Abstract
Plasma renin activity (PRA) may be a surrogate for vascular damage. The authors hypothesize that PRA is associated with cardiovascular and cerebrovascular disease (CED). A cross‐sectional study (January 1, 1998, to December 31, 2009) was performed on hypertensive individuals 18 years and older using multivariable logistic regression models to estimate odds ratios (ORs) for ischemic heart disease (IHD), congestive heart failure (CHF), and CED based on PRA quartiles controlling for age, sex, race, diabetes mellitus (DM), and medication use. Among 7887 individuals (60% women; 34% whites, 23% blacks, and 19% Hispanics; and 29% with DM), the adjusted ORs (95% CI) for IHD were 0.94 (0.80–1.10), 1.09 (0.92–1.29), and 1.18 (1.00–1.39); for CHF were 1.23 (0.99–1.53), 1.27 (1.01–1.61), and 1.41 (1.13–1.77); and for CED were 0.95 (0.78–1.17), 0.77 (0.61–0.97), and 0.97 (0.78–1.20) for the second, third, and fourth quartiles compared with the first quartile. Higher PRA was associated with greater likelihood for prevalent IHD and CHF but not CED in this large ethnically diverse population of hypertensive individuals.
Renin‐angiotensin system (RAS) activity is reflected by the plasma renin activity (PRA) level. The RAS works to physiologically maintain blood pressure (BP) in normal states and in times of physical stress.1 Indivduals with high BP are expected to have suppression of the renin‐angiotensin‐aldosterone system because elevated BP usually results from volume overload or systemically elevated vascular pressures. Consequently, hypertensive individuals in whom PRA is not suppressed are likely to have inappropriately overactive RAS and therefore are prone to detrimental vascular outcomes.
Past observations have been inconsistent in their assessment of PRA as a risk factor for cardiovascular (CV) disease and its related mortality.2, 3, 4, 5, 6, 7, 8 Studies that have demonstrated that PRA is predictive of CV outcomes have been conducted primarily in populations with hypertension and/or preexistent CV disease.2, 3, 4, 5, 9, 10, 11 In patients without hypertension, PRA has not been shown to be prognostic since it would merely reflect the physiology of RAS to maintain normal BP.1, 6, 12, 13, 14 The preponderance of studies that have demonstrated the association between PRA and risk of CV and mortality outcomes were performed as post‐hoc analyses of large heart failure or ischemic heart disease trials wherein PRA was not assessed as the primary or independent predictor.9, 10, 15, 16, 17 While these past observations and studies have examined CV outcomes, the cross‐sectional relationship between PRA and prevalent CV disease has not been well described. We sought to examine whether higher PRA was associated with increased likelihood for prevalent ischemic heart disease (IHD), congestive heart failure (CHF), and cerebrovascular disease (CED) within a large ethnically diverse hypertensive population.
Methods
Study Population
The Kaiser Permanente Southern California (KPSC) health system is a prepaid integrated health plan with 14 medical centers and more than 200 satellite clinics. Geographically, the centers span from San Diego to Bakersfield, California. The patient population is ethnically and socioeconomically diverse, reflective of the underlying population in southern California.18 As of October 2009, KPSC had an active membership of 3.3 million. All members have similar benefit structures with co‐pays and deductibles for medications and healthcare. Members have similar access to all healthcare facilities, procedures, and referrals. The data for this study were collected as part of routine clinical practice wherein individual healthcare providers had determined the need for the laboratory measurements, medications, and procedures. The health information is tracked through electronic health records (EHRs). Each member is given a unique medical record number for tracking of healthcare encounters and outcomes. The study protocol was approved by the regional institutional review board and exempted from informed consent.
This cross‐sectional study covered the period from January 1, 1998, through October 31, 2009. The study population has been previously described.19, 20 The current study cohort included individuals 18 years or older who were identified with hypertension and had documented outpatient measurement of PRA and serum aldosterone. A hypertension diagnosis was based on two separate outpatient International Classification of Diseases, Ninth Revision (ICD‐9) codes specific to hypertension. The accuracy of this identification schema has been previously validated.21
All PRA measurements were made with an activity assay that measures angiotensin I generation in an American College of Pathology/Clinical Laboratory Improvement Act–certified laboratory and are reported as ng/mL/h. The test is performed by Quest Diagnostics Nichols Institute using the Sealey PRA test,22 which utilizes radioimmunoassay for quantification. PRA measurements made in the inpatient setting were excluded for consideration in the study because of the potential confounding by acute volume shifts and physiologic stress that may be present during hospitalizations. If individuals had multiple outpatient PRA measurements, the single first value in the observation period was used and all associated results were determined relative to that PRA result date.
The PRA test is a radioimmunoassay that quantifies the production of angiotensin I. Overall, PRA and absolute renin values show a strong correlation.23 This value is a functional measurement of renin levels as renin is the upstream and rate‐limiting factor for RAS activity. PRA may be a better indicator compared with absolute renin concentration due to the fact that certain conditions such as chronic liver disease are more prone to alter absolute renin levels, whereas PRA values would not be affected.24
PRA values used in this study were single measurements obtained at varying times and with different clinical scenarios. Thus, PRA levels can fluctuate and may not necessarily reflect RAS status but rather physiologic variations from activity and daily rhythm.25, 26 Thus, both serum aldosterone levels and the aldosterone:PRA ratio (ARR) were also used as explanatory variables in an attempt to control for confounding variations in PRA. In particular, the ARR would control for any variations in both PRA and aldosterone, as any clinical scenario that would result in PRA changes would also proportionately affect aldosterone.
Data on age, sex, race/ethnicity (when available), laboratory values, medication use, and comorbidities were extracted from the EHR, which included laboratory databases, disease registries, and electronic medical charts. Race/ethnicity information from the EHR was used to categorize individuals as white, black, Hispanic, Asian, or other. Individuals were categorized as other when they were not classified as any of the above or where no race data were available. The presence of comorbidities was assessed based on inpatient and outpatient ICD‐9 diagnoses codes extracted from the EHR. In order to ensure that comorbidities were reliably captured, we required individuals to have had continuous enrollment in the health plan 3 months prior to and 3 months after the PRA measurement for inclusion in the study analyses.
Antihypertensive medication use was determined as those prescribed within 90 days prior to the PRA result date. Given the potential effects of antihypertensive medications on PRA, these medications were further categorized as either diuretics/natriuretic, RAS blockers, or RAS suppressors (eg, as β‐blockers). All laboratory results reported were those obtained within 3 months prior to or after the PRA measurement.
Analytic Approach
The primary objective was to evaluate the likelihood of prevalent IHD, CHF, and CED based on PRA levels. These morbidities were captured based on ICD‐9 diagnoses codes from the EHRs. The rates of IHD, CHF, and CED and comparisons across PRA levels were reported.
Hypertensive individuals who had PRA measurements were categorized into population‐based PRA quartiles previously established and described (Figure 1).19 The primary objective of the study was to determine whether higher levels of PRA were associated with increased likelihood of existing IHD, CHF, and CED. Each morbidity was treated separately for the analyses and thus the different morbidities were not treated as competing risks. Logistic regression models were employed to estimate odds ratios (ORs) for IHD, CHF, and CED for each PRA quartile compared with the lowest quartile (quartile 1). Multivariable adjustments were performed to control for potential confounders of age older than 59 years, sex, black race, diabetes mellitus (DM), and use of antihypertensive medication classes. PRA was also treated as a continuous variable. Medication classes and associations with morbidities were evaluated.
Figure 1.
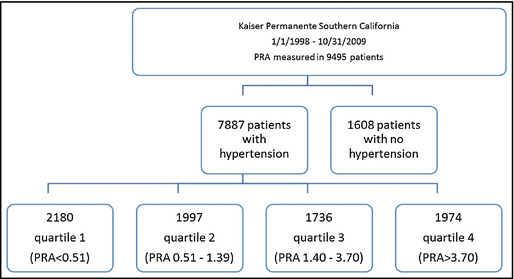
The study cohort was identified from Kaiser Permanente Southern California members aged 18 years and older with diagnosed hypertension and documented plasma renin activity (PRA) and aldosterone measurements. The 7887 individuals who met the inclusion criteria were further categorized by population‐based quartiles based on their PRA values.
Within each PRA quartile, rates of IHD, CHF, and CED and other comorbidities were determined for each quartile and comparisons were made by either chi‐square or Cochran‐Armitage test. Additional data on age, sex, race, and laboratory values were determined among each quartile and trend across the quartiles was investigated.
In secondary analyses, logistic regression models were used to estimate ORs using ARR as the explanatory variable controlling for the same covariates as above.
All analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC).
Results
Cohort Characteristics
A total of 7887 individuals were identified for inclusion in the study cohort. The distribution of the PRA cohort is shown in Figure 2. Overall, values ranged from undetectable to as high as 16.5 ng/mL/h. The median PRA value for the cohort was 1.30 ng/mL/h. The mean BPs in the subset of the cohort who had documented measurements (n=3709) were 141 mm Hg systolic and 79 mm Hg diastolic.27
Figure 2.
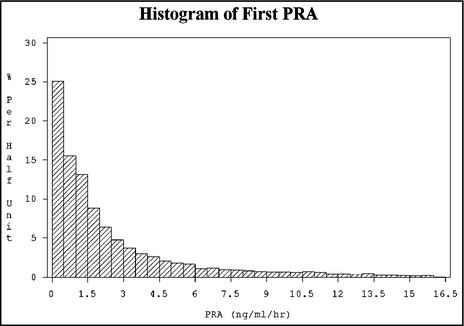
Plasma renin activity (PRA) distribution among the study cohort (N=7887). The median PRA was 1.30 ng/mL/h, with values ranging from undetectable to as high as 16.5 ng/mL/h.
The study population included 59.6% women and was comprised of 29% of patients with DM (Table 1). White patients had the greatest representation at 34.4% followed by blacks (22.7%) and Hispanics (19.0%). Blacks had the greatest proportion in the lowest PRA quartile and their proportion decreased across higher PRA quartiles (29.6%–16.9%). Conversely, whites had the lowest representation in the lowest PRA quartile, which increased with each quartile and had the highest proportion in the highest PRA quartile (31.2%–38.4%). Higher PRA quartiles included younger individuals as demonstrated by a mean age of 55.7 years in the highest quartile compared with a mean age of 60.4 years in the lowest quartile. No meaningful differences between sexes were noted within different PRA quartiles.
Table 1.
Characteristics of Study Cohort by PRA Distribution
| Characteristics | All (N=7887) | Quartile 1 (<0.51) | Quartile 2 (0.51–1.39) | Quartile 3 (1.40–3.70) | Quartile 4 (>3.70) | P Value |
|---|---|---|---|---|---|---|
| (n=2180) | (n=1997) | (n=1736) | (n=1974) | |||
| Aldosterone, ng/dL, median (Q1–Q3) | 10.40 (5.00–19.00) | 11.00 (5.65–19.00) | 9.00 (5.00–16.00) | 10.00 (5.00–18.00) | 12.00 (6.00–23.10) | |
| PRA, ng/mL/h, median (Q1–Q3) | 1.30 (0.43–3.70) | 0.20 (0.19–0.32) | 0.90 (0.74–1.14) | 2.20 (1.80–2.80) | 7.82 (5.10–13.60) | |
| ARR, median (Q1–Q3) | 7.14 (2.40–23.38) | 46.88 (22.11–92.12) | 10.00 (4.62–17.86) | 4.66 (2.27–8.43) | 1.40 (0.56–3.11) | |
| Age, mean (SD), y | 58.0 (15.1) | 60.4 (12.8) | 58.8 (14.8) | 56.8 (16.1) | 55.7 (16.5) | <.001 a |
| Sex | ||||||
| Female, % | 59.6 | 58.2 | 61.2 | 59.0 | 60.0 | .237 b |
| Male, % | 40.4 | 41.8 | 38.8 | 41.0 | 40.0 | |
| Race | ||||||
| White, % | 34.4 | 31.2 | 32.9 | 35.8 | 38.4 | <.001 c |
| Black, % | 22.7 | 29.6 | 24.2 | 18.7 | 16.9 | |
| Hispanic, % | 19.0 | 18.6 | 19.0 | 19.4 | 19.0 | |
| Asian/Pacific, % | 9.3 | 8.3 | 10.6 | 10.4 | 8.1 | |
| Other, % | 14.6 | 12.2 | 13.3 | 15.8 | 17.6 | |
| Diabetes, % | 29.1 | 29.4 | 29.3 | 27.9 | 29.6 | .678 b |
| Ischemic heart disease, % | 22.9 | 24.7 | 21.4 | 22.4 | 22.9 | .080 b |
| Congestive heart failure, % | 9.8 | 9.3 | 9.9 | 9.5 | 10.4 | .645 b |
| Cerebrovascular disease, % | 10.5 | 12.2 | 10.9 | 8.6 | 9.9 | .003 b |
Abbreviations: ARR, aldosterone:PRA ratio; eGFR, estimated glomerular filtration rate; Q, quartile; SD, standard deviation. aTest for linear trend. bCochran‐Armitage trend test. cChi‐square test. Characteristics of the 7887 individuals in the Kaiser Permanente Southern California health system with hypertension and a plasma renin activity (PRA) measurement. The study cohort was categorized and described based on the population distribution quartiles of PRA. The study cohort was racially diverse and had a substantial proportion of patients with diabetes mellitus and cardiovascular disease.
Overall, the study population included 23% IHD, 9.8% CHF, and 10.5% CED patients. CED showed a trend toward higher rates with lower PRA (Table 1).
Among the study cohort, 83% were taking antihypertensive medications (Table 2). Diuretic/natriuretics were the most frequently used medication class, accounting for 70% of the cohort (73% in the highest PRA quartile). RAS blockers (56%) and RAS suppressors (53%) accounted for the next two medication classes that were frequently used.
Table 2.
Antihypertensive Medication Usage
| All (N=7887) | Quartile 1 (<0.51) | Quartile 2 (0.51–1.39) | Quartile 3 (1.40–3.70) | Quartile 4 (>3.70) | P Value | |
|---|---|---|---|---|---|---|
| (n=2180) | (n=1997) | (n=1736) | (n=1974) | |||
| Diuretics/natriuretic, % a | 68 | 70 | 65 | 65 | 70 | <.001 |
| RAS blocker, % b | 54 | 59 | 50 | 48 | 58 | <.001 |
| RAS suppressor, % c | 51 | 68 | 54 | 41 | 39 | <.001 |
| Any medication, % | 83 | 88 | 81 | 80 | 84 | <.001 |
aDiuretics/natriuretic: aldosterone receptor blockers, thiazide diuretics, calcium channel blockers, α‐blockers, and loop diuretics. bRenin‐angiotensin system (RAS) blockers: angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers. cRAS suppressors: β‐receptor blockers, centrally acting α‐antagonists (guanfacine and clonidine) reserpine, methyldopa, and direct renin inhibitors. The majority of the plasma renin activity cohort was taking antihypertensive medication (83%). Diuretic/natriuretics were the most frequently used medication, which was consistent with Kaiser Permanente Southern California hypertension treatment guidelines during the observation period.
Regression Analyses
The crude and multivariable OR for IHD, CHF, and CED using PRA and other explanatory variables are shown in Table 3, Table 4, and Table 5.
Table 3.
Ischemic Heart Disease
| PRA | Crude OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| Quartile 1 | – | – | – | – |
| Quartile 2 | 0.83 (0.72–0.96) | .012 | 0.94 (0.80–1.10) | .410 |
| Quartile 3 | 0.88 (0.76–1.02) | .097 | 1.09 (0.92–1.29) | .314 |
| Quartile 4 | 0.90 (0.78–1.04) | .166 | 1.18 (1.00–1.39) | .047 |
| Every 1‐unit increase in PRA | 1.00 (1.00–1.01) | .173 | 1.01 (1.00–1.02) a | .002 |
| Every 5‐unit increase in PRA | 1.02 (1.00–1.05) | .173 | 1.05 (1.02–1.09) a | <.001 |
| Every 10‐unit increase in PRA | 1.04 (1.00–1.10) | .173 | 1.11 (1.04–1.18) a | <.001 |
| Diabetes | 2.30 (2.06–2.57) | <.001 | 1.77 (1.56–1.99) a | <.001 |
| Age >59 vs 18–59 y | 3.05 (2.72–3.40) | <.001 | 2.46 (2.18–2.77) a | <.001 |
| Male | 1.18 (1.06–1.31) | .003 | 1.28 (1.14–1.44) a | <.001 |
| Black vs non‐black | 0.82 (0.72–0.93) | .002 | 0.88 (0.77–1.00) a | .054 |
| Diuretics/natriuretic | 1.37 (1.21–1.54) | <.001 | 0.91 (0.79–1.05) a | .192 |
| Blocker | 1.58 (1.42–1.76) | <.001 | 1.08 (0.95–1.23) a | .241 |
| Suppressor | 1.96 (1.76–2.19) | <.001 | 1.67 (1.47–1.90) a | <.001 |
Abbreviation: CI, confidence interval. aModeling with plasma renin activity (PRA) as a continuous variable. Estimated odds ratios (ORs) for ischemic heart disease using logistic regression analysis. Crude ORs are listed in the first column and ORs with adjustment for age, sex, black race, diabetes, and medication use are described in the second column.
Table 4.
Congestive Heart Failure
| PRA | Crude OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| Quartile 1 | – | – | – | – |
| Quartile 2 | 1.08 (0.88–1.32) | .477 | 1.23 (0.99–1.53) | .066 |
| Quartile 3 | 1.03 (0.83–1.28) | .799 | 1.27 (1.01–1.61) | .042 |
| Quartile 4 | 1.14 (0.93–1.39) | .226 | 1.41 (1.13–1.77) | .003 |
| Every 1‐unit increase in PRA | 1.01 (1.00–1.02) | .052 | 1.01 (1.00–1.02) a | .030 |
| Every 5‐unit increase in PRA | 1.04 (1.00–1.08) | .052 | 1.05 (1.00–1.09) a | <.001 |
| Every 10‐unit increase in PRA | 1.08 (1.00–1.16) | .052 | 1.09 (1.01–1.18) a | <.001 |
| Diabetes | 2.98 (2.56–3.46) | <.001 | 2.19 (1.86–2.57) a | <.001 |
| Age >59 vs 18–59 y | 2.70 (2.30–3.17) | <.001 | 1.96 (1.66–2.33) a | <.001 |
| Male | 1.38 (1.19–1.60) | <.001 | 1.45 (1.24–1.70) a | <.001 |
| Black vs non‐black | 0.97 (0.82–1.16) | .742 | 1.02 (0.85–1.22) a | .851 |
| Diuretics/natriuretic | 1.77 (1.48–2.13) | <.001 | 1.16 (0.95–1.42) a | .156 |
| Blocker | 2.00 (1.70–2.35) | <.001 | 1.26 (1.05–1.52) a | .012 |
| Suppressor | 1.95 (1.66–2.28) | <.001 | 1.62 (1.35–1.94) a | <.001 |
Abbreviation: CI, confidence interval. aModeling with plasma renin activity (PRA) as a continuous variable. Estimated odds ratios (ORs) for congestive heart failure using logistic regression analysis. Crude ORs are listed in the first column and ORs with adjustment for age, sex, black race, diabetes, and medication use are described in the second column.
Table 5.
Cerebrovascular Disease
| PRA | Crude OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| Quartile 1 | – | – | – | – |
| Quartile 2 | 0.88 (0.73–1.06) | .178 | 0.95 (0.78–1.17) | .638 |
| Quartile 3 | 0.68 (0.55–0.84) | .003 | 0.77 (0.61–0.97) | .024 |
| Quartile 4 | 0.79 (0.65–0.96) | .020 | 0.97 (0.78–1.20) | .759 |
| Every 1‐unit increase in PRA | 0.99 (0.98–1.00) | .036 | 1.00 (0.99–1.01) a | .443 |
| Every 5‐unit increase in PRA | 0.94 (0.89–0.99) | .036 | 0.98 (0.93–1.03) a | .005 |
| Every 10‐unit increase in PRA | 0.89 (0.80–0.99) | .036 | 0.96 (0.86–1.06) a | <.001 |
| Diabetes | 1.63 (1.40–1.89) | <.001 | 1.27 (1.08–1.49) a | .004 |
| Age >59 vs 18–59 y | 2.82 (2.41–3.29) | <.001 | 2.46 (2.08–2.91) a | <.001 |
| Male | 0.90 (0.77–1.04) | .153 | 0.92 (0.78–1.08) a | .290 |
| Black vs non‐black | 0.88 (0.74–1.04) | .133 | 0.92 (0.77–1.10) a | .350 |
| Diuretics/natriuretic | 1.17 (1.00–1.37) | .057 | 0.84 (0.70–1.01) a | .067 |
| Blocker | 1.46 (1.25–1.69) | <.001 | 1.11 (0.93–1.31) a | .252 |
| Suppressor | 1.66 (1.43–1.92) | <.001 | 1.40 (1.18–1.65) a | .001 |
Abbreviation: CI, confidence interval. aModeling with plasma renin activity (PRA) as a continuous variable. Estimated odds ratios (ORs) for cerebrovascular disease using logistic regression analysis. Crude ORs are listed in the first column and ORs with adjustment for age, sex, black race, diabetes, and medication use are described in the second column.
Ischemic Heart Disease
With adjustment for age, sex, black race, DM status, and type of antihypertension medication use, the ORs (95% confidence intervals [CIs]) for IHD were 0.94 (0.80–1.10), 1.09 (0.92–1.29), and 1.18 (1.00–1.39) for PRA quartiles 2, 3, and 4, respectively compared with quartile 1. A linear trend was observed between increasing PRA and presence of IHD wherein each 1‐, 5‐, and 10‐unit increase in PRA was associated with ORs of 1.01 (1.00–1.02), 1.05 (1.02–1.09), and 1.11 (1.04–1.18), respectively (Table 3). Age older than 59 years (2.46 [2.18–2.77]), male sex (1.28 [1.14–1.44]), and presence of DM (1.77 [1.56–1.99]) were associated with IHD. Black race had a lower IHD OR (0.88 [0.77–1.00]) compared with nonblack race. Use of RAS‐suppressing medications was associated with a higher OR for IHD (1.67 [1.47–1.90]) (Table 3, Figure 3).
Figure 3.
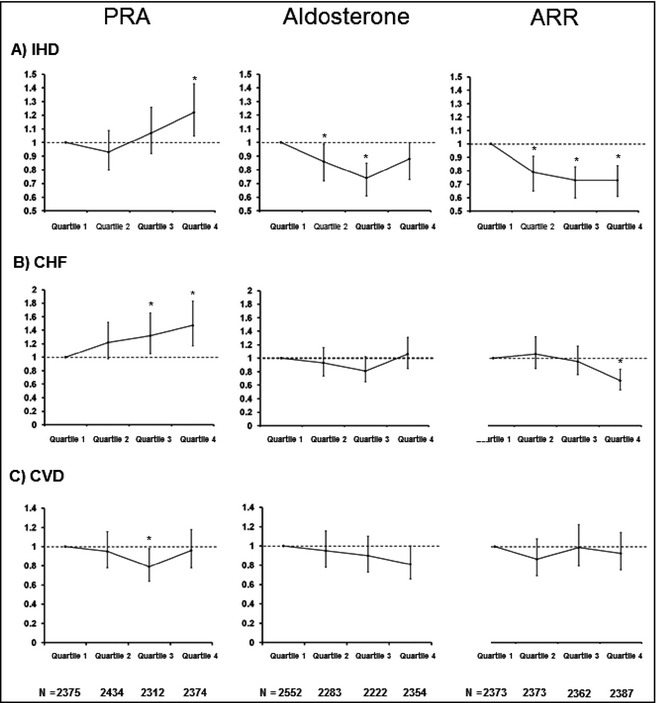
Adjusted odds ratios based on column (1) plasmin renin activity (PRA), column (2) aldosterone, and column (3) aldosterone:renin ratio (ARR). Rows describe the quartiles for (A) ischemic heart disease (IHD), (B) chronic heart failure (CHF), and (C) cerebrovascular disease (CED). The results demonstrate a relationship between higher PRA with IHD and CHF but not CED. ARR demonstrated a similar pattern of association but in an inverse manner. *P<.05.
Congestive Heart Failure
The ORs (95% CIs) for CHF were 1.23 (0.99–1.53), 1.27 (1.01–1.61), and 1.41 (1.13–1.77) for quartiles 2, 3, and 4, respectively, compared with quartile 1. Each 5‐unit increase in PRA was associated with an OR of 1.05 (95 % CI, 1.00–1.09). Age older than 59 years (1.96 [1.66–2.33]), male sex (1.45 [1.24–1.70]), and presence of DM (2.19 [1.86–2.57]) were associated with CHF. In terms of medications, both RAS blocker and RAS suppressor agents were associated with CHF (1.26 [1.05–1.52] and 1.62 [1.35–1.95], respectively (Table 4, Figure 3).
Cerebrovascular Disease
The ORs (95% CIs) for CED were 0.95 (0.78–1.17), 0.77 (0.61–0.97), and (0.97 (0.78–1.20) for quartiles 2, 3, and 4, respectively, compared with quartile 1. Older age (2.46 [2.08–2.91]) and presence of DM (1.27 [1.08–1.49]) were associated with CED. Black race and male sex showed no difference in CED. In terms of medication use, RAS suppressor use was associated with a greater OR for CED (1.40 [1.18–1.65]) (Table 5, Figure 3).
Secondary Analyses
The ORs for IHD, CHF, and CED across quartiles of PRA, serum aldosterone, and ARR are shown in Figure 3. The OR for the three morbidities using ARR as the predictor exhibited a similar trend as PRA but in an inverse manner. Higher ARR was associated with decreased OR for the three morbidities, although the values were not as pronounced as for PRA. Using serum aldosterone alone, there were no significant differences in ORs across the ranges of aldosterone and risk of IHD, CHF, and CED.
Discussion
Summary of findings
In our large ethnically diverse population of more than 7000 hypertensive individuals, we found that higher levels of PRA were associated with prevalent IHD and CHF. For each 5‐unit increase in PRA, there was a 5% increased likelihood for both IHD and CHF. Within our population, we initially observed increased associations at PRA values ≥1.40 ng/mL/h that represented the third quartile. Traditional risk factors including older age, DM, and male sex also demonstrated higher ORs for IHD and CHF. Higher PRA, however, had no association with CED. Black race in our PRA cohort showed lower association with IHD but not CHF or CED. The lower IHD risk may be attributed to the fact that blacks were disproportionately represented in the lower PRA quartiles. When using ARR as the explanatory variable, a similar but inverse trend was observed for IHD and CHF but not for CED throughout the study cohort. Our findings further support the contention that inappropriate upregulation of the RAS may be involved in the pathophysiology and manifestation of CV disease. Our study seeks to examine the possible role of PRA as a biomarker for a sicker population such as patients with prevalent CV disease.
Implications
PRA reflects the state of RAS and may represent a biomarker for adverse biology within certain populations. Physiologically, an increase in RAS maintains “normal BP” by counteracting falls in circulatory volume via an increase in angiotensin II–mediated vasoconstriction.1 Reciprocally, states of volume excess or high BP should lead to suppressed PRA levels. When elevated BP does not suppress PRA, it may represent inappropriate upregulation of renin secretion. The resultant excessive vasoconstriction may lead to vascular injury and poor clinical outcomes. In fact, PRA as a prognosticator for CV and mortality outcomes has been well described.2, 3, 4, 5, 9, 10, 11 We have previously described that elevated PRA levels are also associated with renal disease among a hypertensive and nonhypertensive population.19 Interventional studies that have shown the benefit of targeting RAS support the prominence of RAS's role in vascular diseases and treatment.28, 29, 30, 31 Additionally, reactively high PRA levels induced by excessive diuretic usage are also associated with increased CV mortality.32 Thus, another treatment approach might be able to reduce PRA levels by restoring circulatory volume.
Renin and the use of PRA as a biomarker are more suitable in specific populations. Renin has been examined as a variable to explain various clinical outcomes such as myocardial infarction, stroke, and mortality. Alderman and colleagues4 and Blumenfeld and colleagues8 have found that elevated PRA was associated with higher myocardial infarction rates among hypertensive individuals. Muhlestein and colleagues10 demonstrated that higher PRA was associated with greater rates of myocardial infarction, heart failure, and mortality among a population with known coronary artery disease. Subanalyses of the Heart Outcomes Prevention Evaluation (HOPE) Canadian population and the Valsartan Heart Failure Trial (Val‐HeFT) both revealed that high PRA was a risk for CV mortality.9, 17 Populations with CED have also been described where PRA was associated with worsened risk of CV events.33 Higher PRA among the Framingham offspring population were prone to greater rates of short‐term mortality.7 In contrast, Meade and colleagues6 found no such relationship of PRA and CV outcomes. It is worthy to note that the study population was generally normotensive in the study by Meade and colleagues compared with previous observations on hypertensive and/or known CV disease populations. Similarly, the Anglo‐Scandinavian Cardiac Outcomes Trial (ASCOT) in a hypertensive population did not demonstrate an association with higher baseline PRA and subsequent risk of CV disease and mortality.34 However, the mean BPs at the end of the study were <140/80 mm Hg. Thus, while past PRA outcomes studies appear to be collectively inconsistent, they have been consistently prognostic in patients with known CV disease and risks and have consistently shown lack of predictive value in those without elevated BPs or heart disease.
Potential Limitations
In our study, we attempted to evaluate the value of PRA as a biomarker for CV disease prevalence in a hypertensive population. We hypothesized that inappropriately elevated PRA levels in our hypertensive population would reflect both the existent vascular damage and the underlying role of RAS in CV disease. We report our findings on the assumption that our hypertensive population had elevated BPs where RAS would be expected to be suppressed. Therefore, the fact that BP data were missing for the majority of our study population (61%) confounds the interpretation of our findings and represents an important limitation of our study. While we feel confident in the accuracy of the hypertension cohort identified for our study, uncontrolled or elevated BPs actually represent a small proportion of the KPSC hypertension population.35, 36 Furthermore, causality cannot be inferred from our cross‐sectional observation as morbidities were captured both before and after the PRA measurement. It would be of interest to stratify the population by BPs and determine the prognostic value of PRA on longitudinal CV outcomes. We are currently working to obtain clinical information including BPs on hypertensive individuals with long‐term follow‐up in order to determine the utility of PRA on future CV risks in those with and without elevated BPs.
The potential limitations of our study should further be qualified due to its retrospective observational nature. The findings, though obtained from a real‐world practice environment, may not necessarily represent findings in the entire hypertensive population of KPSC or the general population for that matter. Since PRA and aldosterone are not routine laboratory tests, there is confounding by indication where PRA was measured selectively in certain individuals. The tests were likely ordered based on clinical assessments deemed necessary by practicing clinicians. In addition, the effects of medication use on PRA levels are somewhat underemphasized by our analysis. Most of the medication used to treat hypertension affect the level of PRA.37 We did attempt to account for this by categorizing and controlling for medications according to how they effect PRA levels.1 It is also important to note that angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers significantly lower the effective PRA in vivo,38 which would not be reflected in the measured PRA levels themselves. Given the fact that 54% of the study population was taking an angiotensin‐converting enzyme inhibitor or an angiotensin receptor blocker, the vasculopathic effects or manifestations of upregulated RAS may have been ameliorated to some extent in our study population and thus lowered the actual CV disease rates. To that end, we cannot determine with certainty that all medications were prescribed for the purpose of treating hypertension as certain antihypertensive medications may have been used for other clinical indications such as heart failure, proteinuria, or prostatism.
Strengths
Among the strengths of our study is the large population of hypertensive individuals, which far exceeds any study evaluating PRA and CV disease to date. The study population was sex‐balanced and racially/ethnically representative in the fact that there were ample proportions of minorities including blacks, Hispanics, and Asians. Our findings were drawn from a real‐world practice environment and that aspect may increase the generalizability of our findings.
Conclusions
Higher PRA levels were associated with greater risk for IHD and CHF among a large and ethnically diverse population of hypertensive individuals. However, no association was found for CED. PRA status may reflect the presence of vascular disease in addition to the prognostic potential that has been previously described.
Acknowledgments and Disclosure
The authors would like to thank Federico Calara, PhD, for his valuable contributions in the conceptualization and design of this study. The authors would also like to thank John Laragh, MD, and Jean Sealey, DSc, for their valuable teachings on the physiology of PRA and the medication classifications as it relates to RAS. JJS has previously had research support from Novartis Pharmaceuticals. No other authors have any conflicts of interest relevant to this manuscript. This study was supported by Kaiser Permanente Southern California Regional Research and a research grant by the National Institute of Health grants K24‐DK091419 (KKZ).
J Clin Hypertens (Greenwich). 2014;16:805–813. © 2014 Wiley Periodicals, Inc.
References
- 1. Laragh J. Laragh's lessons in pathophysiology and clinical pearls for treating hypertension. Am J Hypertens. 2001;14(6 Pt 1):491–503. [DOI] [PubMed] [Google Scholar]
- 2. Alderman MH, Madhavan S, Ooi WL, et al. Association of the renin‐sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med. 1991;324:1098–1104. [DOI] [PubMed] [Google Scholar]
- 3. Alderman MH, Cohen HW, Sealey JE, Laragh JH. Plasma renin activity levels in hypertensive persons: their wide range and lack of suppression in diabetic and in most elderly patients. Am J Hypertens. 2004;17:1–7. [DOI] [PubMed] [Google Scholar]
- 4. Alderman MH, Ooi WL, Cohen H, et al. Plasma renin activity: a risk factor for myocardial infarction in hypertensive patients. Am J Hypertens. 1997;10:1–8. [DOI] [PubMed] [Google Scholar]
- 5. Brunner HR, Laragh JH, Baer L, et al. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med. 1972;286:441–449. [DOI] [PubMed] [Google Scholar]
- 6. Meade TW, Cooper JA, Peart WS. Plasma renin activity and ischemic heart disease. N Engl J Med. 1993;329:616–619. [DOI] [PubMed] [Google Scholar]
- 7. Parikh NI, Gona P, Larson MG, et al. Plasma renin and risk of cardiovascular disease and mortality: the Framingham Heart Study. Eur Heart J. 2007;28:2644–2652. [DOI] [PubMed] [Google Scholar]
- 8. Blumenfeld JD, Sealey JE, Alderman MH, et al. Plasma renin activity in the emergency department and its independent association with acute myocardial infarction. Am J Hypertens. 2000;13:855–863. [DOI] [PubMed] [Google Scholar]
- 9. Masson S, Solomon S, Angelici L, et al. Elevated plasma renin activity predicts adverse outcome in chronic heart failure, independently of pharmacologic therapy: data from the Valsartan Heart Failure Trial (Val‐HeFT). J Card Fail. 2010;16:964–970. [DOI] [PubMed] [Google Scholar]
- 10. Muhlestein JB, May HT, Bair TL, et al. Relation of elevated plasma renin activity at baseline to cardiac events in patients with angiographically proven coronary artery disease. Am J Cardiol. 2010;106:764–769. [DOI] [PubMed] [Google Scholar]
- 11. Gonzalez MC, Cohen HW, Sealey JE, et al. Enduring direct association of baseline plasma renin activity with all‐cause and cardiovascular mortality in hypertensive patients. Am J Hypertens. 2011;24:1181–1186. [DOI] [PubMed] [Google Scholar]
- 12. Laragh JH. Vasoconstriction‐volume analysis for understanding and treating hypertension: the use of renin and aldosterone profiles. Am J Med. 1973;55:261–274. [DOI] [PubMed] [Google Scholar]
- 13. Laragh JH, Baer L, Brunner HR, et al. Renin, angiotensin and aldosterone system in pathogenesis and management of hypertensive vascular disease. Am J Med. 1972;52:633–652. [DOI] [PubMed] [Google Scholar]
- 14. Meade T. Review: plasma renin and the incidence of cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2010;11:91–98. [DOI] [PubMed] [Google Scholar]
- 15. Latini R, Masson S, Anand I, et al. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val‐HeFT. Eur Heart J. 2004;25:292–299. [DOI] [PubMed] [Google Scholar]
- 16. Vergaro G, Emdin M. Cardiac angiotensin receptor expression in hypothyroidism: back to fetal gene programme? J Physiol. 2008;586:7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verma S, Gupta M, Holmes DT, et al. Plasma renin activity predicts cardiovascular mortality in the Heart Outcomes Prevention Evaluation (HOPE) study. Eur Heart J. 2011;32:2135–2142. [DOI] [PubMed] [Google Scholar]
- 18. Koebnick C, Langer‐Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sim JJ, Shi J, Calara F, et al. Association of plasma renin activity and aldosterone‐renin ratio with prevalence of chronic kidney disease: the Kaiser Permanente Southern California cohort. J Hypertens. 2011;29:2226–2235. [DOI] [PubMed] [Google Scholar]
- 20. Sim JJ, Bhandari SK, Shi J, et al. Plasma renin activity (PRA) levels and antihypertensive drug use in a large healthcare system. Am J Hypertens. 2012;25:379–388. [DOI] [PubMed] [Google Scholar]
- 21. Bhandari SK, Pashayan S, Liu IL, et al. 25‐hydroxyvitamin D levels and hypertension rates. J Clin Hypertens (Greenwich). 2011;13:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sealey JE. Plasma renin activity and plasma prorenin assays. Clin Chem. 1991;37(10 Pt 2):1811–1819. [PubMed] [Google Scholar]
- 23. Tsutamoto T, Sakai H, Tanaka T, et al. Comparison of active renin concentration and plasma renin activity as a prognostic predictor in patients with heart failure. Circ J. 2007;71:915–921. [DOI] [PubMed] [Google Scholar]
- 24. Campbell DJ, Nussberger J, Stowasser M, et al. Activity assays and immunoassays for plasma renin and prorenin: information provided and precautions necessary for accurate measurement. Clin Chem. 2009;55:867–877. [DOI] [PubMed] [Google Scholar]
- 25. Gordon RD, Wolfe LK, Island DP, Liddle GW. A diurnal rhythm in plasma renin activity in man. J Clin Invest. 1966;45:1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Katz FH, Romfh P, Smith JA. Diurnal variation of plasma aldosterone, cortisol and renin activity in supine man. J Clin Endocrinol Metab. 1975;40:125–134. [DOI] [PubMed] [Google Scholar]
- 27. Noori N, Dukkipati R, Kovesdy CP, et al. Dietary omega‐3 fatty acid, ratio of omega‐6 to omega‐3 intake, inflammation, and survival in long‐term hemodialysis patients. Am J Kidney Dis. 2011;58:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brenner BM, Cooper ME, de ZeeuwD, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 29. Investigators HOPES . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 30. Parving HH, Lehnert H, Brochner‐Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. [DOI] [PubMed] [Google Scholar]
- 31. Wright JT Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. [DOI] [PubMed] [Google Scholar]
- 32. Sealey JE, Alderman MH, Furberg CD, Laragh JH. Renin‐angiotensin system blockers may create more risk than reward for sodium‐depleted cardiovascular patients with high plasma renin levels. Am J Hypertens. 2013;26:727–738. [DOI] [PubMed] [Google Scholar]
- 33. Campbell DJ, Woodward M, Chalmers JP, et al. Prediction of myocardial infarction by N‐terminal‐pro‐B‐type natriuretic peptide, C‐reactive protein, and renin in subjects with cerebrovascular disease. Circulation. 2005;112:110–116. [DOI] [PubMed] [Google Scholar]
- 34. Sever PS, Chang CL, Prescott MF, et al. Is plasma renin activity a biomarker for the prediction of renal and cardiovascular outcomes in treated hypertensive patients? Observations from the Anglo‐Scandinavian Cardiac Outcomes Trial (ASCOT). Eur Heart J. 2012;33:2970–2979. [DOI] [PubMed] [Google Scholar]
- 35. Sim JJ, Bhandari SK, Shi J, et al. Plasma renin activity (PRA) levels and antihypertensive drug use in a large healthcare system. Am J Hypertens. 2011;25:379–388. [DOI] [PubMed] [Google Scholar]
- 36. Sim JJ, Bhandari SK, Shi J, et al. Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clin Proc. 2013;88:1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mulatero P, Rabbia F, Milan A, et al. Drug effects on aldosterone/plasma renin activity ratio in primary aldosteronism. Hypertension. 2002;40:897–902. [DOI] [PubMed] [Google Scholar]
- 38. Sealey JE, Parra D, Rosenstein R, Laragh JH. “Effective” plasma renin activity: a derived measure for assessing residual plasma renin activity in patients taking angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers. Hypertension. 2010;55:e16; author reply e17. [DOI] [PubMed] [Google Scholar]


