Abstract
Clinical long-term outcomes have shown that partial leaflet resection followed by ring annuloplasty is a reliable and reproducible surgical repair technique for treatment of mitral valve (MV) leaflet prolapse. We report a 61-year-old male for three-dimensional transesophageal echocardiography (3D TEE)-based virtual posterior leaflet resection and ring annuloplasty. Severe mitral regurgitation was found and computational evaluation demonstrated substantial leaflet malcoaptation and high stress concentration. Following virtual resection and ring annuloplasty, posterior leaflet prolapse markedly decreased, sufficient leaflet coaptation was restored, and high stress concentration disappeared. Virtual MV repair strategies using 3D TEE have the potential to help optimize MV repair.
Keywords: mitral valve, three-dimensional echocardiography, mitral valve repair, resection, computational evaluation
Mitral valve (MV) leaflet prolapse is frequently associated with varying degrees of mitral regurgitation (MR) due to leaflet malcoaptation. When chordal rupture occurs, leaflet tissue is predisposed to severely anomalous biomechanical conditions and exposed to pathologic tissue deterioration leading to increased tension on the remaining chordae and further leaflet tissue failure.1 Clinical long-term outcomes have shown that partial leaflet resection followed by ring annuloplasty is a reliable and reproducible surgical repair technique for treatment of prolapsed MV leaflets due to chordal rupture.2,3
Virtual MV repair strategies using patient-specific three-dimensional (3D) echocardiography provides a novel methodology to acquire MV geometric data to quantify valvular function. Computational simulation combined with these 3D echocardiographic data provides important biomechanical and physiologic information allowing improved prediction of MV repair and advanced surgical planning.4-6 We report a case of a 61-year-old male where 3D transesophageal echocardiographic (TEE) data was used to perform virtual posterior mitral leaflet resection and ring annuloplasty. Computational MV functional simulations prior to and following the MV repair were performed and effect of the repair on restoration of normal MV function was investigated.
Case Report
A 61-year-old male was referred for evaluation of MV disorders. Real time 3D TEE was performed retrospectively using an iE33 ultrasound system (Philips Healthcare, Andover, MA, USA). TEE clearly revealed severe MR with an anteriorly directed regurgitant jet. The left ventricular ejection fraction was normal (LVEF = 65%). Posterior chordal rupture and a flailed leaflet scallop (P2) were observed.
We have developed novel custom-designed virtual MV modeling and repair simulation protocols using MATLAB (The Mathworks Inc., Natick, MA) and ABAQUS (SIMULIA, Providence, RI) software.7,8 Briefly, the 3D TEE data were transferred to a personal computer to create a computational MV model for virtual MV repair. The mitral leaflets and annulus were created using a 3D non-uniform rational B-spline (NURBS) surface modeling technique followed by adding the marginal and strut chordae except in the P2 scallop region. A triangular portion of the prolapsed posterior leaflet was defined for resection by delineating two excision curves. The nodes and elements in the pre-defined resection region were removed. The excised leaflet edges were combined by imposing nodal displacements, i.e., virtual suturing. A virtual ring annuloplasty protocol was designed following standard guidelines of ring annuloplasty. A prototype annuloplasty ring (Physio II, Edwards Lifesciences, Irvine, CA) of proper size (34 mm) for the MV was modeled into the simulation. The intercommissural and septolateral lines of the ring were aligned to be superimposed on the annulus, and virtual suturing was performed by deforming the annulus to the ring configuration. Following the resection and ring annuloplasty simulations, computational simulation of MV function over the complete cardiac cycle was performed using dynamic finite element analysis methods.8
The patient 2D Doppler ultrasound data revealed a large regurgitant jet directed anteriorly from the P2 prolapse during systole (Fig. 1A). The 3D TEE full volume data clearly demonstrated posterior leaflet prolapse with a flailed P2 scallop (Fig. 1B). Computational simulation of the patient-specific MV function corresponded to the physiologic MR characteristics in the TEE data (Fig. 1C).
Figure 1.

Images of the pathologic MV with chordal rupture and severe MR over the cardiac cycle. A. Two-dimensional TEE with color Doppler, B. 3D TEE with full volume rendering, C. Computational evaluation of MV function demonstrating stress distributions across the leaflets. A = anterior; P = posterior; Al = anterolateral; Pm = posteromedial; LA = left atrium; LV = left ventricle; Ao = aorta.
Virtual triangular posterior leaflet resection clearly demonstrated the location of the excised leaflet tissue (Fig. 2A) followed by successful virtual suturing (Fig. 2B). Virtual ring annuloplasty demonstrated markedly reduced anteroposterior (A-P) distance (Fig. 2C, arrows). The pathologic MV with ruptured chordae demonstrated severe posterior leaflet prolapse (P2 region) resulting in substantial leaflet malcoaptation (Fig. 3A). Maximal leaflet stress (2.8 MPa) appeared near the mitral annulus-aorta junction and over the remaining intact posterior chordae in the pathologic MV. Following virtual resection, the severity of the posterior leaflet prolapse markedly decreased and sufficient leaflet coaptation was restored (Fig. 3B). We found that the extent of leaflet resection affected the consequent outcomes. Maximal stress value was reduced to 2.2 MPa and a large portion of the high stress concentration in the anterior leaflet belly disappeared. Ring annuloplasty further increased contact between the two leaflets resulting in complete leaflet coaptation (Fig. 3C). Maximal stress dramatically decreased to 0.7 MPa and uniform stress distribution was found across both leaflets at the time of ring annuloplasty. The selected annuloplasty ring size (34 mm) in the simulation was based on standard clinical guidelines. There was good agreement to the actual ring size chosen by the surgeon (30 mm) for the operation when considering a slight reduction of the heart and annulus during bypass surgery.
Figure 2.
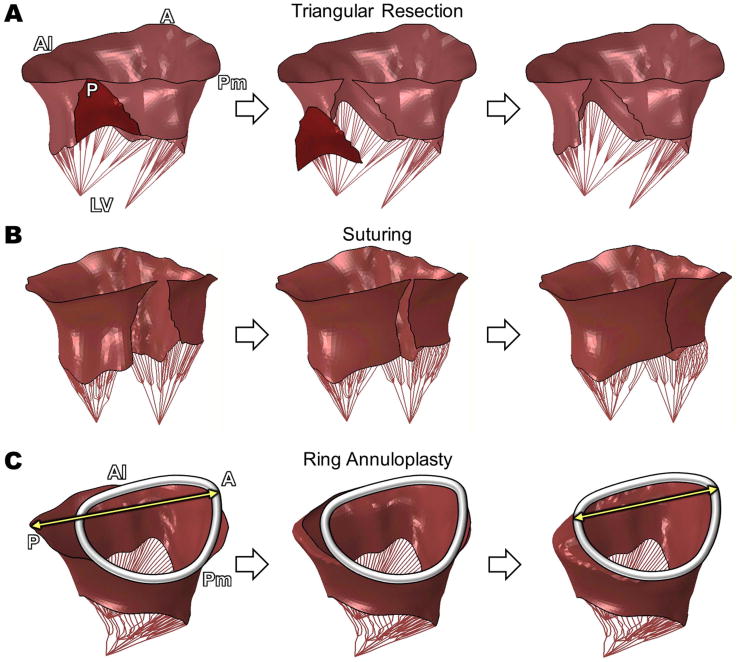
Protocols of A. virtual triangular posterior leaflet resection, B. virtual suturing, and C. virtual ring annuloplasty. A = anterior; P = posterior; Al = anterolateral; Pm = posteromedial; LV = left ventricle.
Figure 3.

Quantitative evaluation of biomechanical characteristics (leaflet contact and stress distributions) of the pathologic MV A. before virtual repair, B. after posterior leaflet resection alone, and C. subsequently after ring annuloplasty. A = anterior; P = posterior; Al = anterolateral; Pm = posteromedial; LV = left ventricle.
Conclusions
We have demonstrated a 3D TEE-based virtual MV repair simulation using a novel computational protocol for mitral leaflet resection followed by ring annuloplasty in a patient with chordal rupture and severe MR. 3D TEE provides a framework for quantitative geometric and physiologic evaluation of valvular function unavailable with other imaging techniques.9,10 It is particularly well-suited for MV evaluation due to transducer location. Combined with 3D TEE, virtual computational MV repair allows evaluation and prediction of physiologic valvular function before and after leaflet resection and/or ring annuloplasty. This includes selection of the optimal annuloplasty ring shape and size, identification of leaflet tissue amount to resect or plicate, and identification of whether chordal repair techniques are necessary, to minimize hemodynamic and physiologic abnormalities following MV repair. This novel virtual MV repair strategy has the potential to help determine patient-specific optimal MV repair techniques preoperatively and allow advanced surgical planning with improved prediction of feasibility and efficacy of MV repair.
Acknowledgments
*This work was supported in part by the National Institutes of Health (R01 HL109597, PI - Hyunggun Kim).
Footnotes
Author Contributions: YR – design, data analysis/interpretation, drafting article; AC – design, data analysis/interpretation; STL – data collection, data interpretation, article revision; DDM – data interpretation, article revision; HK – concept/design, data analysis/interpretation, critical article revision, approval of article.
References
- 1.Carpentier A, Adams DH, Filsoufi F. Carpentier's reconstructive valve surgery. Maryland Heights, Missouri, USA: Elsevier; 2010. [Google Scholar]
- 2.Padala M, Powell SN, Croft LR, et al. Mitral valve hemodynamics after repair of acute posterior leaflet prolapse: quadrangular resection versus triangular resection versus neochordoplasty. J Thorac Cardiovasc Surg. 2009;138:309–315. doi: 10.1016/j.jtcvs.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakamoto Y, Hashimoto K, Okuyama H, et al. Long-term assessment of mitral valve reconstruction with resection of the leaflets: triangular and quadrangular resection. Ann Thorac Surg. 2005;79:475–479. doi: 10.1016/j.athoracsur.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 4.Ender J, Eibel S, Mukherjee C, et al. Prediction of the annuloplasty ring size in patients undergoing mitral valve repair using real-time three-dimensional transoesophageal echocardiography. Eur J Echocardiogr. 2011;12:445–453. doi: 10.1093/ejechocard/jer042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin RP. How do we use imaging to aid considerations for intervention in patients with severe mitral regurgitation? Ann Cardiothorac Surg. 2013;2:779–786. doi: 10.3978/j.issn.2225-319X.2013.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rim Y, Laing ST, Kee P, et al. Evaluation of mitral valve dynamics. JACC Cardiovasc Imaging. 2013;6:263–268. doi: 10.1016/j.jcmg.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rim Y, Laing ST, McPherson DD, et al. Mitral valve repair using ePTFE sutures for ruptured mitral chordae tendineae: a computational simulation study. Ann Biomed Eng. 2014;42:139–148. doi: 10.1007/s10439-013-0908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rim Y, McPherson DD, Chandran KB, et al. The effect of patient-specific annular motion on dynamic simulation of mitral valve function. J Biomech. 2013;46:1104–1112. doi: 10.1016/j.jbiomech.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manda J, Kesanolla SK, Hsuing MC, et al. Comparison of real time two-dimensional with live/real time three-dimensional transesophageal echocardiography in the evaluation of mitral valve prolapse and chordae rupture. Echocardiography. 2008;25:1131–1137. doi: 10.1111/j.1540-8175.2008.00832.x. [DOI] [PubMed] [Google Scholar]
- 10.Sudhakar S, Khairnar P, Nanda NC. Live/real time three-dimensional transesophageal echocardiography. Echocardiography. 2012;29:103–111. doi: 10.1111/j.1540-8175.2011.01525.x. [DOI] [PubMed] [Google Scholar]


