Abstract
Objective
The obesogenic environment is pervasive, yet only some people become obese. We aimed to investigate whether obese individuals show differential neural responses to visual and auditory food cues, independent of cue modality.
Design and Methods
Obese (BMI 29;-41, n=10) and lean (BMI 20-24, n=10) females underwent fMRI scanning during presentation of auditory (spoken word) and visual (photograph) cues representing high energy-density [ED] and low-ED foods. We examined the effect of obesity on whole brain activation, and on functional connectivity with the midbrain/VTA.
Results
Obese compared with lean women showed greater modality-independent activation of the midbrain/VTA and putamen in response to high-ED (vs. low-ED) cues, as well as relatively greater functional connectivity between the midbrain/VTA and cerebellum (p<0.05 corrected).
Conclusions
Heightened modality-independent responses to food cues within the midbrain/VTA and putamen, and altered functional connectivity between the midbrain/VTA and cerebellum, could contribute to excessive food intake in obese individuals.
Keywords: cue reactivity, food responsivity, food stimuli, appetite, dopamine pathway
Introduction
The obesogenic environment is pervasive, but only some individuals develop obesity. One contributing factor could be differential brain responses to food-related cues in the environment, which may promote excessive energy intake. In support, several studies using visual food cues have demonstrated increased activation in obese vs. lean individuals in a number of brain areas including the striatum (e.g. nucleus accumbens, caudate, putamen), limbic regions (e.g. amygdala, hippocampus), and areas within the insular, cingulate, and prefrontal cortices (1, 2, 3). These reports have been highly heterogeneous, likely resulting from variability in methods, statistical approaches, and sample characteristics. However, together they suggest that obese individuals are more ‘neurologically sensitive’ to food (and perhaps particularly high-calorie food) cues in regions associated with reward, emotion, and motivation.
Several questions, though, remain. For example, although we have examined changes in modality-independent responses to high-ED (vs. low-ED) visual and auditory food cues post bariatric surgery (4), no studies have tested for obese vs. lean differences in modality-independent responses (5), i.e. areas that respond differently to high-ED food cues, regardless of cue modality. Networks demonstrating supra-modal activation differences (i.e. differences remaining after extracting effects specific to sensory systems) may capture essential differences in responsivity to food cues, which appear in a range of modalities. Further, although we have reported sex differences in response to auditory food cues within an obese sample (6), no studies have examined both obese and lean individuals’ responses to auditory cues. Auditory food cues abound in the environment (e.g. food commercials) and may be potent neurobehavioral triggers.
Additionally, with the exception of two studies (7, 8) obese-lean comparison studies have conducted only standard univariate activation analyses. These capture the response of individual regions to a particular stimulus. However, regions do not act in isolation, but rather interact with other brain areas. Functional connectivity approaches such as Psychophysiological Interaction [PPI] analysis, which identifies brain regions showing condition;dependent differences in coactivation with a ‘seed’ region (9), may shed additional light on the functional neural networks underlying food cue responsivity. PPI analysis is both a hypothesis-based and exploratory technique: hypothesis-based in that a seed region is selected, and exploratory in that a whole-brain search for regions functionally connected to this seed is conducted.
To address some of these research gaps, we used the same paradigm employed in (4) and (6) to investigate obese vs. lean differences in a) regional brain activation in response to multimodal (i.e. visual and auditory) high;ED vs. low-ED food cues, and b) functional connectivity between a reward-related region of interest and the rest of the brain. Due to its important role in reward and its previous use as a seed in studies of drug addiction (10), as well as its emergence as a region of difference in our bariatric surgery study (4), the region of interest selected was the midbrain in the region of the ventral tegmental area [VTA]. We hypothesized that obese women would show a) greater activation within brain areas associated with reward and motivation, including the midbrain/VTA, and b) greater connectivity between the midbrain/VTA and other reward/motivation regions, in response to auditory and visual high-ED food cues. Our primary focus was group differences independent of modality;specific activations; however we also investigated group differences for visual only, and auditory only, conditions.
Methods and Procedures
Participants
Twenty healthy women (aged 20-27 y) underwent fMRI scanning, 10 of whom were obese (BMI 29-41) and 10 lean (BMI 20-24). All women were right-handed, weight stable, non-smoking, premenopausal, not pregnant or lactating, free of psychological or physical disorders and not taking any medications that affect weight or appetite. None of the participants had diagnostic eating disorders. Exclusion criteria included claustrophobia, and presence of metallic implants and non-removable metallic dental retainers or pacemakers. The scan lasted 45 minutes and was performed between 1-3 pm, 3 hours after supervised consumption of a 650 kcal meal consisting of sandwiches (tuna, chicken or egg salad) with a piece of fruit and a juice or non-diet soda.
Procedure
Participants underwent an fMRI scan during which they were presented with visual and auditory stimuli representing high energy-density [high-ED] foods (e.g. chocolate cake, French fries), low energy-density [low-ED] foods (e.g. green beans) and non-foods (office supplies). The high-ED foods contained ≥3.5 kcal/g and the low-ED foods ≤1 kcal/g. Visual cues were photographs transmitted through eye goggles, and auditory cues were recorded 2-word names similar in content to the visual stimuli, transmitted through a headset and repeated twice to fill the 4-second epoch (e.g. “Chocolate brownie, chocolate brownie”). Most of the photos were taken by a member of the research team, with a small number drawn from the internet, in similar proportions across each stimulus category. The majority of the food pictures were shown against a white plate, with attempts to match for arrangement and color of items across stimulus categories. Stimuli of each condition type were presented in two separate blocks containing similar but not identical stimuli. This resulted in a total of twelve blocks (2 × visual high-ED, 2 × visual low-ED, 2 × visual non-food, 2 × auditory high-ED, 2 × auditory low-ED and 2 × auditory non-food). Within each block, 10 different stimuli of the same type were presented, i.e. 120 stimuli in total. Stimuli within each block were presented consecutively for 4 s each, resulting in a duration of 40 s, together with a 52-second pre-stimulus period (e.g., for visual stimuli, white crosshair centered in a black background) and a 40;second post-stimulus period (2 min 12 s in total). The six blocks containing visual cues were followed by the six blocks containing auditory cues, and stimulus types were presented in pseudorandom order within each set of six blocks, with the constraint that a block of any stimulus type could not be followed by a block of the same stimulus type. This randomization was conducted separately for each subject.
Following each block, participants were asked to verbally rate hunger and desire to eat on a scale from 1 to 10 (1 being Not at all, 10 being Very much). These ratings ensured alertness, and allowed analysis of subjective appetite. After leaving the scanner, participants rated their liking for each high-ED, low-ED and non-food cue using a visual analog scale from -100 to +100 (-100 indicating Dislike extremely, +100 Like extremely. Visual cues were presented as color-printed pictures, and auditory cues as printed two- word phrases.
Imaging acquisition
Scanning was conducted using a 1.5 Tesla twin-speed scanner with quadrature RF head coil. Participants lay supine with the head in a padded restraint and tape strapped across the forehead. Three-plane localization verified head position. Functional T2*-weighted images were obtained using a gradient echo pulse sequence (echo time = 60 msec, repetition time = 4 sec, flip angle = 60°). During each run (each run containing one block), 36 axial whole brain scans were made, each consisting of 25 contiguous slices, parallel to the AC/PC line (19x19 cm field view, 128×128 matrix size, 1.5×1.5 mm in plane resolution). High-resolution anatomical scans were acquired with a T1-weighted SPGR sequence (TR = 19 ms, TE = 5 ms, flip angle = 20°, FoV = 220×220 mm), recording 124 slices at a slice thickness of 1.5 mm and in-plane resolution of 0.86 × 0.86 mm.
Statistical analysis
Desire to eat, hunger (assessed after each block during the scan), and liking ratings (assessed once, post-scan) in relation to high-ED vs. low-ED vs. non-food cues, were compared between obese and lean individuals using repeated measures ANOVA, and paired t-tests were used to further investigate differences between conditions when the F statistic was significant at p < 0.05. Analyses were performed using SPSS 20.
fMRI analysis: 1st level
Images were preprocessed using SPM2 (Wellcome Department of Imaging Neuroscience, London, United Kingdom). Realigned T2-weighted volumes were slice-time corrected, spatially transformed to a standardized brain (Montreal Neurologic Institute) and smoothed with a 8-mm full-width half maximum Gaussian kernel. Data were processed using SPM5. For each subject's 1st level analysis, the 12 blocks were concatenated together to create a single block (396 total time points). Block regressors were included in the GLM to account for the mean of each block within each subject's session. Additional nuisance covariates included motion (obtained from the realignment procedure), as well as global signal and spikes computed using the scn_session_spike_id.m script available in Diagnostics Tools for MATLAB (http://wagerlab.colorado.edu/tools). Regressors-of-interest were created by convolving the onset of each block (auditory and visual high-ED, low-ED, and non-food) with the canonical HRF with duration of 40 seconds. The following contrasts were then created from the resulting estimated parameters and passed to 2nd level analysis (see below): 1) visual high;ED > visual low;ED and 2) auditory high-ED > auditory low-ED. Because our fMRI hypotheses focused on high-ED vs. low;ED food processing, non-food conditions were not included in 2nd level image analyses and will not be presented.
fMRI analysis: PPI
Activity from the midbrain/VTA was extracted from a functionally defined cluster (151 voxels, 1208 mm3 with center of mass at MNI = [1.4, −7.5, −9.0]). The BOLD signal throughout the whole-brain was then regressed on a voxel-wise basis against the product of this time course (physiological variable) and the vector of the psychological variable of interest (i.e. 1* high-ED + −1*low-ED in the visual and auditory conditions separately), with the physiological and the psychological variables serving as regressors of no interest. Both auditory and visual beta maps were subsequently passed to 2nd level random effects analysis (see below).
fMRI analysis: 2nd level
Contrast and beta images generated from the 1st level activation and PPI analyses respectively, were subjected to a 3-way ANOVA. The independent, between-group, factor of interest was obese vs. lean; the dependent, within;subject factor was visual vs. auditory. Since each group contained five individuals with sub;threshold binge eating symptoms (11), we also included binge eating as a factor of no interest.
To obtain regions common to both modalities, we conducted a conjunction (conjunction null hypothesis) of the positive effects of obesity across both visual and auditory cues. Since the likelihood of two independent tests both surviving p < 0.05 (i.e. p < 0.017 (Fisher's method for combining p-values) is much smaller than one test alone,statistical maps for conjoined activation were displayed at p < 0.05 uncorrected, cluster size [k] > 30, to account for the fact that both visual and auditory activation differences were required. In addition, to account for multiple comparisons, we checked each region for whether it appeared in a separate analysis, testing against the global null hypothesis (of 1 or more effects), correcting for multiple comparisons using cluster-extent thresholding. For this, we used the 3dClustSim program in AFNI (v.2010) to generate 1000 Monte Carlo simulations of whole-brain fMRI data and determine the cluster size at which the false positive probability is below a desired alpha level of p < 0.05, i.e. an effective threshold of p < 0.05 corrected. Input parameters to the 3dClustSim program included the same whole-brain mask used for all analyses and the inherent smoothness estimated from the data (obtained from SPM.xVol.FWHM). For an uncorrected p < 0.005 testing against the global null (a reasonable threshold given the requirement of visual and auditory effects), this simulation yielded a cluster size threshold of 58 contiguous voxels, and regions that survived these corrected thresholds are denoted in the tables with an asterisk.
In addition to our conjunction analyses, we conducted group contrasts separately for auditory and visual cues. To comprehensively represent group differences, statistical maps were thresholded at p < 0.001 and k > 10. In addition, to account for multiple comparisons, we used 3dClustSim as described above to determine a cluster size representing an effective p < 0.05 corrected (see above). For an uncorrected p < 0.001, the cluster size threshold was 28. This combination of uncorrected p-value and cluster extent requirement is widely adopted by the neuroimaging community as an acceptable way to balance Type 1 vs. Type 2 errors, and is thought to generally produce replicable data across multiple scanners and studies (12).
To disambiguate the direction of activity for all of the analyses (conjoined, visual only, auditory only), we plotted beta estimates plus 90% confidence intervals for peak voxels in significant clusters for high-ED food responses alone and low-ED food responses alone, for the obese and lean groups separately.
Results
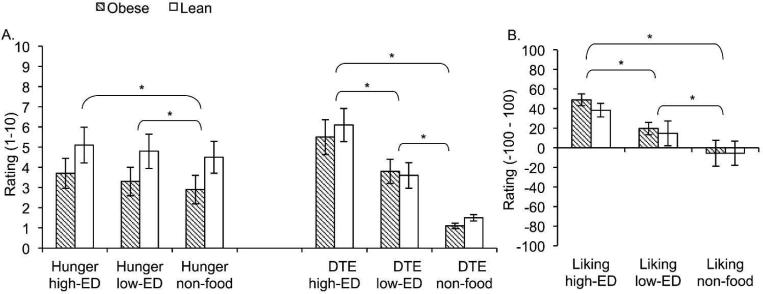
Obese women had higher body weight, BMI and fat percentage than lean women (Table 1). Repeated measures ANOVA revealed a significant difference in hunger scores between high;ED, low-ED, and non;food cues (F [2,32] = 6.1, p = 0.006), with post-hoc t-tests demonstrating significantly higher hunger ratings following high-ED vs. non-food cues (p = 0.01), and low;ED vs. non-food cues (p = 0.007). The pattern was similar for desire to eat ratings (F [2,34] = 46.6, p < 0.001), with scores highest following high-ED, then low-ED, then non-food cues (all post-hoc comparisons p < 0.001). Liking ratings also showed a significant main effect of cue type (F [2,32] = 15.8, p < 0.001, df = 2, 32), reflecting significantly higher liking for high-ED vs. low-ED (p < 0.001), low-ED vs. non;food (p = 0.03), and high;ED vs. non-food (p < 0.001) cues. No weight group interactions were apparent in any of the analyses (Figure 1, Table 2).
Table 1.
Sample characteristics (mean ± SD) for obese and lean groups
| Weight category | N | Age | Weight (kg)a | BMIa | Body fat %a |
|---|---|---|---|---|---|
| Obese | 10 | 22.4 ± 2.0 | 83.3 ± 12.0 | 32.9 ± 5.3 | 41.4 ± 6.7 |
| Lean | 10 | 21 ± 1.2 | 61.5 ± 6.4 | 22.1 ± 1.2 | 28.1 ± 6.4 |
Significant difference between obese and lean p<0.05
Figure 1.
A. Bars represent hunger and desire to eat ratings for all subjects following presentation of high-ED, low-ED and non-food cues. Asterisks represent significant difference based on post;hoc t-tests (p<0.05). B. Bars represent liking ratings for all subjects following presentation of high-ED, low-ED and non;food cues. Asterisks represent significant difference based on post-hoc t-tests (p<0.05).
Table 2.
Hunger, desire to eat and liking ratings (mean ± SD) for each stimulus type in obese and lean groups
| High-ED | Low-ED | Non-foods | ||
|---|---|---|---|---|
| Hunger | Obese | 3.7 ± 2.1 | 3.3 ± 2.0 | 2.9 ± 2.0 |
| Lean | 5.1 ± 2.8 | 4.8 ± 2.7 | 4.5 ± 2.5 | |
| Total* | 4.5 ± 2.5 | 4.1 ± 2.5 | 3.8 ± 2.3 | |
| Desire to eat | Obese | 5.5 ± 2.6 | 3.8 ± 1.8 | 1.1 ± 0.4 |
| Lean | 6.1 ± 2.6 | 3.6 ± 2.0 | 1.5 ± 0.5 | |
| Total* | 5.8 ± 2.5 | 3.7 ± 1.8 | 1.3 ± 0.5 | |
| Liking | Obese | 49.0 ± 18.3 | 19.7 ± 19.0 | −5.6 ± 39.5 |
| Lean | 38.4 ± 21.0 | 14.7 ± 38.0 | −5.5 ± 37.0 | |
| Total* | 43.7 ± 19.9 | 17.2 ± 29.2 | −5.6 ± 37.2 | |
Significant effect of stimulus type, p <0.05
fMRI analyses: Activation
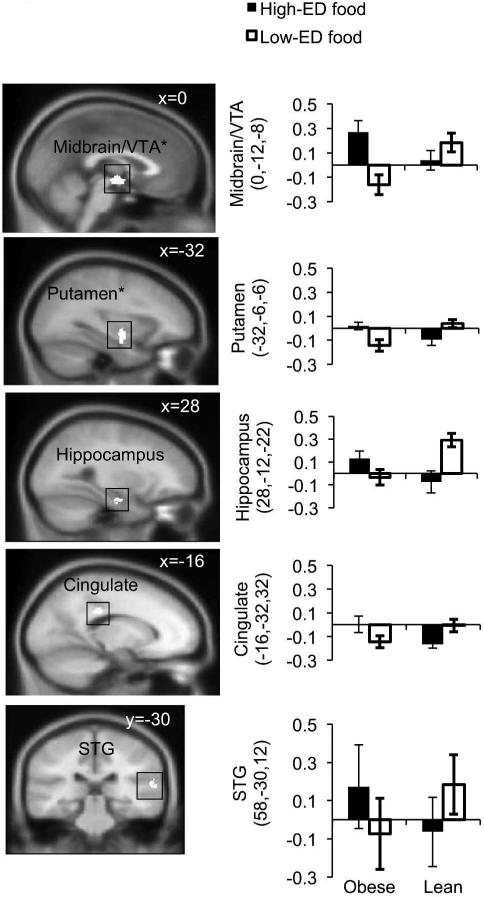
Whole-brain cluster-extent corrected analyses revealed that the greatest conjoined (visual and auditory) activation in response to high-ED (vs. low-ED) foods for obese vs. lean women occurred within the midbrain in the vicinity of the VTA, while the next strongest finding occurred in the putamen (Figure 2, Table 3). Inspection of contrast plots revealed that midbrain/VTA activity was driven by both greater responses to high-ED cues in the obese, and greater responses to low-ED cues in the lean subjects. The activation in this region was also greater for the visual cues alone, and the auditory cues alone (visual: t (32) = 2.54; p = 0.016; auditory: t (32) = 3.24; p = 0.0028), with no influence of binge eating for either modality (visual: F (1,32) = 0.09, p = 0.77; auditory: F (1,32) = 1.42, p = 0.24). Contrast plots for the putamen revealed that activity was driven by deactivation in response to low-ED cues in the obese and in response to high-ED cues among the lean. The activation in the putamen was also relatively greater for the visual and auditory cues alone: (visual: t (32) = 4.34; p = 0.00007; auditory: t (32) = 3.20; p = 0.002), with no evidence for effects of binge eating for either modality (visual: F (1,32) = 0.01, p = 0.93; auditory: F (1,32) = 0.11, p = 0.75).
Figure 2.
Slices depict areas in which there was greater modality-independent activation in response to high-ED (vs. low-ED) food cues in obese compared to lean individuals. Bar graphs show parameter estimates (no contrast) for voxels of each region in obese and lean individuals, for high-ED (vs. block baseline) and low-ED (vs. block baseline). Greater visual and auditory activation (conjunction) is seen in the midbrain/VTA (x=0), putamen (x=-32), posterior cingulate gyrus (cingulate, x=-16), hippocampus (x=28) and superior temporal gyrus (STG, y=-30). MNI coordinate is displayed in upper right corner of each slice. * Significant at p<0.05 corrected (see Methods).
Table 3.
Brain regions showing greater activation for high-ED vs. low-ED foods in obese vs. lean women
| Modality and region | Side | Coordinates |
k | t | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Conjunction of visual and auditory cuesa | ||||||
| Midbrain/VTA* | 0 | −12 | −8 | 151 | 2.54 | |
| Putamen* | L | −32 | −6 | −6 | 122 | 2.66 |
| Hippocampus | R | 28 | −12 | −22 | 40 | 2.44 |
| Posterior cingulate gyrus | L | −16 | −32 | 32 | 36 | 2.95 |
| Superior temporal gyrus | R | 58 | −30 | 12 | 36 | 2.36 |
| Visual cuesb | ||||||
| Superior temporal gyrus* | L | −44 | −36 | 10 | 65 | 4.16 |
| R | 58 | −34 | 12 | 12 | 3.77 | |
| Putamen* | L | −26 | −6 | −6 | 51 | 4.80 |
| OFC | R | 26 | 34 | −10 | 17 | 3.68 |
| Auditory cuesb | ||||||
| Supramarginal gyrus* | L | −58 | −50 | 28 | 46 | 4.73 |
| Parahippocampal gyrus | L | −16 | −40 | −8 | 19 | 4.22 |
| Brainstem | R | 14 | −30 | −32 | 17 | 3.86 |
| Inferior frontal gyrus | R | 44 | 8 | 10 | 12 | 3.99 |
x,y,z = Montreal Neurological Institute coordinates; k = cluster size, i.e. number of voxels within each cluster
Significant at p < 0.05 uncorrected testing against conjunction null hypothesis
Significant at p < 0.001 uncorrected, k > 10
Significant at p< 0.05 corrected (see Methods).
Corrected analyses of the high-ED (vs. low-ED) contrast for each modality separately revealed that obese vs. lean women showed relatively greater high-ED vs. low-ED responses in the superior temporal gyrus [STG] and putamen for the visual modality, and greater high-ED vs. low-ED responses in the supramarginal gyrus for the auditory modality (Supplementary Figure 1, Table 3). Note that because the separate contrasts of visual and auditory cues were conducted at a more stringent threshold (p<0.001 uncorrected, k=10), some of the areas found with the conjunction analysis did not emerge for the contrasts of auditory and visual cues alone.
In uncorrected analyses, we additionally observed greater conjoined activation in the hippocampus, posterior cingulate and STG (Figure 2, Table 3), as well as greater visual activation in the OFC and greater auditory activation in the brainstem, parahippocampal gyrus and inferior frontal gyrus (Supplementary Figure 1, Table 3), among obese vs. lean women in response to high-ED (vs. low-ED) cues.
fMRI analyses: Psychophysiological Interactions (PPI)
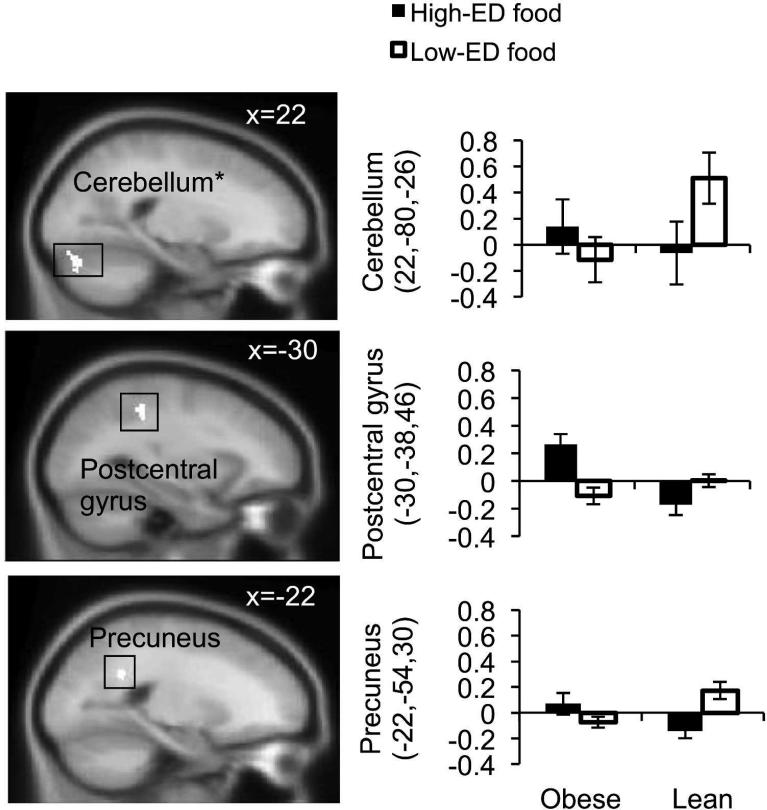
Whole-brain, cluster;extent corrected PPI analyses revealed that obese compared to lean women showed greater functional connectivity with the midbrain/VTA in response to conjoined visual and auditory high-ED vs. low-ED food cues in the cerebellum (Figure 3, Table 4), an effect that was driven by increased midbrain/VTA coupling during the low- ED cues among the lean group, as well as by relatively increased midbrain/VTA coupling during the high-ED cues among the obese group. Corrected analyses of the high-ED (vs. low-ED) contrast for each modality separately revealed that obese vs. lean women showed relatively greater functional connectivity with the midbrain/VTA in the parahippocampal gyrus for the visual modality and the cuneus for the auditory modality (Supplementary Figure 2, Table 4).
Figure 3.
Slices depict areas in which there was greater modality-independent functional connectivity with the midbrain/VTA in response to high-ED (vs. low;ED) food cues in obese vs. lean individuals. Bar graphs show parameter estimates (no contrast) for voxels of each region in obese and lean individuals, for high-ED (vs. block baseline) and low-ED (vs. block baseline). Increased coupling for visual and auditory cues (conjunction) is seen in the cerebellum (x=22), the postcentral gyrus (x=-30) and the precuneus (x=-22). MNI coordinate is displayed in upper right corner of each slice. * Significant at p<0.05 corrected (see Methods).
Table 4.
Brain regions showing greater functional connectivity with midbrain/VTA for high-ED vs. low-ED food cues, in obese vs. lean women
| Modality and region | Side | Coordinates |
k | t | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Conjunction of visual and auditory cuesa | ||||||
| Cerebellum* | R | 22 | −80 | −26 | 148 | 2.58 |
| L | −24 | −62 | −28 | 49 | 2.34 | |
| Postcentral gyrus | L | −30 | −38 | 46 | 43 | 2.32 |
| Precuneus | L | −22 | −54 | 30 | 41 | 2.69 |
| Visual cuesb | ||||||
| Parahippocampal gyrus* | R | 26 | −26 | −20 | 32 | 4.71 |
| Inferior parietal cortex | L | −28 | −56 | 42 | 19 | 4.08 |
| Auditory cuesb | ||||||
| Cuneus* | R | 22 | −66 | 20 | 36 | 4.14 |
| Cerebellum | R | 22 | −54 | −34 | 23 | 3.73 |
x,y,z = Montreal Neurological Institute coordinates; k = cluster size, i.e. number of voxels within each cluster
Significant at p < 0.05 uncorrected testing against the conjunction null hypothesis
Significant at p < 0.001 uncorrected, k > 10
Significant at p < 0.05 corrected (see Methods).
In uncorrected analyses, we additionally observed relatively increased midbrain/VTA connectivity with the postcentral gyrus and precuneus for conjoined cues (Figure 3, Table 4), as well as with the inferior parietal lobe [IPL] for visual cues and with the cerebellum for auditory cues (Supplementary Figure 2 Table 4).
Discussion
Obese compared with lean women showed increased modality-independent responses to high-ED food cues (visual pictures and auditory words) within the midbrain in the vicinity of the VTA, a region with a high concentration of dopaminergic neurons and strong anatomical and functional connections with multiple brain regions involved in reward and motivation (13). This group difference was driven by greater responses to high;ED cues in the obese, as well as greater responses to low;ED cues in the lean. Obese compared with lean women showed increased responses to high-ED food cues within the putamen, a dorsal striatal region involved in reward-based learning and goal-directed action (14); this was driven by deactivation to low;ED cues in obese and to high-ED cues in the lean. Reported regions that survived our applied primary uncorrected thresholds but did not survive corrections for multiple comparisons should be treated as provisional. However, many of these trending effects were indeed in regions one would anticipate based on previous research (e.g. hippocampus, posterior cingulate) (1, 2, 3). Although our sample size is relatively small, significant results by our correction criteria indicate a large effect of obesity relative to the equivalent result for a greater sample size (15).
Given the important role of the midbrain/VTA and putamen in reward, motivation and the learning of appetitive behaviors, our results likely indicate relatively greater responsivity of reward circuitry to high-ED compared with low-ED food cues among obese individuals. Consistent with this interpretation, an fMRI study of healthy adults observed heightened activation in the midbrain, putamen and other regions to conditioned cues signaling delivery of pleasant (vs. aversive or neutral) tastes (16), suggesting a role in anticipation of taste reward, while another found greater putamen activation in obese vs. lean women in response to high-ED compared to low-ED photographs (17). A PET study found that obese vs. lean individuals showed greater rCBF increases in the midbrain in response to a small taste of a liquid meal (18), while satiation with a meal following a prolonged fast has been associated with decreased rCBF in areas including the midbrain and putamen in lean and obese women, consistent with a role in appetitive processing (19). Further, in the only other study to use a conjoined visual/auditory approach, we found that, following gastric bypass surgery, patients had decreased responses to high-ED (vs. low-ED) food cues in the midbrain/VTA and putamen, supporting a role in weight;related food responses (4). Notably, the surgery study also revealed other striatal and prefrontal regions not observed here, which may reflect greater phenotypic differences in an extremely obese population pre and post surgery than between moderately obese and lean individuals.
Our PPI analyses using the midbrain/VTA as a seed region revealed that obese women displayed relatively greater connectivity in response to high ED (vs. low ED) cues in the cerebellum – a region in which others have observed increased activation to food vs. non-food cues in obese vs. lean individuals (20). Although traditionally considered a motor area, the cerebellum is now known to play a broader role in emotion, learning and motivation (21), and shows direct connections to the VTA and limbic system, as well as indirect connections with the NAcc and cingulate (22). The relatively increased midbrain/VTA-cerebellum coupling among the obese group could therefore indicate greater reward-system modulation of motor/emotion circuitry in response to high-ED cues, potentially reflecting greater desire to eat high-ED foods (23, 24). However, it was notable that the cerebellar group difference seemed to be primarily driven by increased midbrain/VTA coupling following the low-ED cues among the lean group. This could indicate relatively greater reward-system modulation of motor/emotion circuits in response to low-ED cues in the lean individuals, possibly reflecting relatively greater desire to eat low-ED foods in this group. We did not see increased connectivity with other regions known to have direct VTA connections, e.g. nucleus accumbens, hippocampus, amygdala, prefrontal cortex. However, given that we were specifically looking at areas of differential connectivity between obese and lean groups, we cannot exclude the possibility that enhanced connectivity in response to high-ED vs. low;ED cues occurred with these areas across all subjects.
A limitation of our design was that auditory blocks always followed visual blocks, preventing a direct comparison of modalities. However, since the order was consistent for all subjects, this would not have biased between;group comparisons, and there was no group x modality interaction for overall midbrain/VTA signal. Although there were some individuals with binge eating symptoms in each group, there was no statistical effect of binge eating in our region of differential activation and binge eating was controlled for as a regressor of no interest in our analyses. Another potential limitation was that we did not control for menstrual cycle phase or oral contraceptive use, which could have influenced appetite and its neural correlates. The relatively small sample size (in combination with our conjoined activation requirement) may also have been a limiting factor and could have prevented us from observing a between-group cerebellum activation difference, as well as significant group differences for the visual condition in regions emerging in previous studies using exclusively visual cues (1, 2, 3). It should additionally be noted that, unlike several previous studies and meta;analyses (1, 2, 3), we focused on contrasts between high-ED vs. low-ED foods. We believe this comparison to be the most relevant for obesity, but differences obtained by this method could potentially be smaller than those resulting from contrasts between all foods and non-foods, or between high-ED foods and non-foods.
Conclusions
Our results suggest that obese individuals show a modality-independent neural bias towards environmental cues representing high-ED foods, such that seeing such a food or hearing its name triggers an appetitive response in the midbrain/VTA and putamen. In addition, we found relatively increased functional coupling between the midbrain/VTA and cerebellum during processing of high;ED food stimuli in obesity. Our results implicate brain regions that may be useful modality-independent targets for novel behavioral or pharmacological interventions to treat and/or prevent obesity.
Supplementary Material
What is already known about this subject
fMRI studies have demonstrated that obese vs. lean individuals show differential brain activation in response to pictures of high energy-density [ED] foods.
Animal and human studies have highlighted the important role of the ventral tegmental area [VTA] of the midbrain in reward and appetite.
Psychophysiological Interaction [PPI] analyses can identify brain regions showing group and condition-dependent differences in coactivation with a seed region.
What this study adds
Obese vs. lean women showed increased amodal midbrain/VTA and putamen responses to auditory and visual cues representing high-ED vs. low-ED foods.
Obese vs. lean women showed increased functional connectivity between the midbrain/VTA and cerebellum in response to high-ED vs. low-ED food cues.
Enhanced midbrain/VTA and putamen responses, and altered midbrain/VTA-cerebellum connectivity, could contribute to overeating and obesity.
Acknowledgments
We are grateful for funding support from the NIH (K99DK088360 [PI: SC], R00DK088360 [PI: SC], R01DK080153 [PI: AG], R01DK074046S2 [PI: AG]) and St. Luke's-Roosevelt (Associate Trustees award [PI: SC]). We would also like to thank Talya Ladell and Mohammed Sharafi for contributions to preliminary data analysis.
Footnotes
Author contributions
S.C. and A.G. formulated hypotheses and supervised the study. S.P.P., L.B. and S.C. analyzed the functional imaging data. S.C. and L.B. analyzed behavioral data, interpreted results and drafted the paper. A.G developed the stimuli for the scans and A.G. and J.H. developed the functional imaging paradigm. A.G. and S.C. contributed funding. All authors contributed to writing and approved the final paper.
Conflict of interest
None of the authors have conflicts of interest.
References
- 1.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks SJ, Cedernaes J, Schioth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One. 2013;8:e60393. doi: 10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy J, Dimitropoulos A. Influence of feeding state on neurofunctional differences between individuals who are obese and normal weight: a meta-analysis of neuroimaging studies. Appetite. 2014;75:103–109. doi: 10.1016/j.appet.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Ochner CN, Kwok Y, Conceicao E, Pantazatos SP, Puma LM, Carnell S, et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253:502–507. doi: 10.1097/SLA.0b013e318203a289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch J, Moreno DR, Kim KH. Interconnected large-scale systems for three fundamental cognitive tasks revealed by functional MRI. J Cogn Neurosci. 2001;13:389–405. doi: 10.1162/08989290151137421. [DOI] [PubMed] [Google Scholar]
- 6.Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D. Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behav Brain Res. 2013;243:91–96. doi: 10.1016/j.bbr.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, et al. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One. 2012;7:e31089. doi: 10.1371/journal.pone.0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, 3rd, Horwitz B. Effective connectivity of a reward network in obese women. Brain Res Bull. 2009;79:388–395. doi: 10.1016/j.brainresbull.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 10.Camara E, Rodriguez-Fornells A, Ye Z, Munte TF. Reward networks in the brain as captured by connectivity measures. Front Neurosci. 2009;3:350–362. doi: 10.3389/neuro.01.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geliebter A, Ladell T, Logan M, Schneider T, Sharafi M, Hirsch J. Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite. 2006;46:31–35. doi: 10.1016/j.appet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 14.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friston K. Ten ironic rules for non-statistical reviewers. Neuroimage. 2012;61:1300–1310. doi: 10.1016/j.neuroimage.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 16.O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 17.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 18.DelParigi A, Chen K, Salbe AD, Reiman EM, Tataranni PA. Sensory experience of food and obesity: a positron emission tomography study of the brain regions affected by tasting a liquid meal after a prolonged fast. Neuroimage. 2005;24:436–443. doi: 10.1016/j.neuroimage.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 19.Gautier JF, Del Parigi A, Chen K, Salbe AD, Bandy D, Pratley RE, et al. Effect of satiation on brain activity in obese and lean women. Obes Res. 2001;9:676–684. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- 20.Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring) 2010;18:254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 21.Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 22.Blatt GJ, Oblak AL, Schmahmann JD. Cerebellar connections with limbic circuits: Anatomy and functional implications. Springer; Netherlands: 2013. [Google Scholar]
- 23.Rissanen A, Hakala P, Lissner L, Mattlar CE, Koskenvuo M, Ronnemaa T. Acquired preference especially for dietary fat and obesity: a study of weight-discordant monozygotic twin pairs. Int J Obes Relat Metab Disord. 2002;26:973–977. doi: 10.1038/sj.ijo.0802014. [DOI] [PubMed] [Google Scholar]
- 24.Ledikwe JH, Blanck HM, Kettel Khan L, Serdula MK, Seymour JD, Tohill BC, et al. Dietary energy density is associated with energy intake and weight status in US adults. Am J Clin Nutr. 2006;83:1362–1368. doi: 10.1093/ajcn/83.6.1362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.