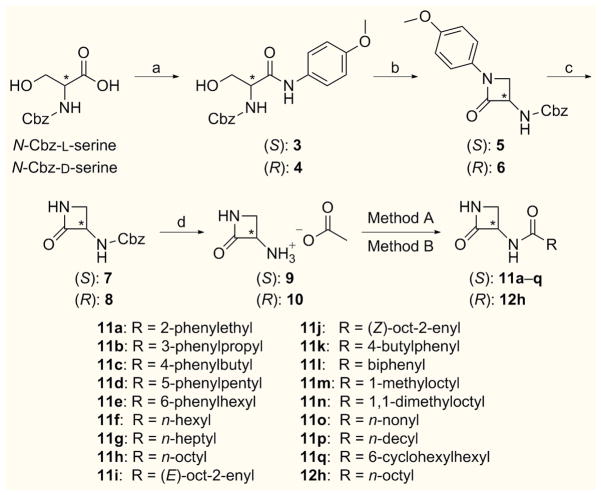
Differently substituted 3–aminoazetidin–2–one derivatives were efficiently accessed in an enantioselective fashion, starting from the corresponding D– or L–N–protected–serine derivatives.
As reported in Scheme 1, the designed synthetic route envisaged both the (S)– and (R)–2–oxoazetidin–3–yl–ammonium acetate (9 and 10),[21] as key intermediates for the synthesis of 3–aminoazetidin–2–one amide derivatives bearing different side chains. Notably, by modifications and optimization of literature procedures,[22] compounds 9 and 10 were prepared in multigram scale, with 40–44% overall yield over four steps.[23]
Scheme 1.
Synthetic pathway for the preparation of amide derivatives 11a–q and 12h. Reagents and conditions: a) p–anisidine, EDC, THF/DCM (3:1), r.t., 16 h [85–92%]; b) ImSO2Im, 0 °C, 1 h, then NaH, DMF, −20 °C, 1 h [75%]; c) CAN, MeCN/H2O (1:1), 0 °C, 1 h [80–85%]; d) 1,4–cyclohexadiene, 10% Pd on charcoal, EtOH, r.t., 12 h, then AcOH, EtOAc [79%]; Method A [for 11a, 11f–h, 11l, 11o and 12h]: RCOCl, Et3N, DCM or DCM/DMF (3:1), r.t., 16 h [15–65%]; Method B [for 11b–e, 11i–k, 11m–n and 11p–q]: RCOOH, TBTU, Et3N, DCM/DMF (3:1), r.t., 16 h [30–62%].
First, benzyloxycarbonyl (Cbz)–protected azetidinones 7 and 8 were prepared following a three–step sequence.[22a] Amide coupling between p–anisidine and N–[(benzyloxycarbonyl)]–L– and D–serine, afforded intermediates 3 and 4, which were converted into the corresponding N–protected α–amino–β–lactams 5 and 6 by a one–pot cyclization reaction, which represents the key step in this synthesis. Subsequent p–methoxyphenyl deprotection with ceric ammonium nitrate in a 1:1 mixture of MeCN/H2O led to β–lactams 7 and 8. Then, Cbz-deprotection[22b] was carried out by catalytic transfer hydrogenation, using 1,4–cyclohexadiene as hydrogen source in the presence of 10% palladium on charcoal.[24] The resulting unprotected 3–aminoazetidin–2–ones were immediately trapped as acetate salts 9 and 10, which could be easily stored for several months.
Coupling reactions of salt 9 with the suitable acyl chloride in the presence of triethylamine afforded amides 11a, 11f–h and 11l and 11o, while reaction of enantiomer 10 with nonanoyl chloride led to compound 12h (Method A, Scheme 1).
Alternatively, the reaction of compound 9 with the appropriate carboxylic acid, activated with TBTU and triethylamine, gave amides 11b–e, 11i–k, 11m–n and 11p–q (Method B, Scheme 1). In all coupling reactions no racemization at the stereogenic α–center was detected.
All the employed acyl chlorides and most of the carboxylic acids were commercially available; carboxylic acids 13, 14 and 15 were prepared as reported in Scheme 2.
Scheme 2.

Syntheses of carboxylic acids 13, 14 and 15. Reagents and conditions: a) LiOH, THF/MeOH/H2O (1:1:1), r.t., 1 h [quant.]; b) Dess–Martin Periodinane, DCM, 0 °C, 1 h, then r.t, 1 h. [94%]; c) NaClO2, NaH2PO4, 2–methyl–2–butene, tBuOH/H2O 4:1, r.t. 1.5 h [quant.]; d) DMSO, oxalyl chloride, DCM, −78°C, 15 min then 17, −78 °C, 1 h, then Et3N, r.t. [quant.]; e) (EtO)3POCH2CO2Et, NaH (95%), THF, 0 °C to r.t. [77%]; f) H2, 10% Pd/C cartridge (H–Cube®, EtOH, 45 °C, 20 bar, flow: 1.0 mL/min), 92%; g) LiOH, THF/MeOH/H2O (1:1:1), r.t., 1 h [quant.].
Compound 13 was synthesized by saponification of commercially available methyl (E)–3–nonenoate (Scheme 2).
The preparation of (Z)–non–3–enoic acid (14) was accomplished in two steps starting from commercially available (Z)–non–3–en–1–ol. Oxidation with Dess–Martin periodinane[25] led to the exclusive formation of (Z)–non–3–enal (16) (Scheme 2). Further oxidation under Pinnick reaction conditions, in the presence of 2–methy–2–butene as scavenger, selectively yielded acid 14 as a single diastereoisomer.
Carboxylic acid 15 was prepared starting from 5–cyclohexylpentan–1–ol (17)[26] which was first oxidized to aldehyde 18. Subsequent Horner–Emmons olefination afforded the α,β–unsaturated ester 19, which was subjected to hydrogenation followed by hydrolysis to furnish compound 15 (Scheme 2).
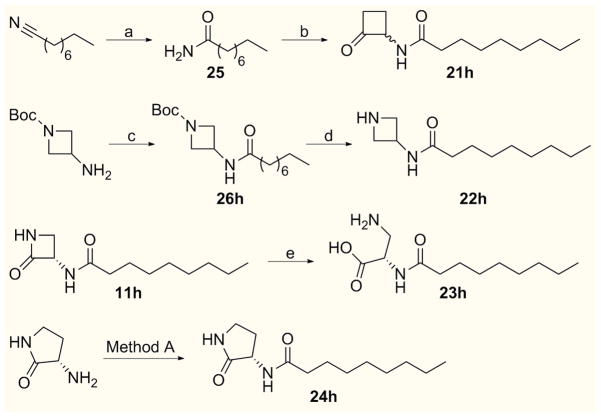
Compounds 21h–24h were synthesized as reported in Scheme 3.
Scheme 3.
Syntheses of compounds 21h-24h. Reagents and conditions: a) 1N NaOH, 35%, H2O2, 60% tBuOH–H2O, r.t., 16 h [82%]; b) 2N HCl.Et2O, 1,2–bis(trimethylsiloxy)cyclobutene, THF, reflux, 3 h [37%]; c) nonanoyl chloride, Et3N, DCM, r.t., 2 h [94%]; d) TFA/DCM (1:3), 0 °C, 30 min, then r.t. 45 min [quant.]; e) 2N HCl, r.t., 30 min then THF, r.t., 16 h [79%]; Method A: nonanoyl chloride, Et3N, DCM, r.t., 16 h [83%].
Cyclobutanone derivative 21h was prepared by the acid–catalyzed reaction of 1,2–bis(trimethylsilanoxy)cyclobutene with amide 25, obtained by alkaline hydrolysis of nonanenitrile (Scheme 3).[19c] The synthesis of compound 22h was accomplished starting from commercially available N–Boc–3–amino–azetidine by standard amide coupling with nonanoyl chloride followed by Boc deprotection with TFA/DCM (Scheme 3). Acid–mediated ring opening of 11h smoothly delivered compound 23h as hydrochloride salt in good yield (Scheme 3). Finally, standard amide coupling of nonanoyl chloride with commercially available (3S)–3–aminopyrrolidin–2–one led to derivative 24h (Scheme 3).
Then, N–methylated amides 27h and 28h were prepared as reported in Scheme 4.
Scheme 4.

Syntheses of N–methylated compounds 27h-28h. Reagents and conditions: a) NaH (60% in mineral oil), MeI, THF, 0° C, 1 h then r.t., 4 h [25%]; b) NaH (95%), MeI, THF, 0° C, 1 h then r.t., 1 h [90%]; c) CAN, MeCN/H2O (1:1), 0 °C, 1 h [92%]; d) H2, 10% Pd/C cartridge (H–Cube, EtOAc, 30 °C, 1.0 bar, flow: 1.0 mL/min) then AcOH, EtOAc [78%]; Method A: nonanoyl chloride, Et3N, DCM, r.t., 16 h [33%].
Alkylation of azetidinone 11h with methyl iodide in the presence of sodium hydride in DMF selectively occurred on the N1–endocyclic nitrogen, providing compound 27h (Scheme 4).
Alkylation of the protected β–lactam 5 with methyl iodide, under standard conditions, afforded compound 29 in good yield. Cleavage of the p–anisyl group followed by catalytic hydrogenation led to the N–methyl acetate salt 31, which gave N–methyl derivative 28h after amide coupling with nonanoyl chloride, using Method A (Scheme 4).
The acetate salt 9 was used to access amine, urea and carbamate derivatives 32, 33 and 34, respectively, as reported in Scheme 5.
Scheme 5.

Syntheses of amine, urea and carbamate derivatives 32–34. Reagents and conditions: a) DMSO, oxalyl chloride, DCM, −78 °C, 15 min then n–nonanol −78 °C, 1 h, then Et3N, r.t. [quant.]; b) 9, Et3N, dichloroethane, 10 min, then Na(OAc)3BH, r.t., 1.5 h [21%]; c) nonyl isocyanate, DMAP, pyridine, r.t., 16 h [59%]; d) DMAP, 2-DPC, DCM, r.t., 16 h [quant.]; e) 9, DIPEA, DCM, r.t., 16 h [36%].
Amine derivative 32 was synthesized by reductive amination of aldehyde 35, obtained by oxidation of n–nonanol, with salt 9, while urea 33 was obtained treating 9 with nonyl isocyanate in the presence of catalytic DMAP (Scheme 5).
Carbamate 34 was synthesized in a two-step sequence. n–Heptanol was reacted with 2–pyridyl carbonate (2–DPC)[19d, 27] and catalytic DMAP to give an isomeric mixture of 2–pyridyl carbonate 36 and 2–oxopyridine–1–carboxylate 37 (65:35 ratio), which was then coupled with 9 affording the desired compound 34 in good yield (Scheme 5).
Structure–Activity Relationship (SAR) and Stability Studies
The aim of the present study was to explore substituted 3–aminoazetidin–2–one derivatives as a novel class of NAAA inhibitors potentially suitable for systemic administration. The new compounds were tested for their ability to inhibit the hydrolysis of N–(4–methyl–2–oxo–chromen–7–yl)–hexadecanamide (PAMCA) by recombinant h–NAAA heterologously expressed in HEK293 cells (see Experimental Section).[16] Median inhibitory concentration (IC50) values are reported in Tables 1, 3 and 4. A few representative compounds were also evaluated for their chemical stability in buffer at pH 7.4 and 5.0[28] and for their stability in mouse plasma (Table 2).
Table 1.
Inhibitory potencies (IC50) on human NAAA of compounds 11a–h[a]
| Compounds | Structure | h–NAAA IC50 (μM) ± S.E.M |
|---|---|---|
| 11a |

|
Not active[b] |
| 11b |

|
73.36 ± 10.68 |
| 11c |

|
27.16 ± 1.89 |
| 11d |
|
12.06 ± 2.18 |
| 11e |

|
0.60 ± 0.17 |
| 11f |

|
27.02 ± 6.15 |
| 11g |
|
1.91 ± 0.28 |
| 11h |
|
0.34 ± 0.03 |
IC50 values are reported as mean values of three or more determinations.
Not active: < 10% inhibition @ 50 μM.
Table 3.
Inhibitory potencies (IC50) on human NAAA of analogues of compound 11h[a]
| Compounds | Structure | h–NAAA IC50 (μM) ± S.E.M |
|---|---|---|
| 12h |
|
74.52 ± 9.08 |
| 21h |
|
Not active[b] |
| 22h |
|
Not active[b] |
| 23h |

|
Not active[b] |
| 24h |

|
Not active[b] |
| 27h |

|
Not active[b] |
| 28h |
|
Not active[b] |
| 32 |
|
Not active[b] |
IC50 values are reported as mean values of three or more determinations.
Not active: < 10% inhibition @ 50 μM.
Table 4.
Inhibitory potencies (IC50) on human NAAA of compounds 11i–q, 33 and 34[a]
| Compounds | Structure | h–NAAA IC50 (μM) ± S.E.M |
|---|---|---|
| 11i |
|
3.09 ± 0.50 |
| 11j |

|
3.90 ± 0.86 |
| 11k |

|
13.85 ± 3.41 |
| 11l |

|
Not active[b] |
| 11m |
|
0.22 ± 0.03 |
| 11n |
|
0.76 ± 0.26 |
| 11o |
|
0.24 ± 0.03 |
| 11p |
|
0.10 ± 0.02 |
| 11q |
|
0.28 ± 0.07 |
| 33 |
|
5.76 ± 0.90 |
| 34 |
|
0.24 ± 0.02 |
IC50 values are reported as mean values of three or more determinations.
Not active: < 10% inhibition @ 50 μM.
Table 2.
Stability data of (S)–OOPP (1) and compounds 11a, 11h.
| Cmpd | Chemical Stability[a], | m–Plasma Stability[a,c] | r–Plasma Stability[a,e] | |
|---|---|---|---|---|
| pH 7.4, t1/2 (min), (%)[d] | pH 5.0, t1/2 (min)[d] | t1/2 (min) | t1/2 (min) | |
| 1 | 30 | 45 | <1 | n.t.[f] |
|
| ||||
| 11a | >1440 (92) | >1440 (97) | >120 | >120 |
|
| ||||
| 11h | >1440 (100) | >1440 (98) | 103 | >120 |
Stability values are reported as mean values of three or more determinations.
Measured after 24 h of incubation at 37 °C in PBS + 10% MeCN at pH 7.4 and 5.0; values in parentheses represent the compound remaining (%) after 24 h (1440 min)
Measured in 100% mouse plasma.
Compound remaining (%) after 24 h (1440 min).
Compounds tested at 2.5 μM in r-plasma added with 2.5% DMSO.
n.t. = Not tested. Compound 1 was reported to have t1/2 < 10 sec in 80% r-plasma. [19c]
We utilized the β–lactam derivative 11a (Table 1), an analogue of the known β-lactone NAAA inhibitor (S)–OOPP (1, Figure 1) ([19a] h–NAAA IC50: 1.29 μM), as starting point for our initial SAR work. Disappointingly, 11a turned out to be inactive. However, the compound showed substantially higher hydrolytic stability (t1/2 > 1440 min at both pH 7.4 and pH 5.0) and promising mouse–plasma stability (t1/2 > 120 min) and rat-plasma stability (t1/2 > 120 min) compared to its β–lactone analogue (S)-OOPP (t1/2 < 1 min) (Table 2).
Based on previous SAR results with the β–lactone class,[19d] we hypothesized that activity might be recovered by increasing the length of the amide side-chain. Therefore, a small set of analogues was prepared where the length of the phenylalkyl chain of 11a was progressively increased from 2 to 6 methylene units (11b–e, Table 1). Confirming our hypothesis, NAAA inhibitory activity, albeit in the high micromolar range, was observed for compounds 11b (IC50 = 73.36 μM) and 11c (IC50 = 27.16 μM), while the 6–phenylhexyl analogue 11e showed submicromolar potency (IC50 = 0.60 μM). Removal of the phenyl group from 11e led to compound 11f (IC50 = 27.02 μM), which showed a >40–fold drop in potency compared to 11e. A good inhibitory activity against NAAA was again recovered by increasing the length of the alkyl chain from six to eight carbon atoms as in analogue 11h (IC50 = 0.34 μM), which turned out to be the most potent derivative within this small series of compounds. Notably, the β–lactam 11h retained a favorable chemical and plasma stability profile (Table 2), proving to be stable to chemical hydrolysis over 24 h (t1/2 > 1440 min), and showing acceptable half–life in mouse and rat plasma (t½ = 103 min and > 120 min, respectively).
These initial results suggested that N–(2–oxoazetidin–3–yl)amide derivatives may hold potential as a new potent and stable class of NAAA inhibitors.
Encouraged by these results, we considered compound 11h as the prototype for this novel class of inhibitors, and synthesized a first set of analogues to identify the key structural features for NAAA inhibition.
We first modified the 3–aminoazetidin–2–one scaffold, while maintaining fixed the amide chain: the stereochemistry at the C(3) position was inverted, the nitrogen and the carbonyl group of the azetidinone ring were replaced by a methylene, the β–lactam ring was hydrolyzed and expanded to the five atoms homologue (γ–lactam). Finally, the endocyclic nitrogen was methylated to assess the stereoelectronic requirements at this position.
The importance of the (S)–configuration for the substituent at C(3) position was clearly demonstrated by the >200–fold loss in potency observed with compound 12h (IC50 = 74.52 μM, Table 3), the (R)–enantiomer of 11h. This stereochemical preference is in agreement with previous observations on serine–derived β–lactone inhibitors.[19a, 19b] Replacement of the endocyclic nitrogen with a methylene (21h), reduction of the azetidinone to an azetidine ring (22h), ring–opening of the azetidinone (23h), and ring–expansion to γ–lactam (24h) all led to loss of activity, as previously observed in the β–lactone series.[19a, 19b] Methylation of the N1–endocyclic nitrogen (27h), abolished the NAAA inhibitory activity. These findings unambiguously indicated that an intact β–lactam moiety was mandatory for NAAA inhibition.
We then focused our attention on the amide function at the C(3) position of the azetidinone ring. The exocyclic nitrogen was methylated, leading to compound 28h, and the amide was replaced by a n–nonylamino group, as in compound 32. Both derivatives turned out to be inactive. A secondary amide at C(3) position of the azetidinone ring appears therefore to be required for NAAA inhibition.
As the next step in our SAR study, we directed our attention to the amide side chain, and explored the importance of the conformational flexibility, the substitution and the length of the alkyl chain.
First, we introduced a steric constraint in compounds 11i–j (Table 4), two analogues of 11h that contain a double bond in the (E) and (Z) configuration, respectively. Both changes resulted in a 10–fold drop in potency, with no preference for the alkene configuration (11i, IC50 = 3.09 μM; 11j, IC50 = 3.90 μM). Further reduction of the side-chain flexibility by introduction of a para-substituted phenyl ring, as in compounds 11k–l, led to a decrease (11k, IC50 = 13.85 μM) or loss (11l) of inhibitory activity. These findings indicated that the insertion of sterically constrained amide chains is detrimental for activity, contrary to what observed with β–lactone amides.[19c]
We also synthesized compounds bearing a branched aliphatic side-chain (11m and 11n). A single methyl group close to the amide function appeared to be well accommodated as compound 11m (IC50 = 0.22 μM), although as a mixture of diastereoisomers, showed a slight increase in potency compared to compound 11h. However, the introduction of a gem-dimethyl group in the same position, as in derivative 11n (IC50 = 0.76 μM), was detrimental for potency.
We then turned our attention to analogues with longer alkyl chains, and prepared compounds containing a decanamide (11o) and undecanamide residue (11p). The compounds showed improved NAAA inhibitory activity (11o, IC50 = 0.24 μM; 11p, IC50 = 0.10 μM). This result supports the idea that linear lipophilic moieties, which resemble the natural enzyme substrate, are well accommodated in the active site.
The insertion of a terminal cyclohexyl moiety, as in amide 11q (IC50 = 0.28 μM), resulted in a slight decrease in potency compared to 11p (Table 4), but a two–fold potency increase with respect to phenyl analogue 11e (Table 1).
Finally, we investigated the impact of the functionality at the N3–exocyclic amino group of the β–lactam on NAAA inhibition by synthetizing the corresponding urea (33) and carbamate (34) analogues of amide 11h (Table 4).
The replacement of the amide moiety with a urea, as in derivative 33 (IC50 = 5.76 μM), resulted in a marked decrease in potency. By contrast, the carbamate 34 (IC50 = 0.24 μM) was interestingly more potent than the amide 11h.
NAAA shows 33–34% identity and 70% similarity to AC, a cysteine amidase that catalyzes the deactivating hydrolysis of the pro-inflammatory lipid messenger, ceramide. We tested therefore the most representative compounds identified in our study (11h, its enantiomer 12h, and 11p) for their selectivity against human AC and FAAH. The selectivity of compounds 11h, 12h and 11p versus AC was evaluated using an UPLC–MS–based assay[19a, 29] in order to measure IC50 values under similar experimental conditions. The results are reported in Table 5.
Table 5.
Inhibitory potencies (IC50) on human NAAA[a], human AC[a] and % inhibitory activity (% inhib) on human FAAH of compounds 11h, 12h and 11p
| Compounds | h–NAAA IC50 (μM) ± SD | h–AC IC50 (μM) ± SD | h–FAAH (% Inhib)[b] |
|---|---|---|---|
| 11h | 0.13 ± 0.03 | 2.53 ± 0.06 | n.a. |
| 12h | 45.54 ± 19.94 | 27.81 ± 5.38 | n.a. |
| 11p | 0.056 ± 0.010 | 0.33 ± 0.10 | n.a. |
IC50 values are measured by LC/MS method and reported as mean values of three or more determinations.
Not active: < 10% inhibition @ 100 μM
Compound 11h showed ca. 19–fold selectivity for NAAA vs. AC (h–NAAA IC50 = 0.13 μM; h–AC IC50 = 2.53 μM) while its enantiomer, 12h, displayed low potency on the two enzymes (h–NAAA IC50 = 45.5 μM; h–AC IC50 = 27.8 μM). Interestingly, lengthening the amide alkyl chain, as in compound 11p, led to a ca. 7–fold increase in potency toward h–AC (IC50 = 0.33 μM) and a ca. 2–fold increase in potency toward h–NAAA (IC50 = 0.056 μM). Lengthening of the alkyl chain appeared therefore to have a more pronounced effect on AC than on NAAA, as indicated by the drop in selectivity vs. AC of analogue 11p with respect to 11h. None of the three selected compounds showed inhibitory activity toward h–FAAH.
The higher selectivity vs. AC of 11h with respect to 11p, coupled with its greater chemical and plasma stability, make this compound a potential probe for further exploration of the functional role of NAAA. To test whether 11h could be administered systemically, we dosed the compound intravenously (3.0 mg kg 1) and orally (10 mg kg 1) in rats, and determined its pharmacokinetic (PK) profile. Relevant PK parameters are reported in Table 6. Although 11h showed a relatively high clearance (Cl = 702 mL min−1 kg−1) by intravenous route, the oral PK profile was characterized by a maximal plasma concentration (Cmax) of 570 ng mL 1 at 15 min postdosing, and a bioavailability of 67% (Table 6). These results support the use of 11h as a NAAA inhibitor for systemic administration.
Table 6.
Plasma pharmacokinetic parameters of compound 11h after a single intravenous (3.0 mg kg−1) or oral administration (10 mg kg−1) in rats
| Parameters | 3.0 mg kg−1 (IV)[a] | 10mg kg−1 (PO)[a] |
|---|---|---|
| Cmax (ng mL−1) | 397 | 570 |
| Tmax (h) | 0.08 | 0.25 |
| AUC (ng h mL−1) | 111 | 247 |
| Cl (mL min−1 kg−1) | 702 | |
| F (%) | 67 |
Cmax = Maximum observed concentration; AUC = Cumulative area under curve for experimental time points (0–24 h); Cl = Systemic clearance based on observed data points (0–24 h); F = Bioavailability.
Compound was dosed in 10% PEG400/10% Tween 80/80% Saline solution; three animals per dose were treated.
Conclusions
In the present work, we report the discovery of 3–aminoazetidin–2–one derivatives as a novel class of NAAA inhibitors. A series of N–(2–oxoazetidin–3–yl)amides were synthesized and tested to identify key structural features for NAAA inhibition. Our results showed that the β–lactam moiety is mandatory for activity, and that alkylation of the endocyclic nitrogen is not tolerated. The (S)–configuration of the acylamino substituent at C(3) position is strongly preferred over the (R)–configuration. The potency of N–(2–oxoazetidin–3–yl)amides as NAAA inhibitors is modulated by the amide alkyl chain, with long and flexible alkyl residues being preferred over sterically constrained chains. Interestingly, we found out that the amino group on the 3–aminoazetidin–2–one scaffold could be further derivatized as a carbamate (34) with retention of NAAA inhibitory activity (Figure 2). Further studies on this new series of derivatives are currently ongoing.
Figure 2.

Summary of key structural features for β-lactam based inhibitors of NAAA.
Among the synthesized compounds, N–[(S)–2–oxoazetidin–3–yl]nonanamide (11h) showed good inhibitory potency (IC50 = 0.34 μM) against NAAA, ca. 19-fold selectivity versus AC - a closely related cysteine amidase - excellent chemical stability in buffer, and acceptable plasma stability. Importantly, this compound showed good oral bioavailability in rats.
Compound 11h and other potent N–(2–oxoazetidin–3–yl)amide analogues represent promising probes that may help further characterize the functional roles of NAAA and assess the therapeutic potential of systemically available NAAA inhibitors.
Experimental Section
Chemistry
Chemicals, Materials and Methods
All the commercially available reagents and solvents were used as purchased from vendors without further purification. Dry solvents (DCM, THF, DMF, Pyridine, dichloroetane) were purchased from Sigma–Aldrich. Optical rotations were measured on a Rudolf Research Analytical Autopol II automatic polarimeter using a sodium lamp (589 nm) as the light source; concentrations are expressed in g/100 mL using MeOH as a solvent and a 1 dm cell. Automated column chromatography purifications were done using a Teledyne ISCO apparatus (CombiFlash Rf) with pre–packed silica gel columns (4 g). Mixtures of increasing polarity of cyclohexane (Cy) and ethyl acetate (EtOAc) were used as eluents. Column chromatography was performed manually on pre–packed silica cartridges (2 g or 5 g) from Biotage or on glass columns using Merck silica gel 60 (230–400 mesh) as stationary phase. Purifications by preparative HPLC–MS were run on a Waters Autopurification system consisting of a 3100 Single Quadrupole Mass Spectrometer equipped with an Electrospray Ionization interface and a 2998 Photodiode Array Detector. HPLC system included a 2747 Sample Manager, 2545 Binary Gradient Module, System Fluidic Organizer and 515 HPLC Pump. The PDA range was 210–400 nm. Purifications were performed on a XBridgeTM Prep C18 OBD (100 × 19 mm i.d., particle size 5 μm) with a XBridgeTM Prep C18 (10 × 19 mm i.d., particle size 5 μm) Guard Cartridge. Mobile phase was 10 mM NH4OAc in MeCN–H2O (95:5) at pH 5. Electrospray ionization in positive and negative mode was used. Hydrogenation reactions were performed using H–Cube® continuous hydrogenation equipment (SS–reaction line version), employing disposable catalyst cartridges (CatCart®) preloaded with the required heterogeneous catalyst. NMR experiments were run on a Bruker Avance III 400 system (400.13 MHz for 1H, and 100.62 MHz for 13C), equipped with a BBI probe and Z–gradients. Spectra were acquired at 300 K, using deuterated dimethylsulfoxide ([D6]DMSO) or deuterated chloroform (CDCl3) as solvents. UPLC–MS analyses were run on a Waters ACQUITY UPLC-MS system consisting of a SQD (Single Quadrupole Detector) Mass Spectrometer equipped with an Electrospray Ionization interface and a Photodiode Array Detector. PDA range was 210–400 nm. Analyses were performed on an ACQUITY UPLC HSS T3 C18 column (50 × 2.1mm i.d., particle size 1.8μm) with a VanGuard HSS T3 C18 pre–column (5 × 2.1 mm i.d., particle size 1.8μm). Mobile phase was either 10 mM NH4OAc in H2O at pH 5 adjusted with AcOH (A) and 10 mM NH4OAc in MeCN–H2O (95:5) at pH 5 (B). Electrospray ionization in positive and negative mode was applied. Accurate mass measurement (HMRS) was performed on a Synapt G2 Quadrupole-Tof Instrument (Waters, USA), equipped with an ESI ion source.
All final compounds, 11a–q, 12h, 21h–24h, 27h–28h, 32–34 showed ≥ 95% purity by NMR and UPLC–MS analysis. The synthesis of reaction intermediates 9, 10, 13–15, 25, 26h, 31, 35–37 is described in the Supporting Information.
General procedures for the synthesis of amide derivatives 11a, 11f–h, 11l, 11o and 12h via Method A (Scheme 1)
Under nitrogen atmosphere, to a cooled (0 °C), stirred suspension of (2–oxoazetidin–3–yl)–ammonium acetate (9 or 10, 1.0 equiv) in dry DCM (0.07 M solution), or in a 3:1 mixture of dry DCM/DMF (0.07 M solution), dry Et3N (2.1 equiv) and the suitable acid chloride (1.1 equiv) were added. The resulting mixture was stirred at r.t. for 16 h, then diluted with DCM and washed with sat. NH4Cl solution, sat. NaHCO3 solution and brine. The organic phase was dried over Na2SO4, filtered, concentrated to dryness and purified according to the specific conditions described in each example.
3–Phenyl–N–[(S)–2-oxoazetidin–3–yl]–propanamide (11a)
The reaction was carried out following Method A, using salt 9 (0.050 g, 0.34 mmol), commercially available hydrocinnamoyl chloride (0.056 mL, 0.38 mmol) and dry Et3N (0.10 mL, 0.71 mmol) in dry DCM (5.0 mL). After work–up, trituration with EtOAc afforded compound 11a (0.080 g, 15%), as a white solid: Rt = 1.40 min; (c = 0.09 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.51 (d, 1H, J = 8.2 Hz), 7.96 (bs, 1H), 7.29–7.24 (m, 2H), 7.22–7.14 (m, 3H), 4.87–4.80 (m, 1H), 3.38 (t, 1H, J = 5.4 Hz), 2.99 (dd, 1H, J = 5.4, 2.6 Hz), 2.81 (t, 2H, J = 7.9 Hz), 2.41 (t, 2H, J = 7.9 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 171.4, 168.0, 141.1, 128.3, 128.2, 125.4, 56.9, 42.9, 36.8, 30.9 ppm; MS (ESI, pos) m/z: 219 [M+H]+, 241 [M+Na]+, 257 [M+K]+; MS (ESI, neg) m/z: 217 [M–H]−; HRMS–ESI: m/z [M+H]+ calcd for C12H15N2O2: 219.1134, found: 219.1136.
N–[(S)–2–Oxoazetidin–3–yl]–heptanamide (11f)
The reaction was carried out following Method A, using salt 9 (0.050 g, 0.34 mmol), commercially available heptanoyl chloride (0.058 mL, 0.38 mmol) and dry Et3N (0.1 mL, 0.71 mmol) in dry DCM (5.0 mL). After work–up, purification by typical silica gel flash chromatography (DCM/MeOH, from 100:0 to 96:4) afforded compound 11f (0.023 g, 34%), as a white solid: Rt = 1.63 min; (c = 0.07 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.43 (d, 1H, J = 8.3 Hz), 7.94 (bs, 1H), 4.82 (ddd, 1H, J = 8.3, 5.4, 2.7 Hz), 3.38 (t, 1H, J = 5.4 Hz), 3.02 (dd, 1H, J = 5.4, 2.7 Hz), 2.08 (t, 2H, J = 7.4 Hz), 1.53–1.42 (m, 2H), 1.32–1.17 (m, 6H), 0.85 (t, 3H, J = 7.0 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 172.7, 168.7, 57.3, 43.3, 35.6, 31.5, 28.7, 25.5, 22.4, 14.4 ppm; MS (ESI, pos) m/z: 199 [M+H]+, 221 [M+Na]+, 237 [M+K]+; MS (ESI, neg) m/z: 197 [M–H]−; HRMS–ESI: m/z [M+H]+ calcd for C10H19N2O2: 199.1447, found: 199.1449.
N–[(S)–2–Oxoazetidin–3–yl]–octanamide (11g)
The reaction was carried out following Method A, using salt 9 (0.060 g, 0.41 mmol), commercially available octanoyl chloride (0.076 mL, 0.45 mmol) and dry Et3N (0.11 mL, 0.86 mmol) in dry DCM (6.0 mL). After work–up, trituration with EtOAc afforded compound 11g (0.019 g, 22%), as a white solid: Rt = 1.88 min; (c = 0.07 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.43 (d, 1H, J = 8.2 Hz), 7.94 (bs, 1H), 4.82 (ddd, 1H, J = 8.2, 5.4, 2.4 Hz), 3.38 (t, 1H, J = 5.4 Hz), 3.02 (dd, 1H, J = 5.4, 2.4 Hz), 2.08 (t, 2H, J = 7.4 Hz), 1.53–1.42 (m, 2H), 1.32–1.17 (m, 8H), 0.85 (t, 3H, J = 7.0 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 172.2, 168.2, 56.8, 42.8, 35.1, 31.1, 28.5, 28.4, 25.1, 22.0, 13.9 ppm; MS (ESI, pos) m/z: 213 [M+H]+, 251 [M+K]+; HRMS–ESI: m/z [M+H]+ calcd for C11H21N2O2: 213.1603, found: 213.1611.
N–[(S)–2–Oxoazetidin–3–yl]–nonanamide (11h)
The reaction was carried out following Method A, using salt 9 (0.60 g, 4.1 mmol), commercially available nonanoyl chloride (0.85 mL, 4.51 mmol) and dry Et3N (1.2 mL, 8.6 mmol) in dry DCM (60 mL). After work–up, trituration with EtOAc afforded compound 11h (0.60 g, 65%), as a white solid: Rt = 2.13 min; (c = 0.07 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.42 (d, 1H, J = 8.3 Hz), 7.94 (bs, 1H), 4.83 (ddd, 1H, J = 8.3, 5.3, 2.7 Hz), 3.38 (t, 1H, J = 5.3 Hz), 3.02 (dd, 1H, J = 5.3, 2.7 Hz), 2.08 (t, 2H, J = 7.3 Hz), 1.53–1.42 (m, 2H), 1.31–1.18 (m, 10H), 0.86 (t, 3H, J = 6.8 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 172.2, 168.2, 56.8, 42.8, 35.1, 31.2, 28.7, 28.6, 28.5, 25.1, 22.1, 13.9 ppm; MS (ESI, pos) m/z: 227 [M+H]+, 249 [M+Na]+, 265 [M+K]+; MS (ESI, neg) m/z: 225 [M–H]−; HRMS-ESI: m/z [M+H]+ calcd for C12H23N2O2: 227.1760, found: 227.1771.
N–[(S)–2–Oxoazetidin–3–yl]–4–phenyl–benzamide (11l)
The reaction was carried out following Method A, using salt 9 (0.13 g, 0.89 mmol), commercially available 4–phenylbenzoyl chloride (0.21 g, 0.98 mmol) and dry Et3N (0.26 mL, 1.87 mmol) in dry DCM/DMF (4.0 mL). After work–up, trituration with EtOAc afforded compound 11l (0.10 g, 42%), as a white solid: Rt = 1.89 min; 1H NMR (400 MHz, [D6]DMSO): δ 9.14 (d, 1H, J = 8.5 Hz), 8.05 (bs, 1H), 7.97 (d, 2H, J = 8.4 Hz), 7.79 (d, 2H, J = 8.4 Hz), 7.74 (d, 2H, J = 7.4 Hz), 7.50 (t, 2H, J = 7.6 Hz), 7.45–7.38 (m, 1H), 5.09 (ddd, 1H, J = 8.5, 5.2, 2.5 Hz), 3.49 (t, 1H, J = 5.2 Hz), 3.27 (dd, 1H, J = 5.2, 2.5 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ 168.6, 166.1, 143.5, 139.5, 132.8, 129.4, 128.5, 127.3, 126.9, 58.5, 43.3; MS (ESI, pos) m/z: 267 [M+H]+, 289 [M+Na]+; MS (ESI, neg) m/z: 265 [M–H]−; HRMS–ESI: m/z [M+H]+ calcd for C16H15N2O2: 267.1134, found: 267.1133.
N–[(S)–2–Oxoazetidin–3–yl]–decanamide (11o)
The reaction was carried out following Method A, using salt 9 (0.050 g, 0.34 mmol), commercially available decanoyl chloride (0.077 mL, 0.38 mmol) and dry Et3N (0.11 mL, 0.71 mmol) in dry DCM (6.0 mL). After work–up, purification by typical silica gel flash chromatography (DCM/MeOH, from 100:0 to 96:4) afforded compound 11o (0.051 g, 63%), as a white solid: Rt = 2.31 min; (c = 0.07 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.43 (d, 1H, J = 8.4 Hz), 7.94 (s, 1H), 4.82 (ddd, 1H, J = 8.4, 5.4, 2.7 Hz), 3.38 (t, 1H, J = 5.4 Hz), 3.02 (dd, 1H, J = 5.4, 2.7 Hz), 2.08 (t, 2H, J = 7.5 Hz), 1.53–1.42 (m, 2H), 1.33–1.16 (m, 12H), 0.86 (t, 3H, J = 7.1 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 172.7, 168.7, 57.3, 43.3, 35.6, 31.7, 29.3, 29.2, 29.1, 29.0, 25.5, 22.6, 14.4 ppm; MS (ESI, pos) m/z: 241 [M+H]+, 263 [M+Na]+, 279 [M+K]+; MS (ESI, neg) m/z: 239 [M–H]−; HRMS–ESI: m/z [M+H]+ calcd for C13H25N2O2: 241.1916, found: 241.1920.
N–[(R)–2–Oxoazetidin–3–yl]–nonanamide (12h)
The reaction was carried out following Method A, using salt 10 (0.090 g, 0.62 mmol), commercially available nonanoyl chloride (0.13 mL, 0.68 mmol) and dry Et3N (0.18 mL, 1.03 mmol) in dry DCM (9.0 mL). After work–up, trituration with EtOAc afforded compound 12h (0.074 g, 53%), as a white solid: Rt = 2.13 min; (c = 0.07 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.42 (d, 1H, J = 8.3 Hz), 7.94 (bs, 1H), 4.83 (ddd, 1H, J = 8.3, 5.3, 2.7 Hz), 3.38 (t, 1H, J = 5.3 Hz), 3.02 (dd, 1H, J = 5.3, 2.7 Hz), 2.08 (t, 2H, J = 7.3 Hz), 1.53–1.42 (m, 2H), 1.31–1.18 (m, 10H), 0.86 (t, 3H, J = 6.8 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 172.2, 168.2, 56.8, 42.8, 35.1, 31.2, 28.7, 28.6, 28.5, 25.1, 22.1, 13.9 ppm; MS (ESI, pos) m/z: 227 [M+H]+, 249 [M+Na]+, 265 [M+K]+; MS (ESI, neg) m/z: 225 [M–H]−; HRMS–ESI: m/z [M+H]+ calcd for C12H23N2O2: 227.1760, found: 227.1766.
General procedures for the synthesis of amide derivatives 11b–e, 11i–k, 11m–n and 11p–q via Method B (Scheme 1)
Under nitrogen atmosphere, to a cooled (0 °C) solution of the suitable carboxylic acid (1.1 equiv) in dry DCM (0.07 M solution) or in a 3:1 mixture of dry DCM/DMF (0.07 M solution), dry Et3N (2.2 equiv) was added followed by addition of N,N,N′,N′–tetramethyl–O–(benzotriazol–1–yl)uronium tetrafluoro borate (TBTU, 1.1 equiv). The resulting reaction mixture was stirred for 10 min then (S)-2–oxoazetidin–3–yl)–ammonium acetate (9, 1.0 equiv) was added. The reaction mixture was stirred at r.t. for 16 h, then diluted with DCM and washed with sat. NH4Cl solution, sat. NaHCO3 solution and brine. The organic phase was dried over Na2SO4, filtered, concentrated to dryness and purified according to the specific conditions described in each example.
N–[(S)–2-Oxoazetidin–3–yl]–4–phenyl–butanamide (11b)
The reaction was carried out following Method B, using salt 9 (0.060 g, 0.41 mmol), commercially available 4–phenylbutanoic acid (0.074 g, 0.45 mmol), TBTU (0.144 g, 0.45 mmol) and dry Et3N (0.12 mL, 0.90 mmol) in dry DCM/DMF (6.0 mL). After work–up, purification by silica gel column chromatography (Cy/EtOAc, from 100:0 to 10:90) afforded compound 11b (0.032 g, 34%), as a white solid: Rt = 1.62 min; (c = 0.09 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.46 (d, 1H, J = 8.4 Hz,), 7.94 (bs, 1H), 7.33–7.24 (m, 2H), 7.20–7.16 (m, 3H), 4.82 (ddd, 1H, J = 8.4, 5.4, 2.5 Hz), 3.39 (t, 1H, J = 5.4 Hz), 3.03 (dd, 1H, J = 5.4, 2.5 Hz), 2.55 (t, 2H, J = 7.5 Hz), 2.12 (t, 2H, J = 7.5 Hz), 1.79 (p, 2H, J = 7.5 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 171.9, 168.4, 141.7, 128.9, 126.0, 125.7, 56.9, 43.1, 34.5, 34.6, 26.9 ppm; MS (ESI, pos) m/z: 233 [M+H]+, 250 [M+Na]+, 271 [M+K]+; HRMS–ESI: m/z [M+H]+ calcd for C13H17N2O2: 233.129, found: 233.1299.
5–Phenyl–N–[(S)–2–oxoazetidin–3–yl]–pentanamide (11c)
The reaction was carried out following Method B, using salt 9 (0.050 g, 0.34 mmol), commercially available 5–phenylpentanoic acid (0.067 g, 0.38 mmol), TBTU (0.12 g, 0.38 mmol) and dry Et3N (0.10 mL, 0.71 mmol) in dry DCM (6.0 mL). After work–up, trituration with Et2O afforded compound 11c (0.028 g, 33%), as a white solid: Rt = 1.79 min; (c = 0.07 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.46 (d, 1H, J = 8.3 Hz), 7.94 (bs, 1H), 7.30–7.23 (m, 2H), 7.21–7.13 (m, 3H), 4.82 (ddd, 1H, J = 8.3, 5.4, 2.6 Hz), 3.38 (t, 1H, J = 5.4 Hz), 3.02 (dd, 1H, J = 5.4, 2.6 Hz), 2.56 (t, 2H, J = 7.2 Hz), 2.12 (t, 2H, J = 6.8 Hz), 1.60–1.45 (m, 4H) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 172.6, 168.6, 142.5, 128.7, 128.6, 126.1, 57.3, 43.31, 35.4, 35.3, 30.9, 25.2 ppm; MS (ESI, pos) m/z: 247 [M+H]+, 269 [M+Na]+, 285 [M+K]+; MS (ESI, neg) m/z: 245 [M–H]−; HRMS–ESI: m/z [M+H]+ calcd for C14H19N2O2: 247.1447, found: 247.1458.
N–[(S)–2–Oxoazetidin–3–yl]–6–phenyl–hexanamide (11d)
The reaction was carried out following Method B, using salt 9 (0.060 g, 0.41 mmol), commercially available 6–phenylhexanoic acid (0.084 mL, 0.45 mmol), TBTU (0.144 g, 0.45 mmol) and dry Et3N (0.12 mL, 0.90 mmol) in dry DCM/DMF (6.0 mL). After work–up, trituration with Et2O afforded compound 11d (0.032 g, 30%), as a white solid: Rt = 1.98 min; (c = 0.07 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.44 (d, 1H, J = 8.2 Hz), 7.94 (bs, 1H), 7.29–7.23 (m, 2H), 7.20–7.13 (m, 3H), 4.82 (ddd, 1H, J = 8.2, 5.4, 2.5 Hz), 3.38 (t, 1H, J = 5.4 Hz), 3.01 (dd, 1H, J = 5.4, 2.5 Hz), 2.58–2.52 (m, 2H), 2.08 (t, 2H, J = 7.4 Hz), 1.60–1.42 (m, 4H), 1.32–1.20 (m, 2H) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 172.5, 168.5, 142.2, 128.3, 128.2, 125.6, 56.8, 42.8, 35.5, 35.4, 30.7, 28.2, 24.9 ppm; MS (ESI, pos) m/z: 261 [M+H]+, 283 [M+Na]+, 299 [M+K]+; HRMS–ESI: m/z [M+H]+ calcd for C15H21N2O2: 261.1603, found: 261.1603.
7–Phenyl–N–[(S)–2–oxoazetidin–3–yl]–heptanamide (11e)
The reaction was carried out following Method B, using salt 9 (0.030 g, 0.21 mmol), commercially available 7–phenylheptanoic acid (0.065 mL, 0.23 mmol), TBTU (0.073 g, 0.23 mmol) and dry Et3N (0.06 mL, 0.46 mmol) in dry DCM (3.0 mL). After work–up, trituration with EtOAc afforded compound 11e (0.022 g, 38%), as a white solid: Rt = 2.19 min; (c = 0.07 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.43 (d, 1H, J = 8.4 Hz), 7.94 (bs, 1H), 7.29–7.23 (m, 2H), 7.20–7.13 (m, 3H), 4.82 (ddd, 1H, J = 8.4, 5.3, 2.6 Hz), 3.38 (t, 1H, J = 5.3 Hz), 3.02 (dd, 1H, J = 5.3, 2.6 Hz), 2.59–2.53 (m, 2H), 2.08 (t, 2H, J = 7.4 Hz), 1.60–1.42 (m, 4H), 1.32–1.21 (m, 4H) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 172.7, 168.6, 142.8, 128.7, 128.6, 126.0, 57.4, 43.3, 35.6, 35.5, 31.4, 28.9, 28.8, 25.5 ppm; MS (ESI, pos) m/z: 275 [M+H]+, 297 [M+Na]+, 313 [M+K]+; HRMS–ESI: m/z [M+H]+ calcd for C16H23N2O2: 275.1760, found: 275.1766.
(E)–N–[(S)–2–Oxoazetidin–3–yl]–non–3–enamide (11i)
The reaction was carried out following Method B, using salt 9 (0.050 g, 0.34 mmol), (E)–3–nonenoic acid (13) (0.058 g, 0.37 mmol), TBTU (0.12 g, 0.37 mmol) and dry Et3N (0.10 mL, 0.75 mmol) in dry DCM (5.0 mL). After work–up, trituration with EtOAc afforded compound 11i (0.035 g, 46%), as a white solid: Rt = 2.04 min; (c = 0.07 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.45 (d, 1H, J = 8.4 Hz), 7.96 (bs, 1H), 5.54–5.41 (m, 2H), 4.81 (ddd, 1H, J = 8.4, 5.5, 2.6 Hz), 3.38 (t, 1H, J = 5.5 Hz), 3.03 (dd, 1H, J = 5.5, 2.6 Hz), 2.83 (d, 2H, J = 5.6 Hz), 2.01–1.93 (m, 2H), 1.37–1.19 (m, 6H), 0.86 (t, 3H, J = 7.1 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 171.0, 168.5, 133.5, 124.0, 57.3, 43.2, 39.6, 32.3, 31.3, 28.9, 22.4, 14.4; MS (ESI, pos) m/z: 225 [M+H]+, 247 [M+Na]+, 263 [M+K]+; HRMS–ESI: m/z [M+H]+ calcd for C12H21N2O2: 225.1603, found: 225.1614.
(Z)–N–[(S)–2–Oxoazetidin–3–yl]–non–3–enamide (11j)
The reaction was carried out following Method B, using salt 9 (0.050 g, 0.34 mmol), (Z)–non–3–enoic acid (14), (0.059 g, 0.38 mmol), TBTU (0.12 g, 0.38 mmol) and dry Et3N (0.10 mL, 0.71 mmol) in dry DCM (3.0 mL). After work–up, trituration with EtOAc afforded compound 11j (0.034 g, 45%), as a white solid: Rt = 1.98 min; (c = 0.07 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.50 (d, 1H, J = 8.3 Hz), 7.97 (bs, 1H), 5.52–5.42 (m, 2H), 4.82 (ddd, 1H, J = 8.3, 5.4, 2.5 Hz), 3.39 (t, 1H, J = 5.4 Hz), 3.03 (dd, 1H, J = 5.4, 2.5 Hz), 2.94–2.86 (m, 2H), 2.04–1.96 (m, 2H), 1.37–1.18 (m, 6H), 0.86 (t, 3H, J = 7.1 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 170.4, 168.0, 131.8, 122.9, 56.9, 42.7, 34.0, 30.8, 28.5, 26.8, 21.9, 13.9 ppm; MS (ESI, pos) m/z: 225 [M+H]+, 247 [M+Na]+, 263 [M+K]+; MS (ESI, neg) m/z: 223 [M–H]−; HRMS–ESI: m/z [M+H]+ calcd for C12H21N2O2: 225.1603, found: 225.1612.
4–Butyl–N–[(S)–2–oxoazetidin–3–yl]–benzamide (11k)
The reaction was carried out following Method B, using salt 9 (0.060 g, 0.41 mmol), commercially available 4–butylbenzoic acid (0.080 g, 0.45 mmol), TBTU (0.144 g, 0.45 mmol) and dry Et3N (0.12 mL, 0.90 mmol) in dry DCM (6.0 mL). After work–up, trituration with Et2O afforded compound 11k (0.029 g, 29%), as a white solid: Rt = 2.07 min; (c = 0.07 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.99 (d, 1H, J = 8.4 Hz,), 8.02 (bs, 1H), 7.78 (d, 2H, J = 8.2 Hz), 7.29 (d, 2H, J = 8.2 Hz), 5.05 (ddd, 1H, J = 8.4, 5.4, 2.6 Hz,), 3.46 (t, 1H, J = 5.4 Hz), 3.24 (dd, 1H, J = 5.4, 2.6 Hz), 2.63 (t, 2H, J = 7.5 Hz), 1.63–1.50 (m, 2H), 1.37–1.20 (m, 2H), 0.89 (t, 3H, J = 7.4 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ 168.3, 166.0, 146.3, 131.1, 128.3, 127.4, 57.3, 42.5, 34.6, 32.8, 217, 13.7 ppm; MS (ESI, pos) m/z: 247 [M+H]+, 269 [M+Na]+, 285 [M+K]+; HRMS–ESI: m/z [M+H]+ calcd for C14H19N2O2: 247.1447, found: 247.1457.
(2R)- and (2S)-2-Methyl-N-[(3S)-2-oxoazetidin-3-yl]nonanamide (11m)
The reaction was carried out following Method B, using salt 9 (0.045 mg, 0.31 mmol), 2-methylnonanoic acid (0.059 mg, 0.34 mmol), TBTU (0.109 mg, 0.34 mmol) and dry Et3N 0.090 mL, 0.68 mmol) in dry DCM (4.5 mL). After work-up, purification by silica gel flash chromatography using a Teledyne ISCO apparatus (Cy/EtOAc from 90:10 to 0:100) afforded compound 11m (0.024 g, 32%), as a mixture (1:1 ratio) of isomers, as a trasparent liquid: Rt = 2.28 min; 1H NMR (400 MHz, [D6]DMSO): δ 8.41 (t, 2H, J = 8.0 Hz), 7.94 (bs, 2H), 4.85-4.78 (m, 2H), 3.44–3.36 (m, 2H), 3.01 (ddd, 2H, J = 8.0, 5.2, 2.7 Hz), 2.30-2.15 (m, 2H), 1.50-1.44 (m, 2H), 1.23 (s, 22H), 0.99-0.97 (m, 6H), 0.85 (t, 6H, J = 6.8 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 175.8, 168.2, 56.9, 43.0, 42.9, 33.8, 31.2, 29.0, 28.9, 28.7, 26.9, 26.8, 22.1, 17.9, 17.8, 14.0 ppm; MS (ESI, pos) m/z: 241 [M+H]+, 258 [M+NH4]+, 279 [M+K]+; HRMS-ESI: m/z [M+H]+ calcd for C13H25N2O2: 241.1916, found: 241.1923.
2,2-Dimethyl-N-[(S)-2-oxoazetidin-3-yl]nonanamide (11n)
The reaction was carried out following Method B, using salt 9 (0.045 mg, 0.31 mmol), commercially available 2,2-dimethylnonanoic acid (0.064 mg, 0.34 mmol), TBTU (0.144 g, 0.45 mmol) and dry Et3N (0.090 mL, 0.68 mmol) in dry DCM/DMF (3:1, 4.4 mL). After work-up, purification by preparative HPLC afforded compound 11n (0.026 g, 33%), as a trasparent liquid: Rt = 2.47 min; (c = 0.10 in MeOH); 1H NMR (400 MHz, [D6]DMSO) δ 8.01 (d, 1H, J = 8.4 Hz), 7.88 (bs, 1H), 4.79 (ddd, 1H, J = 8.4, 5.4, 2.8 Hz), 3.34 (t, 1H, J = 5.4 Hz), 3.09 (dd, 1H, J = 5.4, 2.8 Hz), 1.45–1.36 (m, 2H), 1.29-1.07 (m, 10H), 1.04 (s, 6H), 0.84 (t, 3H, J = 6.9 Hz); 13C NMR (100 MHz, [D6]DMSO): δ = 177.0, 168.6, 57.1, 42.4, 41.5, 40.6, 31.3, 29.6, 28.7, 25.3, 25.2, 24.2, 22.1, 14.0 ppm; MS (ESI, pos) m/z: 255 [M+H]+, 272 [M+NH4]+, 277 [M+Na]+; HRMS-ESI: m/z [M+H]+ calcd for C14H27N2O2: 255.2073, found: 255.2084.
N–[(S)–2–Oxoazetidin–3–yl]–undecanamide (11p)
The reaction was carried out following Method B, using salt 9 (0.060 g, 0.41 mmol), commercially available undecanoic acid (0.084 g, 0.45 mmol), TBTU (0.144 g, 0.45 mmol) and dry Et3N (0.12 mL, 0.90 mmol) in dry DCM (6.0 mL). After work–up, trituration with EtOAc afforded compound 11p (0.065 g, 62%), as a white solid: Rt = 2.54 min; (c = 0.08 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.43 (d, 1H, J = 8.3 Hz), 7.94 (bs, 1H), 4.82 (ddd, 1H, J = 8.3, 5.4, 2.5 Hz), 3.38 (t, 1H, J = 5.4 Hz), 3.02 (dd, 1H, J = 5.4, 2.5 Hz), 2.08 (t, 2H, J = 7.4 Hz), 1.54–1.42 (m, 2H), 1.33–1.18 (m, 14H), 0.84 (t, 3H, J = 6.4 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ 172.3, 168.2, 55.9, 42.9, 35.2, 31.3, 29.0, 28.9, 28.8, 28.7, 28.6, 25.1, 22.1, 14.0 ppm; MS (ESI, pos) m/z: 255 [M+H]+, 293 [M+K]+; HRMS–ESI: m/z [M+H]+ calcd for C14H27N2O2: 255.2073, found: 255.2083.
6–Cyclohexyl–N–[(S)–2–oxoazetidin–3–yl]–hexanamide (11q)
The reaction was carried out following Method B, using salt 9 (0.050 g, 0.34 mmol), 7-cyclohexylheptanoic acid (15) (0.08 g, 0.38 mmol), TBTU (0.12 g, 0.38 mmol) and dry Et3N (0.100 mL, 0.75 mmol) in dry DCM (6.0 mL). After work–up, trituration with Et2O afforded compound 11q (0.033 g, 35%), as a white solid: Rt = 2.73 min; (c = 0.07 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ = 8.42 (d, 1H, J = 8.3 Hz), 7.94 (bs, 1H), 4.85–4.79 (m, 1H), 3.38 (t, 1H, J = 5.3 Hz), 3.02 (dd, 1H, J = 5.3, 2.7 Hz), 2.08 (t, 2H, J = 7.4 Hz), 1.70–1.55 (m, 5H), 1.53–1.42 (m, 2H), 1.28–1.07 (m, 12H), 0.89–0.77 (m, 2H) ppm; 13C NMR (100 MHz, [D6]DMSO) δ = 172.7, 168.6, 57.3, 43.3, 37.5, 37.4, 35.6, 33.4, 29.6, 29.1, 26.7, 26.6, 26.3, 25.5 ppm; MS (ESI, pos) m/z: 281 [M+H]+, 303 [M+Na]+, 319 [M+K]+; MS (ESI, neg) m/z: 279 [M–H]−; HRMS–ESI: m/z [M+H]+ calcd for C16H29N2O2: 281.2229, found: 281.2237.
N–(2–Oxocyclobutyl)nonanamide (21h)
Under nitrogen atmosphere, amide 25 (0.210 g, 1.34 mmol) was dissolved in dry THF (15.0 mL) and commercially available 2N HCl–Et2O solution (15.0 mL) was added at 0 °C. Then, commercially available bis(trimethylsilyloxy) cyclobutene (0.33 mL, 1.27 mmol) was added in one portion. The solution was refluxed for 3 h and then concentrated to dryness. Purification by typical silica flash chromatography (Cy/EtOAc, 60:40) and preparative HPLC afforded compound 21h (0.110 g, 37%), as a white solid: Rt = 2.48 min; 1H NMR (400 MHz, [D6]DMSO): δ 8.27 (d, 1H, J = 7.9 Hz), 4.77 (dt, 1H, J = 10.3, 7.9 Hz), 2.93–2.80 (m, 1H), 2.79–2.68 (m, 1H), 2.19 (qd, 1H, J = 10.3, 4.5 Hz), 2.06 (t, 2H, J = 7.3 Hz), 2.02–1.91 (m, 1H), 1.52–1.40 (m, 2H), 1.32–1.17 (m, 10H), 0.86 (t, 3H, J = 6.8 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 207.5, 172.3, 64.0, 41.6, 35.2, 31.7, 29.2, 29.1, 29.0, 25.5, 22.5, 18.8, 14.4 ppm. MS (ESI, pos) m/z: 226 [M+H]+, 248 [M+Na]+, 264 [M+K]+; HRMS–ESI: m/z [M+H]+ calcd for C13H24NO2: 226.1807, found: 226.1814.
N–(Azetidin–3–yl)nonanamide (22h)
To a cooled (0 °C), stirred solution of Boc-derivative 26 (0.060 g, 0.19 mmol) in DCM (3.0 mL), a 1:3 mixture of TFA/DCM (4.0 mL) was added dropwise The reaction mixture was stirred at 0 °C for 30 min, then at r.t. for additional 30 min. The solution was diluted with sat. Na2CO3 solution until neutralization. The organic phase was separated and the aqueous phase was extracted from DCM (15.0 mL) and EtOAc (15.0 mL). The combined organic phases were dried over Na2SO4, filtered and concentrated to dryness, affording compound 22h (0.095 g, quant.), as a colorless oil: Rt = 1.92 min; 1H NMR (400 MHz, [D6]DMSO): δ = 8.62 (bs, 1H), 8.50 (d, 1H, J = 6.5 Hz), 4.55 (sex, 1H, J = 15.1, 7.6 Hz), 4.11–4.04 (m, 1H), 3.92–3.85 (m, 1H), 2.08 (t, 2H, J = 7.4 Hz), 1.55–1.40 (m, 2H), 1.32–1.17 (m, 10H), 0.86 (t, 3H, J = 6.8 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 172.9, 52.6, 41.4, 35.6, 31.7, 29.2, 29.1, 29.0, 25.4, 22.5, 14.4 ppm; MS (ESI, pos) m/z: 213 [M+H]+, 235 [M+Na]+; HRMS–ESI: m/z [M+H]+ calcd for C12H25N2O: 213.1967, found: 213.1977.
[(S)–3–Hydroxy–2–(nonanoylamino)–3–oxo–propyl]ammonium chloride (23h)
β–lactam amide 11h (0.040 g, 0.18 mmol) was suspended in 2N HCl (3.0 mL) and it was vigorously stirred at room temperature for 30 min, then THF (2.0 mL) was added to the suspension to dissolve any insoluble residue. The resulting solution was stirred at room temperature for 16 h. Solvents were evaporated. Trituration with EtOAc afforded compound 23h (0.040 g, 79%), as a white solid: Rt = 1.68 min; (c = 0.11 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 13.00 (bs, 1H), 8.31 (d, 1H, J = 8.0 Hz), 8.17 (bs, 3H), 4.47 (dt, 1H, J = 8.0, 5.2 Hz), 3.19 (dd, 1H, J = 13.0, 5.2 Hz), 3.00 (dd, 1H, J = 13.0, 8.9 Hz), 2.15 (t, 2H, J = 7.6 Hz), 1.56–1.46 (m, 2H), 1.33–1.19 (m, 10H), 0.90–0.82 (m, 3H) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 173.4, 171.3, 50.4, 35.7, 31.7, 29.3, 29.1, 25.4, 22.6, 14.4 ppm; MS (ESI, pos) m/z: 245 [M+H]+, 267 [M+Na]+, 283 [M+K]+; HRMS–ESI: m/z [M+H]+ calcd for C12H25N2O3: 245.1865, found: 245.1873.
N-[(3S)-2-Oxopyrrolidin-3-yl]-nonanamide (24h)
The reaction was carried out following Method A, using commercially available (3S)-3-aminopyrrolidin-2-one (0.100 g, 1.0 mmol), commercially available nonanoyl chloride (0.24 mL, 1.10 mmol) and dry Et3N (0.15 mL, 1.10 mmol) in dry DCM (8 mL). After work-up, trituration with Et2O afforded compound 24h (0.20 g, 83%), as a white solid: Rt = 2.09 min; 1H NMR (400 MHz, [D6]DMSO): δ 7.99 (d, 1H, J = 8.3 Hz), 7.76 (bs, 1H), 4.27 (dt, 1H, J = 10.3, 8.3 Hz), 3.20-3.11 (m, 2H), 2.32-2.23 (m, 1H), 2.07 (t, 2H, J = 7.4 Hz), 1.81-1.69 (m, 1H), 1.53-1.43 (m, 2H), 1.31-1.20 (m, 10H), 0.85 (t, 3H, J = 6.6 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 174.5, 172.2, 49.3, 38.0, 35.2, 31.2, 28.7, 28.6, 28.5, 25.2, 22.1, 13.9 ppm; MS (ESI, pos) m/z: 241 [M+H]+; MS (ESI, neg) m/z: 239 [M–H]−; HRMS-ESI: m/z [M+H]+ calcd for C13H25N2O2: 241.1916, found: 241.192.
N-[(S)-1-methyl-2-oxo-azetidin-3-yl]nonanamide (27h)
Under nitrogen atmosphere, to a cooled (0° C) solution of amide 11h in dry THF (2.5 mL), a suspension of NaH (60% mineral oil, 0.015 g, 0.362 mmol) in dry THF (4.0 mL) was added dropwise. The mixture was warmed up to r.t., stirred for additional 20 min and cooled again to 0°C. MeI (0.022 mL, 0.362 mmol) was added dropwise and the resulting reaction mixture was maintained at 0°C for 3 h, warmed up to r.t. and stirred for additional 2 h and 30 min. The reaction mixture was diluted with DCM (25 mL), and water (2.0 mL). The aqueous layer was extracted with DCM (3 x10 mL) and the combined organic layers were dried over Na2SO4, filtered and concentrated to dryness. Purification by preparative HPLC afforded pure compound 27h (0.020 g, 25% yield), as a white solid: Rt = 2.23 min; (c = 0.07 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ = 8.42 (d, 1H, J = 8.1 Hz), 4.81 (ddd, 1H, J = 8.1, 5.2, 2.4 Hz), 3.46 (t, 1H, J = 5.2 Hz), 3.08 (dd, 1H, J = 5.2, 2.4 Hz), 2.73 (s, 3H), 2.07 (t, 2H, J = 7.4 Hz), 1.55–1.42 (m, 2H), 1.33–1.17 (m, 10H), 0.86 (t, 3H, J = 6.8 Hz); 13C NMR (100 MHz, [D6]DMSO: 172.2, 167.1, 56.0, 49.0, 35.1, 31.2, 28.7, 28.6, 28.5, 28.1, 25.1, 22.1, 13.9 ppm; MS (ESI, pos) m/z: 241 [M+H]+, 263 [M+Na]+, 279 [M+K]+; HRMS-ESI: m/z [M+H]+ calcd for C13H25N2O2: 241.1916, found: 241.1918
N–Methyl–N–[(S)–2–oxoazetidin–3–yl]–nonanamide (28h)
The reaction was carried out following Method A, using salt 31 (0.050 g, 0.31 mmol), commercially available nonanoyl chloride (0.076 mL, 0.34 mmol) and dry Et3N (0.091 mL, 0.66 mmol) in dry DCM (5.0 mL). After work–up, purification by preparative HPLC afforded compound 28h (0.027 g, 33%), as a 1:1 mixture of two rotamers, as an oil: Rt = 2.31 min; (c = 0.12 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 8.14 (bs, 1H), 8.07 (bs, 1H), 5.50–5.45 (m, 1H), 5.33–5.27 (m, 1H), 3.43 (t, 1H, J = 5.8 Hz), 3.35 (t, 1H, J = 5.8 Hz), 3.22 (dd, 1H, J = 5.8, 2.5 Hz), 3.17 (dd, 1H, J = 5.8, 2.5 Hz), 2.90 (s, 3H), 2.74 (s, 3H), 2.42–2.23 (m, 4H), 1.53–1.40 (m, 4H), 1.33–1.16 (m, 20H), 0.86 (t, 6H, J = 7.0 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 172.6, 172.2, 167.4, 166.8, 64.6, 62.0, 32.6, 32.4, 31.4, 31.2, 28.8, 28.7, 28.6, 28.0, 24.9, 24.4, 22.1, 14.0 ppm; MS (ESI, pos) m/z: 241 [M+H]+, 263 [M+Na]+, 279 [M+K]+; HRMS–ESI: m/z [M+H]+ calcd for C13H25N2O2: 241.1916, found: 241.1918.
(S)–3–(Nonylamino)azetidin–2–one (32)
Under nitrogen atmosphere, to a stirred suspension of salt 9 (0.050 g, 0.34 mmol) in dry dichloroetane (3.5 mL), dry Et3N (0.050 mL, 0.38 mmol) was added. The resulting suspension was stirred for 10 min, then a solution of aldehyde 35 (0.049 g, 0.34 mmol) in dry dichloroetane (0.5 mL) and Na(OAc)3BH (0.100 g, 0.48 mmol) were added to the reaction mixture. The solution was stirred for 90 min, then diluted with EtOAc (20.0 mL) and washed with sat. NaHCO3 solution (10.0 mL). The organic phase was dried over Na2SO4, filtered and concentrated to dryness. Purification by typical silica flash chromatography (DCM/MeOH, from 97:3 to 95:5) afforded compound 32 (0.015 g, 21%), as a white solid: Rt = 2.37 min; 1H NMR (400 MHz, [D6]DMSO): δ 7.72 (bs, 1H), 4.00 (bs, 1H), 3.24 (t, 1H, J = 5.2 Hz), 2.92 (dd, 1H, J = 5.6, 2.4 Hz), 2.63–2.52 (m, 2H), 1.42–1.16 (s, 14H), 0.86 (d, 3H, J = 7.0 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 168.5, 67.5, 46.5, 43.0, 31.8, 30.3, 29.5, 29.4, 29.1, 27.2, 22.6, 14.4 ppm; MS (ESI, pos) m/z: 213 [M+H]+, 235 [M+Na]+; HRMS–ESI: m/z [M+H]+ calcd for C12H25N2O: 213.1967, found: 213.1977.
1–Heptyl–3–[(S)–2–oxoazetidin–3–yl]–urea (33)
To a solution of salt 9 (0.047 g, 0.32 mmol) in dry pyridine (4.0 mL), DMAP (0.048 g, 0.39 mmol) and, subsequently, heptyl isocyanate (0.057 mL, 0.36 mmol) were added. The reaction mixture was stirred at r.t. for 16 h and concentrated to dryness. Trituration with DCM afforded compound 33 (0.040 g, 59%), as a white solid: Rt = 1.90 min; (c = 0.08 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 7.83 (bs, 1H), 6.50 (d, 1H, J = 8.4 Hz), 5.94 (t, 1H, J = 5.4 Hz), 4.80–4.63 (m, 1H), 3.34 (t, 1H, J = 5.4 Hz), 3.03–2.99 (m, 1H), 2.99–2.92 (m, 2H), 1.31–1.14 (m, 10H), 0.94–0.81 (m, 3H) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 169.4, 157.0, 57.9, 43.8, 31.3, 29.9, 28.4, 26.3, 22.0, 13.9 ppm; MS (ESI, pos) m/z: 228 [M+H]+, 250 [M+Na]+; HRMS–ESI: m/z [M+H]+ calcd for C11H22N3O2: 228.1712, found: 228.1718.
Heptyl N–[(S)–2–oxoazetidin–3–yl]–carbamate (34)
Under nitrogen atmosphere, to a suspension of salt 9 (0.040 g, 0.27 mmol) in dry DCM (4.0 mL), DIPEA (0.053 mL, 0.32 mmol) was added dropwise. Subsequently, the crude mixture (0.179 g) containing 37 (0.064 g, 0.27 mmol) in dry DCM (2.0 mL) was added. The reaction mixture was stirred at r.t. for 15 h, then concentrated to dryness. Purification by typical silica gel flash chromatography (Cy/EtOAc, from 100:0 to 40:60) afforded compound 34 (0.022 g, 36%), as a white solid: Rt = 2.18 min; (c = 0.05 in MeOH); 1H NMR (400 MHz, [D6]DMSO): δ 7.90 (bs, 1H), 7.78 (d, 1H, J = 8.6 Hz), 4.58–4.62 (m, 1H), 3.95 (t, 2H, J = 6.7 Hz), 3.37 (t, 1H, J = 5.4 Hz), 3.07 (dd, 1H, J = 5.4, 2.7 Hz), 1.59–1.48 (m, 2H), 1.35–1.21 (m, 8H), 0.86 (t, 3H, J = 6.9 Hz) ppm; 13C NMR (100 MHz, [D6]DMSO): δ = 168.2, 155.6, 64.1, 58.3, 42.6, 31.2, 28.6, 28.3, 25.3, 22.0, 13.9 ppm; MS (ESI, pos) m/z: 229 [M+H]+, 251 [M+Na]+, 267 [M+K]+; HRMS–ESI: m/z [M+Na]+ calcd for C11H20N2O3Na: 251.1372, found: 251.1374.
Pharmacology
Fluorogenic h-NAAA Assay
Hek293 cells stably transfected with the human NAAA coding sequence cloned from a human spleen cDNA library were used as enzyme source. Recombinant HEK-h-NAAA pellets were resuspended in homogenizing buffer, and sonicated. Samples were spinned at 800×g for 15 min at 4°C and the resultant supernatants were then ultracentrifuged at 12,000×g for 30 min at 4°C. The pellets were resuspended in PBS pH 7.4 on ice and subjected to two freeze/thaw cycles at −80°C. The suspension was finally centrifuged at 105,000×g for 1 h at 4°C. Protein concentration was measured and samples aliquoted and stored at −80°C until use. The assay was run in Optiplate 96-wells black plates (Perkin Elmer Inc., Boston, MA, USA), in a total reaction volume of 200 μL. NAAA protein preparation (20 μg) was pre-incubated for 10 min with various concentrations of test compounds or vehicle control (5 % DMSO) in 100 mM citrate/phosphate buffer (pH 4.5) containing 3 mM DTT, 0,1 % Triton X-100, 0,05 % BSA, 150 mM NaCl. N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide (PAMCA)[16] was used as a substrate (5 μM) and the reaction carried over for 30 min at 37°C. The samples were then read in a Perkin Elmer Envision plate reader (Perkin Elmer Inc., Boston, MA, USA) using an excitation wavelength of 360 nm and emission 460 nm. IC50 values were calculated by non-linear regression analysis of log[concentration]/inhibition curves using GraphPad Prism 5 (GraphPad Software Inc., CA–USA) applying a standard slope curve fitting.
In vitro chemical stability
Chemical stability of selected compounds was evaluated under physiological pH conditions (0.01 M phosphate-buffered saline, pH 7.4) and acidic pH conditions (0.01 M phosphate buffer, pH 5.0) for up to 24 h. Both buffers were added with 10% of CH3CN. Stock solutions of each compound (10 mM) were prepared freshly in DMSO. Each compound was incubated at a final concentration of 1 μM (1% DMSO) in both preheated buffers (37°C). The sample solutions were divided into aliquots in glass vials (preheated at 37°C) for each time point. The samples were maintained at 37°C in the UPLC-MS autosampler during the study (no shaking). A reference solution of each compound (final concentration: 1 μM at 1% DMSO) in preheated CH3CN (37°C) was prepared from the stock solutions (10 mM in DMSO) and maintained at 37°C in the UPLC-MS autosampler during the study (no shaking). The analyses were performed on a Waters ACQUITY UPLC/MS TQD system consisting of a TQD (triple quadrupole detector) mass spectrometer equipped with an electrospray ionization interface and a photodiode array detector. The 24 h time course analysis for 1, 11a and 11h was carried out by UPLC-UV on the same instrument described above. A calibration curve in the 0.2–50 μM concentration range was prepared for each parent compound by serial dilution in CH3CN (R2 values were > 0.999 for all compounds). The concentration of the rearranged and hydrolyzed products of each parent was then calculated on the corresponding calibration curve, assuming no changes in the molar absorptivity values (ε). To test this assumption, each parent was fully hydrolyzed with 1 M NaOH solution and the concentration of the corresponding product was calculated on the parent compound calibration curve. The measured concentration matched the expected 30 μM value. The analyses were run on an ACQUITY UPLC BEH C18 1.7 μm 2.1 × 50mm column with a VanGuard BEH C18 1.7μm pre-column at 40 °C. The mobile phase was 0.1% HCOOH in H2O (A) and 0.1% HCOOH in CH3CN (B) using the following gradient: 0–0.5 min.: 5% B, 0.5-2.5 min.: 5-100% B, 2.5-2.7 min.: 100% B, 2.7-2.8 min.: 100-5% B, 2.8-3.5: 5%B with flow rate at 0.5 mL/min.
In vitro mouse plasma stability
Compounds were diluted in mouse plasma added with 5% DMSO to help solubilization. Plasma was already pre-heated at 37° C (10 min). The final compound concentration was 2 μM. At time points (immediately after dilution, 5, 15, 30, 60, 120, min) a 40 μL aliquot of the incubation solution was diluted in 120 μL of cold CH3CN spiked with Warfarin 200 nM, as internal standard. After 30 sec of vortexing, the solution was centrifuged at 3500g for 15 min at 4°C and the surnatant transferred for LC-MS analysis on a Waters ACQUITY UPLC/MS TQD system consisting of a TQD (Triple Quadrupole Detector) Mass Spectrometer equipped with an Electrospray Ionization interface. Briefly: 3 μL of the surnatant were injected on a reversed phase column (BEH C18 2.1X50 mm) and separated with a linear acetonitrile gradient. Compounds were quantified on the basis of their MRM (Multiple Reaction Monitoring) peak areas. The response factors, calculated on the basis of the internal standard peak area, were then plotted over time. When possible, response vs time profiles were fitted with Prism (GraphPad Software, Inc., USA) to estimate compound half lives in plasma.
In vitro rat plasma stability
Compounds were added to blank rat plasma pre-incubated at 37°C. Final molecule concentration was 2 μM. Final DMSO concentration was 2.5%. The mixture was kept at 37°C under shaking. Aliquots (50 μL) were taken at various timepoints (0, 5, 15, 30, 60 and 120 minutes) and crashed with 150μL of acetonitrile spiked with 200 nM warfarin (internal standard). After vortexing and centrifugation, 3 μL of surnatant were analyzed by LC-MS/MS by multiple reaction monitoring (MRM). The corresponding time Vs response factor profiles were fitted with PRISM (Graphpad, CA, USA) to derive the experimental half-life of the compounds.
Supplementary Material
Acknowledgments
The authors thank Dr Giuliana Ottonello and Sine Mandrup Bertozzi for determination of compounds’ stability in buffer and in mouse and rat plasma, Dr Federica Vacondio for providing the experimental protocol for chemical stability studies, Luca Goldoni for NMR technical support, Silvia Venzano for compounds handling, Luisa Mengatto for compounds’ testing on h-NAAA, Dr Natalia Realini and Clara Albani for compounds’ testing on h-AC and h-FAAH, respectively.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemmedchem.org or from the author.
Contributor Information
Dr. Tiziano Bandiera, Email: tiziano.bandiera@iit.it.
Prof. Daniele Piomelli, Email: daniele.piomelli@iit.it.
References
- 1.a) Calignano A, La Rana G, Giuffrida A, Piomelli D. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]; b) LoVerme J, La Rana G, Russo R, Calignano A, Piomelli D. Life Sci. 2005;77:1685–1698. doi: 10.1016/j.lfs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Piomelli D, Giuffrida A, Calignano A, de Fonseca FR. Trends Pharmacol Sci. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- 3.a) Berdyshev E, Boichot E, Corbel M, Germain N, Lagente V. Life Sci. 1998;63:Pl125–Pl129. doi: 10.1016/s0024-3205(98)00324-5. [DOI] [PubMed] [Google Scholar]; b) D’Agostino G, La Rana G, Russo R, Sasso O, Iacono A, Esposito E, Raso GM, Cuzzocrea S, Lo Verme J, Piomelli D, Meli R, Calignano A. J Pharmacol Exp Ther. 2007;322:1137–1143. doi: 10.1124/jpet.107.123265. [DOI] [PubMed] [Google Scholar]
- 4.a) Mazzari S, Canella R, Petrelli L, Marcolongo G, Leon A. Eur J Pharmacol. 1996;300:227–236. doi: 10.1016/0014-2999(96)00015-5. [DOI] [PubMed] [Google Scholar]; b) Calignano A, La Rana G, Piomelli D. Eur J Pharmacol. 2001;419:191–198. doi: 10.1016/s0014-2999(01)00988-8. [DOI] [PubMed] [Google Scholar]; c) Darmani NA, Izzo AA, Degenhardt B, Valenti M, Scaglione G, Capasso R, Sorrentini I, Di Marzo V. Neuropharmacology. 2005;48:1154–1163. doi: 10.1016/j.neuropharm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 5.a) Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. Mol Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]; b) LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, Meli R, Hohmann A, Calignano A, Piomelli D. J Pharmacol Exp Ther. 2006;319:1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]; c) Khasabova IA, Xiong Y, Coicou LG, Piomelli D, Seybold V. J Neurosci. 2012;32:12735–12743. doi: 10.1523/JNEUROSCI.0130-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Petrosino S, Cristino L, Karsak M, Gaffal E, Ueda N, Tuting T, Bisogno T, De Filippis D, D’Amico A, Saturnino C, Orlando P, Zimmer A, Iuvone T, Di Marzo V. Allergy. 2010;65:698–711. doi: 10.1111/j.1398-9995.2009.02254.x. [DOI] [PubMed] [Google Scholar]; b) Kemeny L, Koreck A, Kis K, Kenderessy-Szabo A, Bodai L, Cimpean A, Paunescu V, Raica M, Ghyczy M. Skin Pharmacol Phys. 2007;20:155–161. doi: 10.1159/000098702. [DOI] [PubMed] [Google Scholar]; c) Hesselink JM, Hekker TA. J Pain Res. 2012;5:437–442. doi: 10.2147/JPR.S32143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piomelli D. Trends Endocrin Met. 2013;24:332–341. doi: 10.1016/j.tem.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung D, Saghatelian A, Simon GM, Cravatt BF. Biochemistry-Us. 2006;45:4720–4726. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Ueda N, Yamanaka K, Yamamoto S. J Biol Chem. 2001;276:35552–35557. doi: 10.1074/jbc.M106261200. [DOI] [PubMed] [Google Scholar]; b) Tsuboi K, Takezaki N, Ueda N. Chem Biodivers. 2007;4:1914–1925. doi: 10.1002/cbdv.200790159. [DOI] [PubMed] [Google Scholar]
- 10.Ueda N, Tsuboi K, Uyama T. Prog Lipid Res. 2010;49:299–315. doi: 10.1016/j.plipres.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Ueda N. J Biol Chem. 2005;280:11082–11092. doi: 10.1074/jbc.M413473200. [DOI] [PubMed] [Google Scholar]
- 12.a) Tsuboi K, Zhao LY, Okamoto Y, Araki N, Ueno M, Sakamoto H, Ueda N. Biochim Biophys Acta Mol Cell Biol Lipids. 2007;1771:623–632. doi: 10.1016/j.bbalip.2007.03.005. [DOI] [PubMed] [Google Scholar]; b) Tsuboi K. Inflamm Regener. 2007;27:18–27. [Google Scholar]
- 13.Zhao LY, Tsuboi K, Okamoto Y, Nagahata S, Ueda N. Biochim Biophys Acta Mol Cell Biol Lipids. 2007;1771:1397–1405. doi: 10.1016/j.bbalip.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Rossocha M, Schultz-Heienbrok R, von Moeller H, Coleman JP, Saenger W. Biochemistry-Us. 2005;44:5739–5748. doi: 10.1021/bi0473206. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Zhao LY, Uyama T, Tsuboi K, Tonai T, Ueda N. Biochim Biophys Acta Mol Cell Biol Lipids. 2008;1781:710–717. doi: 10.1016/j.bbalip.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 16.West JM, Zvonok N, Whitten KM, Wood JT, Makriyannis A. J Proteome Res. 2012;11:972–981. doi: 10.1021/pr200735a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Petrocellis L, Ligresti A, Moriello AS, Allara M, Bisogno T, Petrosino S, Stott CG, Di Marzo V. Brit J Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Vandevoorde S, Tsuboi K, Ueda N, Jonsson KO, Fowler CJ, Lambert DM. J Med Chem. 2003;46:4373–4376. doi: 10.1021/jm0340795. [DOI] [PubMed] [Google Scholar]; b) Tsuboi K, Hilligsmann C, Vandevoorde S, Lambert DM, Ueda N. Biochem J. 2004;379:99–106. doi: 10.1042/BJ20031695. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Saturnino C, Petrosino S, Ligresti A, Palladino C, De Martino G, Bisogno T, Di Marzo V. Bioorg Med Chem Lett. 2010;20:1210–1213. doi: 10.1016/j.bmcl.2009.11.134. [DOI] [PubMed] [Google Scholar]; d) Yamano Y, Tsuboi K, Hozaki Y, Takahashi K, Jin XH, Ueda N, Wada A. Bioorg Med Chem. 2012;20:3658–3665. doi: 10.1016/j.bmc.2012.03.065. [DOI] [PubMed] [Google Scholar]; e) Li Y, Yang L, Chen L, Zhu C, Huang R, Zheng X, Qiu Y, Fu J. Plos One. 2012;7:1–7. doi: 10.1371/journal.pone.0043023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solorzano C, Zhu CG, Battista N, Astarita G, Lodola A, Rivara S, Mor M, Russo R, Maccarrone M, Antonietti F, Duranti A, Tontini A, Cuzzocrea S, Tarzia G, Piomelli D. P Natl Acad Sci USA. 2009;106:20966–20971. doi: 10.1073/pnas.0907417106.Solorzano C, Antonietti F, Duranti A, Tontini A, Rivara S, Lodola A, Vacondio F, Tarzia G, Piomelli D, Mor M. J Med Chem. 2010;53:5770–5781. doi: 10.1021/jm100582w.Duranti A, Tontini A, Antonietti F, Vacondio F, Fioni A, Silva C, Lodola A, Rivara S, Solorzano C, Piomelli D, Tarzia G, Mor M. J Med Chem. 2012;55:4824–4836. doi: 10.1021/jm300349j.Ponzano S, Bertozzi F, Mengatto L, Dionisi M, Armirotti A, Romeo E, Berteotti A, Fiorelli C, Tarozzo G, Reggiani A, Duranti A, Tarzia G, Mor M, Cavalli A, Piomelli D, Bandiera T. J Med Chem. 2013;56:6917–6934. doi: 10.1021/jm400739u.Vitale R, Ottonello G, Petracca R, Bertozzi SM, Ponzano S, Armirotti A, Berteotti A, Dionisi M, Cavalli A, Piomelli D, Bandiera T, Bertozzi F. Chem Med Chem. 2014;9:323–336. doi: 10.1002/cmdc.201300416.f) Compounds 1 and 2 were tested using the reported assay conditions, giving IC50 = 1.29±0.215 μM and IC50 = 0.047±0.005 μM, respectively.
- 20.Sasso O, Moreno-Sanz G, Martucci C, Realini N, Dionisi M, Mengatto L, Duranti A, Tarozzo G, Tarzia G, Mor M, Bertorelli R, Reggiani A, Piomelli D. Pain. 2013;154:350–360. doi: 10.1016/j.pain.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.3-Aminoazetidin-2-one has been described in Matsuo T, Masuya YK, Noguchi N, Ochiai M. Chem Pharm Bull. 1983;31:1874–1884. doi: 10.1248/cpb.31.1874.
- 22.Hanessian S, Couture C, Wiss H. Can J Chem. 1985;63:3613–3617.Deprotection of benzyl N-(2-oxoazetidin-3-yl) carbamate was adapted from Felix AM, Lambros TJ, Tzougraki C, Meienhofer J. J Org Chem. 1978;33:4194–4196.. Other deprotection procedures on the same substrate were reported in Nakaguchi O, Hemmi K, Shiokawa Y, Hashimoto M, Kamiya T. Chem Pharm Bull. 1987;35:3464–3466. doi: 10.1248/cpb.35.3464.
- 23.All the intermediates were obtained in almost pure form after simple work-up and/or trituration with solvent.
- 24.The use of EtOH as protic solvent turned out to be critical for the reaction.
- 25.McGinley CM, Jacquot C, van der Donk WA. Bioorg Med Chem Lett. 2007;17:4049–4052. doi: 10.1016/j.bmcl.2007.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.5-Cyclohexylpentan-1-ol 23 was obtained starting from the corresponding commercially available carboxylic acid by standard reduction.
- 27.a) Ghosh AK, Duong TT, Mckee SP. Tetrahedron Lett. 1991;32:4251–4254. doi: 10.1016/S0040-4039(00)92141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shiina I, Suenaga Y, Nakano M, Mukaiyama T. Bull Chem Soc Jpn. 2000;73:2811–2818. [Google Scholar]
- 28.The in vivo h-NAAA inhibitory assay was performed at pH 5.0.
- 29.Realini N, Solorzano C, Pagliuca C, Pizzirani D, Armirotti A, Luciani R, Costi MP, Bandiera T, Piomelli D. Sci Rep-UK. 2013;3 doi: 10.1038/srep01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.