Abstract
Autophagy is a highly conserved degradative process through which cells overcome stressful conditions. Inasmuch as faulty autophagy has been associated with aging, neuronal degeneration disorders, diabetes, and fatty liver, autophagy is regarded as a potential therapeutic target. This review summarizes the present state of knowledge concerning the role of zinc in the regulation of autophagy, the role of autophagy in zinc metabolism, and the potential role of autophagy as a mediator of the protective effects of zinc. Data from in vitro studies consistently support the notion that zinc is critical for early and late autophagy. Studies have shown inhibition of early and late autophagy in cells cultured in medium treated with zinc chelators. Conversely, excess zinc added to the medium has shown to potentiate the stimulation of autophagy by tamoxifen, H2O2, ethanol and dopamine. The potential role of autophagy in zinc homeostasis has just begun to be investigated.Increasing evidence indicates that autophagy dysregulation causes significant changes in cellular zinc homeostasis. Autophagy may mediate the protective effect of zinc against lipid accumulation, apoptosis and inflammation by promoting degradation of lipid droplets, inflammasomes, p62/SQSTM1 and damaged mitochondria.Studies with humans and animal models are necessary to determine whether autophagy is influenced by zinc intake.
Keywords: autophagy, zinc, inflammation, lipophagy, alcohol, apoptosis
Introduction
Autophagy is an evolutionary conserved catabolic process used by eukaryotic cells for thedegradation of damaged or superfluous proteins and organelles (Chen and Klionsky 2011). There are three forms of autophagy: macroautophagy, microautophagy and chaperone-mediated autophagy (Chen and Klionsky 2011). Among these three types of autophagy, macroautophagy,hereafter referred as autophagy is the best characterized and is the focus of this review. Autophagy involves the sequestering of cellular constituents in double-membrane vesicles (autophagosomes) and subsequent delivery to lysosomes for degradation. Autophagy can be nonselective or selective. Nonselective autophagy is used for the turnover of bulk cytoplasm under starvation conditions (Kirkin et al. 2009). Selective autophagy targets damaged or superfluous mitochondria (mitophagy) (Narendra et al. 2008), peroxisomes (pexophagy) (Iwata et al. 2006), lipid droplets (lipophagy) (Singh et al. 2009) and microbes (xenophagy) (Baxt et al. 2013). Autophagy is activated in response to adverse environment conditions such as the deprivation of nutrients, hypoxia, pathogen infection, radiation and oxidative stress as a survival mechanism (Kroemer et al. 2010). This process plays a role in cellular homeostasis, development and longevity. Under many conditions, autophagy is considered as a physiologic cytoprotective or pro-survival mechanism; however, completely uncontrolled or excessive autophagy has been associated with cell death (Galluzzi et al. 2008). The characterization of the regulation of autophagy has become relevant because defective autophagy has been linked to aging (Pyo et al. 2013; Cuervo 2008), neurodegenerative disorders (Nilsson and Saido 2014; Orr and Oddo 2013), cancer (Lozy and Karantza 2012), fatty liver and diabetes (Czaja et al. 2013; Quan et al. 2013). In this review, we describe the potential role of zinc in the regulation of autophagy, the interrelationship between autophagy and zinc metabolisms as well as the potential role of autophagy as mediator of protective effects of zinc.
Autophagy process and regulation
In mammalian cells, autophagy is controlled by the orchestration of more than 30 autophagy related genes (Atg) (Feng et al. 2014; Glick et al. 2010). Autophagy begins with the formation of the “isolation membrane” also known as phagophore. Phagophore membranes seem to originate from endoplasmic reticulum (Hayashi-Nishino et al. 2009), plasma membrane (Ravikumar et al. 2010), trans-Golgi (Ge et al. 2013) and mitochondria (Hailey et al. 2010). However, since autophagosomal membranes have a relative lack of transmembrane proteins, phagophore membranes could also originate from de novo membrane formation from cytosolic lipids (Lamb et al. 2013; Fengsrud et al. 2000). Phagophores expand and sequester damaged mitochondria and organelles, lipid droplets and portions of the cytoplasm, eventually closing to become autophagosomes (Mizushima 2007). Subsequently, autophagosomes mature by fusing with late endosomes and lysosomes, thereby forming autolysosomes. In autolysosomes, the cargo is exposed to lysosomal enzymes to allow its breakdown and resulting nutrients are released into the cytosol (Mizushima 2007). Autophagic flux is used to denote the process of autophagosome synthesis, delivery of autophagic substrates to the lysosome, and degradation of autophagic substrates inside the lysosome (Klionsky et al. 2012). Phagophore formation is regulated by multiple signaling events (for a review see Glick et al. 2010 and Mizushima 2010). In mammalians cells, the formation of phagophore is initiated by the UNC51-like kinase 1/2 (ULK1/2) complex and the coiled-coil-myosin-like-BCL2-interacting protein/class III phosphatidylinositol-3-kinase (Beclin1-PI3K) complex (Jung et al. 2009; Kihara et al. 2001). The ULK1/2 complex comprises ULK1 or ULK2, Atg13, FIP200 and Atg101. In nutrient-replete conditions, mammalian target of rapamycin (mTORC1) prevents induction of autophagy by phosphorylating the ULK1/2 complex (Jung et al. 2009). However, upon mTORC1 inhibition by starvation, mTORC1 dissociates from ULK1/2 complex, leading to dephosphorylation and catalytic activation of ULK1(or ULK2), which in turn causes phosphorylation of Atg13 and FIP200 (Jung et al. 2009). The ULK1/2 complex controls the trafficking of Atg9, the only Atg protein with transmembrane domains (Kihara et al. 2001; Young et al. 2006; Zavodszky et al. 2013). Atg9 may deliver lipids to phagophore by cycling between organelles. The Beclin1-PIK3 core complex consists of Vps34, p150 and Beclin1 and generates phosphatidylinositol 3-phosphate (PI3P), which triggers the recruitment of FYVE domain containing protein 1(DFCP1) and WD-repeat protein interacting with phosphoinositides (WIPI) to promote double membrane vesicle nucleation at the site of phagophore assembly (Kihara 2001; Burd and Emr 1998; Jeffries et al. 2004; Axe et al. 2008). PI3P is essential for phagophore expansion and recruitment of other Atg proteins to phagophore (Kihara et al. 2001; Devereaux et al. 2013; Axe et al. 2008). Regulatory proteins interact with Vps34-Beclin1 complex to either promote or suppress autophagy. Atg14L, Ambra1, BIF-1 and UVRAG bind to Vps34-Beclin1 complex and activate autophagy, whereas Rubicon and Bcl2 binding to Beclin-1 inhibits autophagy (Fimia et al. 2007; Matsunaga et al. 2009; Liang et al. 2008; Zhong et al. 2009; Pattingre et al. 2005). During glucose starvation, 5′ adenosine monophosphate-activated protein kinase (AMPK) coordinates the activation of the Beclin1-PI3K complex through phosphorylation of Vps34 and Beclin1 (Kim et al. 2013). The ULK1/2 complex has been shown to activate the Beclin1-PI3K complex through phosphorylation of Ambra1, under amino acid starvation conditions (Di Bartolomeo et al. 2010). The expansion of phagophore is mediated by two ubiquitin-like systems acting at the Atg5-Atg12-Atg16 conjugation and the processing of microtubule associated protein 1 light chain 3 (LC3) (Romanov et al. 2012). Atg7, an E1-like enzyme, and Atg10, an E2 like enzyme, catalyze the conjugation of Atg12 and Atg5. Conjugated Atg5-Atg12 complexes with Atg16 (Atg16L in mammals), forming the Atg5-Atg12-Atg16 complex which associates with the extending phagophore (Mizushima et al. 1998; Kuma et al 2002; Mizushima et al. 2003). The Atg5-Atg12-Atg16 complex dissociates from membrane just after the completion of autophagosomes (Mizushima et al. 2003). Atg12-Atg5-Atg16 is required for the recruitment of LC3II at the phagophores (Suzuki et al. 2001). LC3 is synthetized as pro-LC3 which is cleaved by Atg4 into LC3I (Tanida et al. 2004). The carboxy-terminal of LC3I is conjugated to phosphatidylethanolamine to generate LC3II (Ichimura et al. 200). When autophagy is induced, most LC3I is converted to LC3II (Huang et al. 2000). LC3II is likely the marker most commonly used to assess autophagic flux (Klionsky et al. 2012). LC3II plays a role in the selection of cargo for degradation by interacting with adaptor molecules such as p62/SQSTM, also known as sequestosome-1 (Pankiv et al. 2008). p62/SQSTM1 is the best characterized adaptor molecule and associates with polyubiquitinated proteins and aggregates through its ubiquitin-binding domain (UBD) (Pankiv et al. 2008).
Autophagy is stimulated by a variety of stimuli, such as nutrient starvation, pathogen associated molecular patterns (PAMPs), danger associated molecular patterns (DAMPs), oxidative stress, endoplasmic reticulum stress (ER stress) and mitochondria damage (for a review see Kroemer et al. 2010).
Regulation of autophagy by zinc
It has been known for decades that zinc plays a key role in the regulation of apoptosis and cell cycle (Truong-Tran et al. 2001; Wong et al., 2007). Nevertheless, the role of zinc in autophagy has just begun to be investigated. Growing evidence is supporting the notion that zinc is a positive regulator of autophagy. In vitro studies have consistently shown that zinc is critical for basal and induced autophagy (Hung et al. 2013; Hwang et al. 2010; Lee and Koh, 2010; Liuzzi and Yoo, 2013). Excess zinc in medium (20 to 200 μM) has shown to enhance autophagy induced by tamoxifen in MCF-7 breast cancer cells (Hwang et al., 2010), H2O2 in astrocytes (Lee and Koh, 2010), ethanol in human hepatoma cells (Liuzzi and Yoo 2013) and Dopamine in PC12 cells and cultured neurons (Hung et al. 2013). On the other hand, zinc depletion caused by treatment with either the cell permeable zinc chelator TPEN (N,N,N’N’-tetrakis(-)[2-pyridylmethyl]-ethylenediamine) or Chelex-100 was shown to suppress basal and induced autophagy in MCF-7 cells (Hwang et al., 2010), astrocytes and human hepatoma cells (Lee and Koh, 2010; Liuzzi and Yoo, 2013). The role of zinc as positive regulator of autophagy is further substantiated by the finding that the zinc ionophore PCI-5002 radiosensitizes non-small cell lung cancer cells by potentiating autophagy (Kim et al. 2011).
The mechanisms by which zinc modulates autophagy are yet to be defined. Results from studies with extracellular-signal-regulated kinases (ERK1/2) inhibitors demonstrate that phosphorylation of ERK1/2 is necessary for the regulation of basal and induced autophagy by zinc (Hwang et al. 2010; Liuzzi and Yoo 2013). ERK1/2 has been shown to activate autophagythrough at least two pathways. ERK1/2 can activate the Beclin1-PI3K complex by phosphorylating the negative regulator of Beclin1, Bcl-2 (Botti et al. 2006). In addition ERK1/2 activation was shown to activate autophagy by promoting disassembly of mTORC1 complex through a non-classical pathway (Wang et al. 2009). Reportedly, Metallothionein, a small molecular weight protein that binds zinc, is the potential source of zinc for autophagy activation during oxidative stress. This assumption comes from the fact that astrocytes from metallothionein 3 (MT3) knockout mice exhibited lower induction of autophagy by H2O2 (Lee and Koh, 2010). Although this has not been investigated yet, zinc can potentially regulate autophagy by modulating the expression of genes involved in autophagy. Zinc is known to induce the expression of genes through activation of the metal-responsive transcription factor (MTF1) (Lichtlen and Schaffner 2001). Thus, genes involved in autophagy containing binding sites for MTF1 can be potentially induced by zinc. According to bioinformatics analysis (www.genomatix.de; Cartharius et al. 2005), the promoters of ATG7 and DFCP1 contain four binding sites for MTF1 within 2.0 kb upstream of the transcription start site. This suggests that the expression these two genes is likely regulated by zinc and MTF1 activation. However, experimental tests are needed to confirm this prediction.
Zinc may also regulate autophagy through regulation of microRNA expression (Ryu et al. 2011) and modulation of methylation of genes (Sharif et al. 2012).
Aside from regulating the initiation of autophagy (early autophagy), zinc likely plays animportant role in the degradation of the cargo in autolysosomes (late autophagy). There is some evidence suggesting that zinc is needed for proper lysosomal function. Julien et al. (2011) reported that zinc deficiency caused accumulation of lipofuscin, an indicator of incomplete lysosomal digestion, in retinal epithelium of rats. Additionally, astrocytes from mice lacking metallothionein 3 (MT3) exhibited reduced acid phosphatase and neuraminidase activities, decreased expression of cathepsin D and L as well as increased lysosomal accumulation of cholesterol and lipofuscin (Lee and Koh 2010). Low cellular zinc may also affect lysosome function by causing disruption of the integrity of this organelle. Lysosome integrity was found to be disrupted in macrophages treated with TPEN (Summersgill et al. 2014). Lastly, treatment with 4 μM TPEN for 72h caused impaired degradation of LC3II and p62/SQSTM in human hepatoma cells (Liuzzi and Yoo 2013), a clear indication that zinc depletion blocks the degradation of cargo in autolysosomes.
Nevertheless, it is important to mention that there are no studies evaluating whether zinc intake affects autophagy in humans or animal models.
Regulation of zinc homeostasis by autophagy
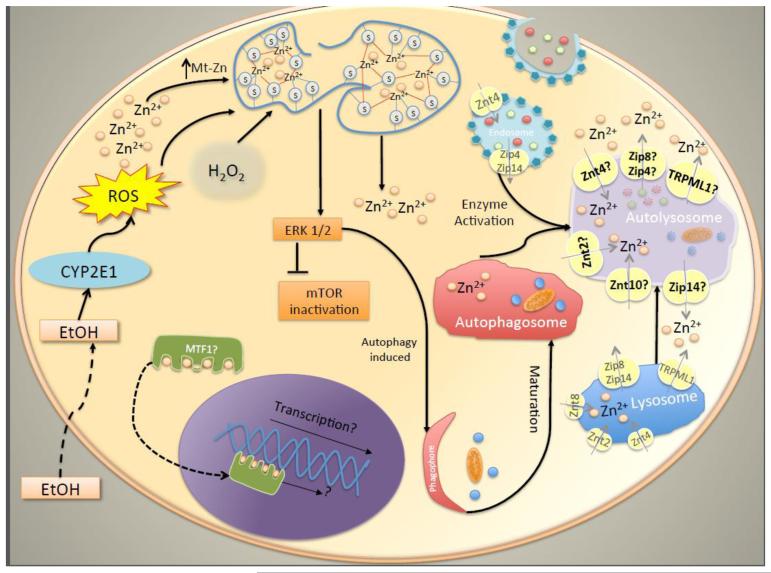
Recent studies have shown that autophagy dysregulation causes significant changes in cellular zinc homeostasis. Ni et al. (2011) examined the effect of the autophagy inhibitor 3-methyladenine (3-MA) on the regulation of the zinc transporters ZnT1, ZnT2 and ZnT3 in rat hippocampus following recurrent neonatal seizures and found that 3-MA, an inhibitor of Vps34, attenuated seizure-induced ZnT1 and ZnT2 expression. Of particular interest is the zinc transporter ZnT10, this zinc transporter was found to be downregulated by 3-MA in human hepatoma cells (Liuzzi and Yoo 2013). ZnT10 has been located to the Golgi apparatus (Bosomworth et al. 2012), a potential source of membrane for autophagosomes. However, another group reported that ZnT10 localizes in endosomes of smooth muscle cells (Patrushev et al 2012). Notably, mutations in ZnT10 gene have been associated with liver cirrhosis, dystonia, polycythemia, and hypermanganesemia (Tuschl et al. 2012). Lastly, the expression of ZnT10 has been found to be downregulated in brain of subjects with Alzheimer’s disease (Bosomworth et al. 2013), a condition that has been shown to be associated with defective autophagy (Orr and Oddo 2013; Nilsson and Saido 2014). Autophagy levels in cells have been shown to correlate with labile zinc levels in cells. Hwang et al. (2010) reported that the activation of autophagy by tamoxifen induced accumulation of labile zinc in autophagosomes and lysosomes in MCF-7 cells. In addition, Lee et al. (2010) found that the induction of autophagy by H2O2 caused a similar redistribution of labile zinc in astrocytes. Interestingly, inhibition of autophagy with 3-MA has been shown to reduce labile zinc in astrocytes and human hepatoma cells (Hung et al., 2013; Liuzzi and Yoo 2013). It is not clear how zinc is transported into the autophagosomes or what is the role of this metal in these compartments. Autophagosomes are believed to be virtually devoid of transmembrane proteins (Fengsrud et al 2000); thus it is possible that zinc inside these structures originates from the sequestered cell components. However, the presence of zinc transporters in autophagosomes membrane cannot be ruled out. Since endosomes and lysosomes fuse with autophagosomes, it is conceivable that the zinc transporters present in endosomes and lysosomes such as Zip4, Zip14, Zip8, ZnT10 and ZnT4 and the TRPML1 channel (Kambe 2011; Kukic et al. 2013) may also be present in autolysosomes (Fig.1). Zip4, Zip14, Zip8 and TRPML1 would promote zinc efflux from the autolysosomes, whereas ZnT10 and ZnT4 would facilitate zinc entry into autolysosomes.
Figure 1. Schematic diagram of mechanisms of regulation of autophagy by zinc and potential localization of zinc transporters in autolysosomes.
H2O2 treatment or reactive oxidative species (ROS) released from ethanol metabolization by CYP2E1 causes release of zinc from metallothionein which activates phosphorylation of ERK1/2. High zinc levels in cells can induce the expression of genes involved in autophagy by activation of MTF1. Zinc transporters present in endosomes and lysosomes are likely present in autolysosomes membrane.
Autophagy likely serves as a mechanism by which cells can recycle zinc from damaged or superfluous organelles and cargo degraded in autolysosomes. Nevertheless, since autophagy seems to be downregulated during zinc depletion, the recycling of zinc by autophagy may not be a relevant mechanism of adaptation to low zinc conditions.
Autophagy as a mediator of protective effects of zinc
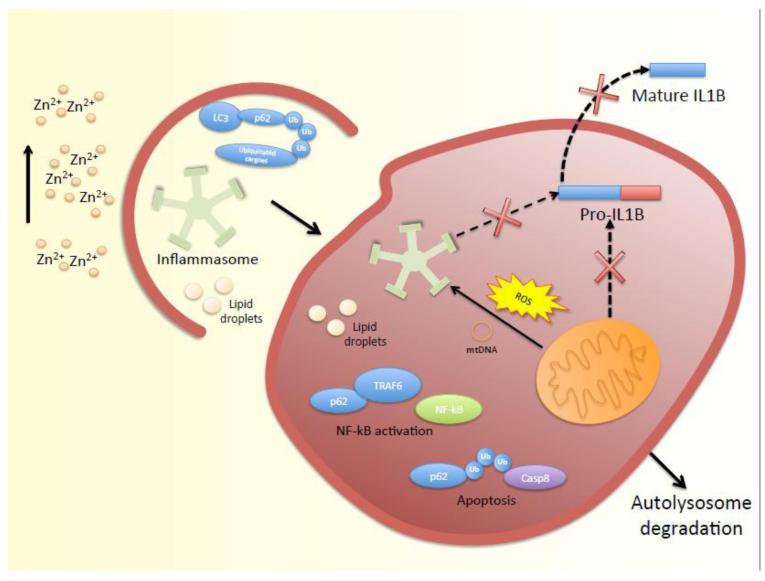
Recent studies have revealed new functions of autophagy such as regulation of lipid metabolism and suppression of inflammation and apoptosis. Until recently, mobilization of lipids was attributed exclusively to cytosolic lipases; however, reports now demonstrate a role for autophagosomes in the delivery of lipids to lysosomes for degradation, which is called lipophagy (Singh et al. 2009). Consistent with this idea, induction of autophagy with rapamycin was shown to prevent hepatic steatosis produced by binge ethanol in mice (Ding et al. 2010). Genetic and functional studies indicate that autophagy has an anti-inflammatory function (Deretic et al. 2013; Rathinam et al. 2012; Wellcome Trust Case Control Consortium 2007; Rioux et al. 2007; Hampe et al. 2007). It has been proposed that autophagy suppresses inflammatory response by promoting degradation of inflammasomes, inflammasomes agonists and p62/SQSTM1 (Manley et al. 2013; Rathinam et al. 2012; Harris et al. 2011). Autophagosomes can sequester and degrade inflammasome components, including NOD-like receptor 3 (NLRP3), apoptosis-associated speck-like protein containing a CARD (ASC), and caspase-1. Inflammasomes are large caspase-1 multiprotein complexes that promote the maturation of pro-inflammatory cytokines pro-IL-1β and pro-IL-18 (Stutz et al. 2009). p62/SQSTM1 is a substrate of autophagy that is known to promote NF-kB activation through its interaction with TRAF6 (Manley et al. 2013; Dickson et al 2004). Thus, degradation of p62/SQSTM1 by autophagy prevents NF-κB activation and hence attenuates inflammation. In addition, autophagosomes can degrade inflammasome agonist sources such as depolarized mitochondria. Of note, the link between autophagy and inflammation is supported by genome wide association studies (GWAS) that have reported an association between a polymorphism in ATG16L gene and Crohn’s disease, a progressive inflammatory bowel disease in humans (Wellcome Trust Case Control Consortium 2007; Rioux et al. 2007; Hampe et al. 2007).
Inasmuch as p62/SQSTM1 promotes the aggregation of ubiquitinated caspase-8 which leads to full activation of this enzyme and apoptosis, p62/SQSTM1 degradation by autophagy can also alleviate apoptosis (Zhang et al 2013; Manley et al. 2013). Zinc is known to confer protection against lipid accumulation, inflammation and apoptosis (Kang et al. 2009; Prasad 2009; Prasad et al. 2011; Truong-Tran et al. 2001). Given the potential role of zinc in autophagy, the protective effects of zinc could be mediated through autophagy. The potential mechanisms by which autophagy mediates the protective effects of zinc against lipid droplets accumulation, inflammation and apoptosis are delineated below (Fig.2). Consistent with this idea, a recent study demonstrated that the induction of autophagy by zinc and dopamine prevented apoptosis and cell death in cultured neurons (Hung et al. 2013). Moreover, Summersgill et al. (2014) showed that zinc depletion induced the activity of Caspase-1 activity in cultured mouse macrophages; however, they did not measured autophagic flux in these cells. It is conceivable that zinc alleviates inflammatory response initiated by lipopolysaccharide (LPS) by promoting autophagy. On the other hand, the inhibition of autophagy by zinc depletion may result in enhance LPS induced inflammation. This is consistent with previous reports that have shown that inflammatory response is enhanced in zinc deficient animals treated with LPS (Cui et al. 1998; Miyazaki et al. 2012). Interestingly, cellular zinc depletion has been shown to enhance NF-κB DNA-binding activity in human hepatoma and endothelial cells (Otsu et al. 2004; Hennig et al. 1999). The hyperactivation of NF-κB during zinc deficiency could be the result of impaired autophagic degradation of p62/SQSTM1.
Figure 2. Potential mechanisms of regulation of lipid metabolism, inflammation andapoptosis by zinc through modulation of autophagy.
Zinc prevents steatosis and apoptosis by promoting removal of lipid droplets, damaged mitochondria (Mitophagy) and degradation of p62/SQSTM1. Removal of p62/SQSTM1 prevents activation of Caspase 8 and NF-kB. IL-1β maturation is prevented by zinc through induction of autophagy and subsequent degradation of inflammasomes.
It should be noted, however, that inhibitory action of zinc on inflammation could also bemediated by autophagy-independent mechanisms. It has been proposed that zinc suppresses activation of NF-kB through induction of the zinc finger protein A20, a negative regulator of NF-kB (Prasad et al. 2011). Moreover, zinc antioxidant activity is believed to block inflammatory response (Prasad 2009).
Conclusions
Current evidence suggests that zinc is critical for basal and induced autophagy. Excess zinc appears to potentiate autophagy induced by tamoxifen, alcohol, H2O2 and Dopamine. On the other hand, zinc depletion seems to affect both early and late autophagy. However, in vivo studies supporting this hypothesis are still lacking. Studies with animals or whole organisms will help determine whether zinc intake affects autophagy. Since relatively high doses of zinc or severe zinc depletion models were utilized in the studies above mentioned, it is not clear whether classical low levels of zinc supplementation or marginal zinc deficiency would affect autophagy rate. The characterization of the role of zinc transporters in autophagy will help develop new therapeutic approaches to alleviate conditions associated with impaired autophagy. In addition, the study of the role of autophagy in zinc homeostasis will expand our knowledge on the molecular mechanisms of zinc homeostasis. Autophagy could be a mediator of the protective role of zinc against lipid accumulation, apoptosis and inflammation. The potential synergistic effect of ethanol and zinc on the induction of autophagy warrants further research. Moderate ethanol intake in combination with zinc supplementation could be used to alleviate chronic inflammation. Chronic inflammation has been implicated in diseases such as diabetes, cancer and vascular disease (Esser et al. 2014; Landskron et al. 2014; Virdis et al. 2014). Nevertheless, acute inflammation is a protective mechanism that helps eliminate invading organisms and promotes the healing process (Serhan 2010). Therefore, the therapeutic stimulation of autophagy to block acute inflammation may not be advisable. Lastly, it should be kept in mind that while moderate stimulation of autophagy confers cytoprotection, excessive stimulation of autophagy causes cell death (Galluzzi et al. 2008).
Acknowledgements
Liang Guo is supported by NIH HL58541 and Merck MISP36106/IIS50298. Juan Liuzzi is supported by NIH 1R03AA022451-01.
References Cited
- Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;82(4):685–701. doi: 10.1083/jcb.200803137. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxt LA, Garza-Mayers AC, Goldberg MB. Bacterial subversion of host innate immune pathways. Science. 2013;340(6133):697–701. doi: 10.1126/science.1235771. 697-701. doi: 10.1126/science.1235771. [DOI] [PubMed] [Google Scholar]
- Bosomworth HJ, Thornton JK, Coneyworth LJ, Ford D, Valentine RA. Efflux function, tissue-specific expression and intracellular trafficking of the Zn transporter ZnT10 indicate roles in adult Zn homeostasis. Metallomics. 2012;4(8):771–9. doi: 10.1039/c2mt20088k. doi: 10.1039/c2mt20088k. [DOI] [PubMed] [Google Scholar]
- Bosomworth HJ1, Adlard PA, Ford D, Valentine RA. Altered expression of ZnT10 in Alzheimer’s disease brain. PLoS One. 2013;8(5):e65475. doi: 10.1371/journal.pone.0065475. doi: 10.1371/journal.pone.0065475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botti J, Djavaheri-Mergny M, Pilatte Y, Codogno P. Autophagy signaling and the cogwheels of cancer. Autophagy. 2006;2(2):67–73. doi: 10.4161/auto.2.2.2458. [DOI] [PubMed] [Google Scholar]
- Burd CG, Emr SD. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol Cell. 1998;2(1):2157–162. doi: 10.1016/s1097-2765(00)80125-2. [DOI] [PubMed] [Google Scholar]
- Cartharius K1, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21(13):2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- Chen Y, Klionsky DJ. The regulation of autophagy-unanswered questions. J Cell Sci. 2011;124(Pt 2):161–70. doi: 10.1242/jcs.064576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM. Autophagy and aging keeping that old broom working. Trends Genet. 2008 Dec;4(12):604–612. doi: 10.1016/j.tig.2008.10.002. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Takagi Y, Wasa M, Iiboshi Y, Inoue M, Khan J, Sando K, Nezu R, Okada A. Zinc deficiency enhances interleukin-1alpha-induced metallothionein-1 expression in rats. J Nutr. 1998;128(7):1092–8. doi: 10.1093/jn/128.7.1092. [DOI] [PubMed] [Google Scholar]
- Czaja MJ, Ding WX, Donohue TM, Jr, Friedman SL, Kim JS, Komatsu M, Lemasters JJ, Lemoine A, Lin JD, Ou JH, Perlmutter DH, Randall G, Ray RB, Tsung A, Yin XM. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9(8):1131–58. doi: 10.4161/auto.25063. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13(10):722–37. doi: 10.1038/nri3532. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereaux K, Dall’Armi C, Alcazar-Roman A, Ogasawara Y, Zhou X, Wang F, Yamamoto A, De Camilli P, Di Paolo G. Regulation of mammalian autophagy by class II and III PI 3-kinases through PI3P synthesis. PLoS One. 2013;8(10):e76405. doi: 10.1371/journal.pone.0076405. doi: 10.1371/journal.pone.0076405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol. 191(1):155–68. doi: 10.1083/jcb.201002100. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson KM, Bhakar AL, Barker PA. TRAF6-dependent NF-kB transcriptional activity during mouse development. Dev Dyn. 2004;231(1):122–7. doi: 10.1002/dvdy.20110. [DOI] [PubMed] [Google Scholar]
- Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, Lu B, Stolz DB, Clemens DL, Yin XM. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139(5):1740–52. doi: 10.1053/j.gastro.2010.07.041. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;(14):S0168–8227. doi: 10.1016/j.diabres.2014.04.006. 00187-9. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24:24–4. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fengsrud M, Erichsen ES, Berg TO, Raiborg C, Seglen PO. Ultrastructural characterization of the delimiting membranes of isolated autophagosomes and amphisomes by freeze-fracture electron microscopy. Eur J Cell Biol. 2000;79(12):871–82. doi: 10.1078/0171-9335-00125. [DOI] [PubMed] [Google Scholar]
- Fimia GM, Stoykova A, Romagnoli A, Giunta L, Di Bartolomeo S, Nardacci R, Corazzari M, Fuoco C, Ucar A, Schwartz P, et al. Ambra1 regulates autophagy and development of the nervous system. Nature. 2007;447(7148):1121–1125. doi: 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Morselli E, Vicencio JM, Kepp O, Joza N, Tajeddine N, Kroemer G. Life, death and burial: multifaceted impact of autophagy. Biochem Soc Trans. 2008;36(Pt 5):786–90. doi: 10.1042/BST0360786. doi: 10.1042/BST0360786. [DOI] [PubMed] [Google Scholar]
- Ge L, Melville D, Zhang M, Schekman R. The ER–Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis. Elife. 2013;2:e00947. doi: 10.7554/eLife.00947. doi: 10.7554/eLife. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms (2010) J Pathol. 221(1):3–12. doi: 10.1002/path.2697. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailey DW1, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141(4):656–667. doi: 10.1016/j.cell.2010.04.009. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Gen. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- Harris J, Hartman M, Roche C, Zeng SG, O’Shea A, Sharp FA, Lambe EM, Creagh EM, Golenbock DT, Tschopp J, Kornfeld H, Fitzgerald KA, Lavelle EC. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J Biol Chem. 2011;286(11):9587–97. doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Nishino M, et al. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nature Cell Biol. 2009;11(12):1433–1437. doi: 10.1038/ncb1991. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Toborek M, McClain CJ. Antioxidant-like properties of zinc in activated endothelial cells. J Am Coll Nutr. 1999;18(2):152–8. doi: 10.1080/07315724.1999.10718843. [DOI] [PubMed] [Google Scholar]
- Huang W-P, Scott SV, Kim J, Klionsky DJ. The itinerary of a vesicle component, Aut7p/Cvt5p, terminates in the yeast vacuole via the autophagy/Cvt pathways. J Biol Chem. 2000;275(8):5845–585. doi: 10.1074/jbc.275.8.5845. [DOI] [PubMed] [Google Scholar]
- Hung HH, Huang WP, Pan CY. Dopamine- and zinc-induced autophagosome formation facilitates PC12 cell survival. Cell Biol Toxicol. 2013;29(6):415–29. doi: 10.1007/s10565-013-9261-2. [DOI] [PubMed] [Google Scholar]
- Hwang JJ, Kim HN, Kim J, Cho DH, Kim MJ, Kim YS, Kim Y, Park SJ, Koh JY. Zinc(II) ion mediates tamoxifen-induced autophagy and cell death in MCF-7 breast cancer cell line. Biometals. 2010;23(6):997–1013. doi: 10.1007/s10534-010-9346-9. doi: 10.1007/s10534-010-9346-9. [DOI] [PubMed] [Google Scholar]
- Ichimura Y, Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408(6811):488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- Iwata J, et al. Excess peroxisomes are degraded by autophagic machinery in mammals. J. Biol. Chem. 2006;281(7):4035–4041. doi: 10.1074/jbc.M512283200. [DOI] [PubMed] [Google Scholar]
- Jeffries TR1, Dove SK, Michell RH, Parker PJ. PtdIns-specific MPR pathway association of a novel WD40 repeat protein, WIPI49. Mol. Biol. Cell. 2004;15(6):2652–2663. doi: 10.1091/mbc.E03-10-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien S, Biesemeier A, Kokkinou D, Eibl O, Schraermeyer U. Zinc deficiency leads to lipofuscin accumulation in the retinal pigment epithelium of pigmented rats. PLoS One. 2011;6(12):e29245. doi: 10.1371/journal.pone.0029245. doi: 10.1371/journal.pone.0029245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULKAtg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20(7):1992–2003. doi: 10.1091/mbc.E08-12-1249. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambe T. An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway. Biosci Biotechnol Biochem. 2011;75(6):1036–43. doi: 10.1271/bbb.110056. [DOI] [PubMed] [Google Scholar]
- Kang X, Zhong W, Liu J, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology. 2009;50(4):1241–50. doi: 10.1002/hep.23090. doi: 10.1002/hep.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A, Yukiko Kabeya, Yoshinori Ohsumi, Tamotsu Yoshimori. Beclin–phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2(4):330–335. doi: 10.1093/embo-reports/kve061. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Speirs CK, Jung DK, Lu B. The zinc ionophore PCI-5002 radiosensitizes non-small cell lung cancer cells by enhancing autophagic cell death. J Thorac Oncol. 2011;6(9):1542–52. doi: 10.1097/JTO.0b013e3182208fac. doi: 10.1097/JTO.0b013e3182208fac. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, et al. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152(1-2):290–303. doi: 10.1016/j.cell.2012.12.016. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34(3):259–69. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdalla F, Abeliovich P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2012;8(4):445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–93. doi: 10.1016/j.molcel.2010.09.023. doi: 10.1016/j.molcel.2010.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukic I, Lee JK, Coblentz J, Kelleher SL, Kiselyov K. Zinc-dependent lysosomal enlargement in TRPML1-deficient cells involves MTF-1 transcription factor and ZnT4 (Slc30a4) transporter. Biochem J. 2013;451(2):155–63. doi: 10.1042/BJ20121506. doi: 10.1042/BJ20121506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Mizushima N, Ishihara N, Ohsumi Y. Formation of the ~350-kDa Apg12-Apg5·Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J Biol Chem. 2002;277(21):18619–18625. doi: 10.1074/jbc.M111889200. [DOI] [PubMed] [Google Scholar]
- Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14(12):759–74. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J Immunol Res. 20142014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Koh JY. Roles of zinc and metallothionein-3 in oxidative stress-induced lysosomal dysfunction, cell death, and autophagy in neurons and astrocytes. Mol Brain. 2010;3(1):30. doi: 10.1186/1756-6606-3-30. doi: 10.1186/1756-6606-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Lee JS, Inn KS, Gack MU, Li Q, Roberts EA, Vergne I, Deretic V, Feng P, Akazawa C, et al. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol. 2008;10(7):776–787. doi: 10.1038/ncb1740. doi: 10.1038/ncb1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtlen P, Schaffner W. The “metal transcription factor” MTF-1: biological facts and medical implications. Swiss Med Wkly. 2001;131(45-46):647–52. doi: 10.4414/smw.2001.09672. [DOI] [PubMed] [Google Scholar]
- Liuzzi JP, Yoo C. Role of zinc in the regulation of autophagy during ethanol exposure in human hepatoma cells. Biological Trace Element Research. 2013;156(1-3):350–6. doi: 10.1007/s12011-013-9816-3. doi: 10.1007/s12011-013-9816-3. [DOI] [PubMed] [Google Scholar]
- Lozy F, Karantza V. Autophagy and cancer cell metabolism. Semin Cell Dev Biol. 2012;23(4):395–401. doi: 10.1016/j.semcdb.2012.01.005. doi: 10.1016/j.semcdb.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley S, Williams JA, Ding WX. Role of p62/SQSTM1 in liver physiology and pathogenesis. Exp Biol Med (Maywood) 2013;238(5):525–38. doi: 10.1177/1535370213489446. doi: 10.1177/1535370213489446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, Maejima I, Shirahama-Noda K, Ichimura T, Isobe T, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11(4):385–396. doi: 10.1038/ncb1846. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- Miyazaki T1, Takenaka T, Inoue T, Sato M, Miyajima Y, Nodera M, Hanyu M, Ohno Y, Shibazaki S, Suzuki H. Lipopolysaccharide-induced overproduction of nitric oxide and overexpression of iNOS and interleukin-1β proteins in zinc-deficient rats. Biol Trace Elem Res. 2012;145(3):375–81. doi: 10.1007/s12011-011-9197-4. doi: 10.1007/s12011-0. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395(6700):395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, Takao T, Natsume T, Ohsumi Y, Yoshimori T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci. 2003;116(Pt 9):1679–88. doi: 10.1242/jcs.00381. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22(2):132–9. doi: 10.1016/j.ceb.2009.12.004. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen D’F, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183(5):795–803. doi: 10.1083/jcb.200809125. Nature Cell Biol. 12 (8): 747-757. doi: 10.1038/ncb2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H, Feng X, Xiao ZJ, Tao LY, Jin MF. Dynamic pattern of gene expression of ZnT-4, caspase-3, LC3, and PRG-3 in rat cerebral cortex following flurothyl-induced recurrent neonatal seizures. Biol Trace Elem Res. 2011;143(3):1607–15. doi: 10.1007/s12011-011-8982-4. doi: 10.1007/s12011-011-8982-4. [DOI] [PubMed] [Google Scholar]
- Nilsson P, Saido TC. Dual roles for autophagy: Degradation and secretion of Alzheimer’s disease Aβ peptide. Bioessays. 2014;36(6):570–8. doi: 10.1002/bies.201400002. doi: 10.1002/bies.201400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr ME, Oddo S. Autophagic/lysosomal dysfunction in Alzheimer’s disease. Alzheimers Res Ther. 2013;5(5):53. doi: 10.1186/alzrt217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu K, Ikeda Y, Fujii J. Accumulation of manganese superoxide dismutase under metal-depleted conditions: proposed role for zinc ions in cellular redox balance. Biochem J. 2004;377(Pt 1):241–8. doi: 10.1042/BJ20030935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282(33):24121–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Patrushev N1, Seidel-Rogol B, Salazar G. Angiotensin II requires zinc and downregulation of the zinc transporters ZnT3 and ZnT10 to induce senescence of vascular smooth muscle cells. PLoS One. 2012;7(3):e33211. doi: 10.1371/journal.pone.0033211. doi: 10.1371/journal.pone.0033211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Prasad AS. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care. 2009;12(6):646–52. doi: 10.1097/MCO.0b013e3283312956. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
- Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB. Nutrition. 2011;27(7-8):816–23. doi: 10.1016/j.nut.2010.08.010. doi: 10.1016/j.nut.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Pyo JO, Yoo SM, Jung YK. The Interplay between Autophagy and Aging. Diabetes Metab J. 2013;37(5):333–339. doi: 10.4093/dmj.2013.37.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan W, Jung HS, Lee MS. Role of autophagy in the progression from obesity to diabetes and in the control of energy balance. Arch Pharm Res. 2013;36(2):223–9. doi: 10.1007/s12272-013-0024-7. doi: 10.1007/s12272-013-0024-7. [DOI] [PubMed] [Google Scholar]
- Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nature Immuno. 2012;l13(4):333–332. doi: 10.1038/ni.2237. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol. 2010;12(8):747–57. doi: 10.1038/ncb2078. doi: 10.1038/ncb2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux JD, Xavier R, Taylor K, Silverberg M, Goyette P, Huett A, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Gen. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov J, Walczak M, Ibiricu I, Schüchner S, Ogris E, Kraft C, Martens S. Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. EMBO J. 2014;31(22):4304–17. doi: 10.1038/emboj.2012.278. doi: 10.1038/emboj.2012.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu MS, Langkamp-Henken B, Chang SM, Shankar MN, Cousins RJ. Genomic analysis, cytokine expression, and microRNA profiling reveal biomarkers of human dietary zinc depletion and homeostasis. Proc Natl Acad Sci U S A. 2011;108(52):20970–5. doi: 10.1073/pnas.1117207108. doi: 10.1073/pnas.1117207108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177(4):1576–91. doi: 10.2353/ajpath.2010.100322. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif R1, Thomas P, Zalewski P, Fenech M. The role of zinc in genomic stability. Mutat Res. 2012;733(1-2):111–2. doi: 10.1016/j.mrfmmm.2011.08.009. doi: 10.1016/j.mrfmmm.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Singh R, Xiang Y, Wang Y, et al. Autophagy regulates adipose mass and differentiation in mice. J Clin Inv. 2009;119(11):3329–3339. doi: 10.1172/JCI39228. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz A1, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119(12):3502–11. doi: 10.1172/JCI40599. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summersgill H, England H, Lopez-Castejon G, Lawrence CB, Luheshi NM, Pahle J, Mendes P, Brough D. Zinc depletion regulates the processing and secretion of IL-1β. Cell Death Dis. 2014;5:e104. doi: 10.1038/cddis.2013.547. doi: 10.1038/cddis.2013.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20(21):5971–5981. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Sou YS, Ezaki J, Minematsu-Ikeguchi N, Ueno T, Kominami E. HsAtg4B/HsApg4B/autophagin-1 cleaves the carboxyl termini of three human Atg8 homologues and delipidates microtubule-associated protein light chain 3- and GABAA receptor-associated protein-phospholipid conjugates. J. Biol. Chem. 2004;279(35):36268–36276. doi: 10.1074/jbc.M401461200. [DOI] [PubMed] [Google Scholar]
- Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in caspase activation and apoptotic cell death. Biometals. 2001;14(3-4):315–30. doi: 10.1023/a:1012993017026. [DOI] [PubMed] [Google Scholar]
- Tuschl K1, Clayton PT, Gospe SM, Jr, Gulab S, Ibrahim S, Singhi P, Aulakh R, Ribeiro RT, Barsottini OG, Zaki MS, Del Rosario ML, Dyack S, Price V, Rideout A, Gordon K, Wevers RA, Chong WK, Mills PB. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am J Hum Genet. 90(3):457–66. doi: 10.1016/j.ajhg.2012.01.018. doi: 10.1016/j.ajhg.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virdis A, Dell’Agnello U, Taddei S. Impact of inflammation on vascular disease in hypertension. Maturitas. 2014;(14):S0378–5122. doi: 10.1016/j.maturitas.2014.04.012. 00131-5. doi: 10.1016/j.maturitas.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Wang J, Whiteman MW, Lian H, Wang G, Singh A, Huang D, Denmark T. A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating Beclin 1. J Biol Chem. 2009;284(32):21412–24. doi: 10.1074/jbc.M109.026013. doi:10.1074/jbc.M109.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Zhao Y, Schoene NW, Han CT, Shih RS, Lei KY. Zinc deficiency depresses p21 gene expression: inhibition of cell cycle progression is independent of the decrease in p21 protein level in HepG2 cells. Am J Physiol Cell Physiol. 2007;292(6):C2175–84. doi: 10.1152/ajpcell.00256.2006. [DOI] [PubMed] [Google Scholar]
- Young AR1, Chan EY, Hu XW, Köchl R, Crawshaw SG, High S, Hailey DW, Lippincott-Schwartz J, Tooze SA. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119(Pt 18):3888–900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
- Zavodszky E, Vicinanza M, Rubinsztein DC. Biology and trafficking of ATG9 and ATG16L1, two proteins that regulate autophagosome formation. FEBS Lett. 2013;587(13):1988–96. doi: 10.1016/j.febslet.2013.04.025. doi: 10.1016/j.febslet.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Zhang YB, Zhao W, Zeng RX. Autophagic degradation of caspase-8 protects U87MG cells against H2O2-induced oxidative stress. Asian Pac J Cancer Prev. 2013;14(7):4095–9. doi: 10.7314/apjcp.2013.14.7.4095. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, Heintz N, Yue Z. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol. 2009;11(4):468–476. doi: 10.1038/ncb1854. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]