Abstract
Objective
High fat diet (HFD) contributes to the increased prevalence of obesity and hyperlipidemia in young adults, a possible cause for their recent increase in stroke. Isoflurane post-treatment provides neuroprotection. We determined whether isoflurane post-treatment induced neuroprotection in HFD-fed mice.
Design and Methods
Six-week old CD-1 male mice were fed HFD or regular diet (RD) for 5 or 10 weeks. Their hippocampal slices (400 µm) were subjected to oxygen-glucose deprivation (OGD). Some slices were exposed to isoflurane for 30 min immediately after OGD. Some mice had a 90-min middle cerebral arterial occlusion and were post-treated with 2% isoflurane for 30 min.
Results
OGD time-dependently induced cell injury. This injury was dose-dependently reduced by isoflurane. The effect was apparent at 1% or 2% isoflurane in RD-fed mice but required 3% isoflurane in HFD-fed mice. HFD influenced the isoflurane effects in DG. OGD increased carboxyl-terminal modulator protein (CTMP), an Akt inhibitor, and decreased Akt signaling. Isoflurane reduced these effects. LY294002, an Akt activation inhibitor, attenuated the isoflurane effects. HFD increased CTMP and reduced Akt signaling. Isoflurane improved neurological outcome in the RD-fed mice but not in the HFD-fed mice.
Conclusions
HFD attenuated isoflurane post-treatment-induced neuroprotection possibly due to decreased prosurvival Akt signaling.
Keywords: Akt, high fat diet, isoflurane, neuroprotection, post-treatment
Introduction
We and others have shown that isoflurane application after brain ischemia reduced ischemic brain injury (1, 2, 3, 4). This phenomenon is called isoflurane post-treatment-induced neuroprotection. However, this effect has only been shown in healthy animals. Brain ischemia/stroke often occurs in patients with co-morbidities and risk factors for stroke. Thus, experts have recommended to test the effectiveness of neuroprotective strategies in animals with co-morbidities and risk factors for stroke in the preclinical stage (5).
Obesity and its associated conditions, such as hyperlipidemia, are significant risk factors for stroke. There is an increased prevalence of obesity in recent years. Now about one third of American adults and 20% of teenagers are obese (6, 7). High fat diet (HFD) contributes to the pandemic of obesity in the USA (8). In fact, increased prevalence of obesity and its associated pathological conditions in young adults is considered a major cause for the increased stroke in this age group in recent years (9, 10). However, there are very few studies on the effectiveness of various neuroprotective strategies under obese condition.
We have shown an important role of activation of Akt (2), a survival protein kinase (11), in the isoflurane post-treatment-induced neuroprotection. Recently, it has been found that increased carboxyl-terminal modulator protein (CTMP) contributes to the ischemic brain cell death/injury (12). CTMP binds and inhibits Akt (13). However, it is not known whether neuroprotective strategies can affect the expression of CTMP.
We designed this study to determine whether isoflurane post-treatment induced neuroprotection in obese mice and whether isoflurane post-treatment affects CTMP expression to provide neuroprotection. We fed 6-week old mice with HFD for 10 weeks to simulate teenager onset obesity in humans. Ex vivo and in vivo studies were performed to determine the effectiveness of isoflurane post-treatment effects in these mice.
Methods
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Virginia, Charlottesville, VA, USA. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23) revised in 2011.
Animals and diet feeding
Male 6-week old CD1 mice were randomly assigned to two dietary groups: animals in the regular diet (RD, 4.5% calories supplied by fat) group and those in the high fat diet (HFD, 45% calories supplied by fat) group received a diet containing 1% cholesterol, 10% egg yolk powder, 5% lard, 0.5% sodium cholate and 83.5% RD for 10 weeks.
Preparation of hippocampal slices
Similar to our previous method (1, 14), hippocampal coronal slices at 400 µm thickness were freshly prepared from the brains of male CD1 mice fed with RD or HFD for 10 weeks. They were placed into a tissue holder and immersed in circulating artificial cerebrospinal fluid (aCSF) continuously bubbled with 5% CO2 and 95% O2 (oxygenated aCSF) at room temperature for at least 1 h for recovery of the synaptic function [4] and then transferred to oxygenated aCSF at 37°C for 15 min before used for experiments. The aCSF contained 116 mM NaCl, 26.2 mM NaHCO3, 5.4 mM KCl, 1.8 mM CaCl2, 0.9 mM MgCl2, 0.9 mM NaH2PO4, and 5.6 mM glucose, pH 7.4.
Oxygen-glucose deprivation (OGD)
Ischemia was simulated in vitro by OGD at 37°C. This was performed as we described previously.1,13 After the OGD, slices were recovered in circulating oxygenated aCSF at 37°C for 5 h to allow cell injury and death that may not be evident immediately after the OGD episode to become apparent.
Isoflurane post-treatment
Isoflurane post-treatment was performed immediately after a 20-min OGD. Isoflurane was delivered by the carrier gases (5% CO2 and 95% O2) through an isoflurane vaporizer. Isoflurane concentrations in the aCSF were determined by gas chromatography as we described before (15, 16, 17). The aqueous isoflurane concentration was approximately 0.22, 0.44 and 0.66 mM, respectively, for 1, 2 and 3% isoflurane in the gas phase.
Administration of LY294002
LY294002, an Akt activation inhibitor, was added to make the final concentration at 50 µM in the aCSF. LY294002 was present for 50 min started immediately before the OGD application.
Quantification of cell injury in hippocampal slices
As we described before (14), cell injury was detected by incubating the slices with fluorescent propidium iodide (PI, 2.3 µM) for 20 min. PI binds to DNA in cells with significant membrane damage and PI binding has been used to measure cell injury in hippocampal slices (14, 18, 19). PI fluorescence emission from the CA1, CA3 and dental gyrus (DG) was viewed using a fluorescence microscope (Nikon, Tokyo, Japan) with a 10 × lens coupled to a camera (Diagnostic Imaging, Spot II, San Carlos, CA). PI image optical density was measured with Image J software (National Institutes of Health, Bethesda, MD). All slices were analyzed at the same time by a blinded observer. For each slice, randomly selected 10 areas from CA1, CA3 and DG were analyzed.
Hippocampal tissue sampling and preparation of cytosolic fraction
Mice on RD or HFD for 5 and 10 weeks were euthanized by 5% isoflurane and transcardially perfused with saline. The hippocampus was harvested. The cytosolic fraction of the whole hippocampus or hippocampal slices after being exposed to various experimental conditions was prepared as we previously described (20, 21). Briefly, brain tissue was placed in a ice-cold buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, and 0.05% NP40; pH 7.9) containing protease and phosphatase inhibitor cocktails and homogenized by 30 strokes of gentle pounding in a glass tissue grinder. Homogenates were centrifuged at 13,000 rpm for 15 min at 4°C. The supernatants were cytosolic fractions.
Western blotting
Proteins of 30 to 50 µg per lane were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene fluoride membrane. Membranes were incubated with the following primary antibodies overnight at 4°C: the anti-CTMP antibody (1:1000, catalog number: SAB3500057; Sigma-Aldrich, St. Louis, MO), anti-Akt antibody (1:1000, catalog number: 9272; Cell Signaling Technology, Danvers, MA), anti-phospho-Akt (Ser473) antibody (1:1000, catalog number: 9271; Cell Signaling Technology), anti-glycogen synthase kinase 3β (GSK-3β) antibody (1:1000, catalog number: 9315; Cell Signaling Technology), anti-phospho-GSK-3β (Ser9) antibody (1:1000, catalog number: 9336, Cell Signaling Technology), anti-Forkhead box O3 (FoxO3a) antibody (1:1000, catalog number: 2497; Cell Signaling Technology), anti-phospho-FoxO3a (Ser253) antibody (1:1000, catalog number: 9466; Cell Signaling Technology), anti-β-actin polyclonal antibody (1:2000; catalog number: A2228; Sigma-Aldrich), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (1:5000, catalog number: G9545; Sigma-Aldrich). The protein bands were visualized with the enhanced chemiluminescence method using a Genomic and Proteomic Gel Documentation Systems from Syngene (Frederick, MD, USA). The densities of interesting protein bands were normalized to those of GAPDH or actin. The phospho-Akt, phospho-GSK-3β and phospho-FoxO3a were normalized by the total Akt, GSK-3β and FoxO3a. The results from animals under various dietary conditions and durations were then normalized by those from animals fed with RD for 5 weeks. The results from hippocampal slices exposed to various experimental conditions were then normalized to the corresponding data of the control slices from the same animals. Samples from the same animals were always run on the same Western blots.
Transient middle cerebral arterial occlusion (MCAO) and the neurological outcome assessment
As we described previously (20), MCAO in mice was achieved by an intraluminal filament under isoflurane anesthesia. The animal was allowed to wake-up immediately after MCAO was achieved. They were re-anesthetized briefly 90 min later to withdraw the suture. Mice assigned randomly to the MCAO only group were allowed to wake-up immediately and then placed in a chamber with pure oxygen for 30 min. Mice in the isoflurane post-treatment group were placed in a box filled with 2% isoflurane in oxygen for 30 min immediately after the onset of reperfusion. During surgery to create MCAO and isoflurane exposure, temporalis muscle temperatures of mice were strictly maintained at 37 ± 0.2°C. Their pulse oximeter oxygen saturation (SpO2) was monitored continuously.
The neurological deficit scores of mice were evaluated 24 h after the MCAO as we described before (3, 20). Mice were then sacrificed by 5% isoflurane. Their brains were cut into 1-mm thick coronal slices. Slices were stained with 1% 2,3,5-triphenyltet-razolium chloride solution to evaluate edema index and infarct volume. The infarct area, the left and right cerebral hemispheres in each brain slice were quantified using Image J. Edema index = right hemisphere volume/left hemisphere volume. Corrected brain infarct volume in percentage = [left hemisphere volume − (right hemisphere volume − right infarction volume)] × 100 /left hemisphere volume.
Statistical analysis
Data are means ± S.E.M. (n ≥ 5 for each experimental condition). Comparison of the data of age-matched RD-mice with those of HFD-fed mice was performed by t-test. Time-course and dose-response data from RD- or HFD-fed mice were analyzed by repeated measures one-way analysis of variance followed by the Tukey test after confirmation of normal distribution of the data or by repeated measures one-way analysis of variance on ranks followed by the Tukey test when the data were not normally distributed. This analysis was chosen because slices from the same mouse were used for various experimental conditions on that day. Comparison of dose-response data of RD-fed mice with those of HFD-fed mice was performed by two-way repeated measures analysis of variance. Other data were analyzed by one-way analysis of variance followed by the Tukey test or by one-way analysis of variance on ranks followed by the Tukey test. P value < 0.05 was considered significant.
Results
HFD feeding significantly increased the body weights
HFD feeding for 5 weeks increased the body weights from 37.2 ± 0.5 g of RD-fed mice to 43.2 ± 1.0 g (n = 6 for each group, P < 0.05). HFD feeding for 10 weeks increased the body weights from 41.0 ± 1.1 g of RD-fed mice to 57 ± 1.3 g (n = 6 for each group, P < 0.05).
Isoflurane post-treatment dose-dependently attenuated OGD-induced cell injury
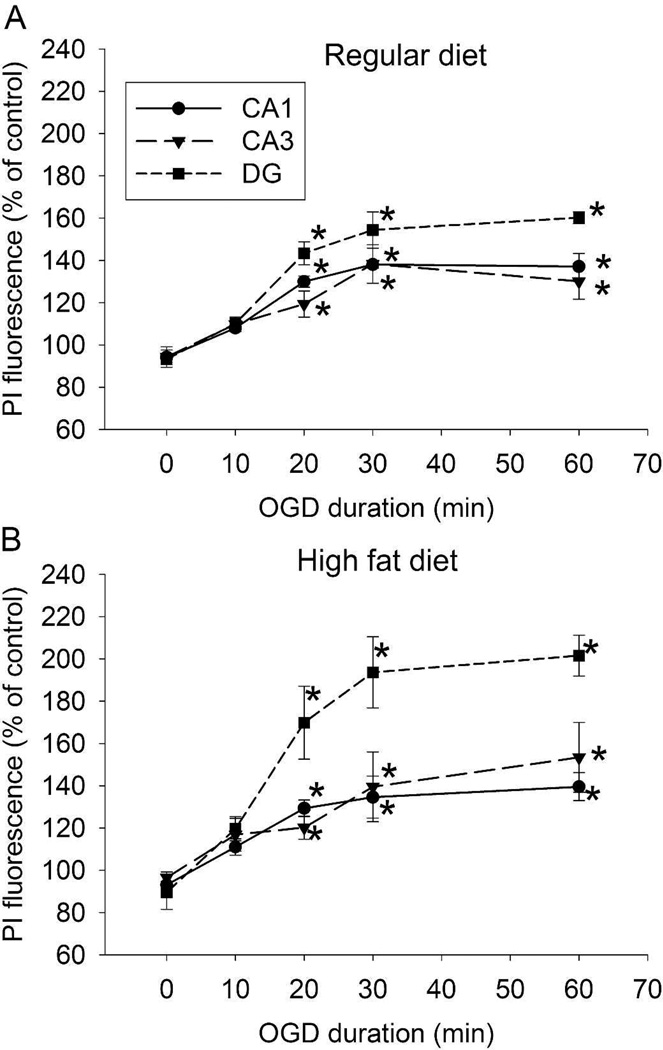
Slices from RD- and HFD-fed mice had increased PI staining in the CA1, CA3 and DG regions after 20-min OGD, suggesting that a 20-min OGD causes significant cell injury. The injury in RD- and HFD-fed mouse slices was maximized by 30-min OGD (Figs. 1A and 1B). Thus, a 20-min OGD was used in other experiments.
Fig. 1.
Time-course of OGD. Hippocampal slices were freshly prepared from 16-week old CD-1 mice that fed HFD or RD for 10 weeks. The slices were subjected to various lengths of OGD and then PI staining at 5 h after the OGD. A: data of RD-fed mice. B: data of HFD-fed mice. Results are mean ± S.E.M. (n = 5). * P < 0.05 compared with control.
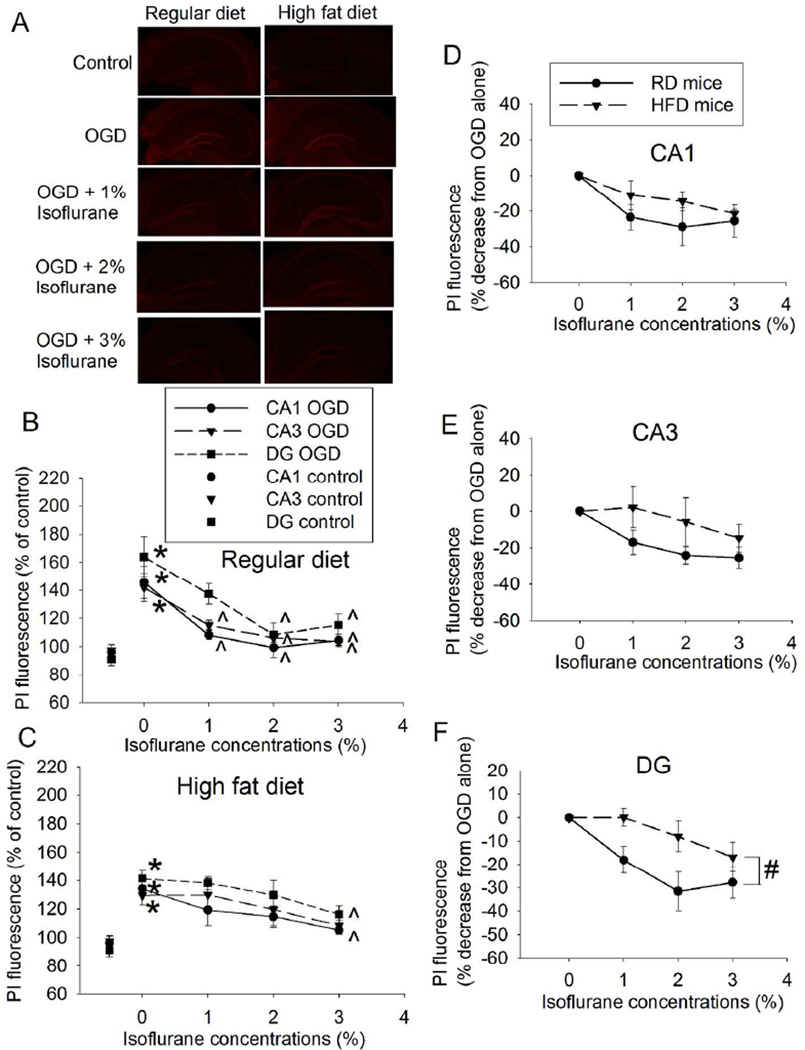
Isoflurane post-treatment dose-dependently reduced injury in the hippocampal slices of RD- and HFD-fed mice. The effects were significant at 1% isoflurane in the CA1 and CA3 and at 2% isoflurane in the DG of RD-fed mice (Figs. 2A and 2B). However, the protective effects in the hippocampal slices of HFD-fed mice were not significant until 3% isoflurane (Figs. 2A and 2C). These results suggest that isoflurane post-treatment-induced neuroprotection is easier to be induced in the RD-fed mouse hippocampal slices than that in the HFD-fed mouse slices. To further determine the role of HFD feeding in the isoflurane effect, isoflurane post-treatment-induced percentage decrease of OGD-caused cell injury was analyzed. HFD had a significant effect on isoflurane post-treatment-induced protection in DG [F(1,8) = 5.501, P = 0.047], while the HFD effect in the CA1 and CA3 did not reach significance yet [F(1,8) = 1.379, P = 0.274 for CA1; F(1,8) = 2.378, P = 0.162 for CA3] (Figs. 2D to 3F). Together, these results suggest that HFD attenuates isoflurane post-treatment-induced neuroprotection, especially in the DG.
Fig. 2.
Dose-response of isoflurane post-treatment-induced neuroprotection in hippocampal slices. Slices were freshly prepared from 16-week old CD-1 mice that fed HFD or RD for 10 weeks. The slices were subjected to a 20-min OGD and then exposed to various concentrations of isoflurane for 30 min. PI staining was performed at 5 h after the OGD. A: representative images of PI staining. B: data of RD-fed mice. C: data of HFD-fed mice. Based on the data presented in panels B and C, the isoflurane post-treatment-induced percentage decrease of PI fluorescence intensity in the presence of OGD was calculated with the following formula: (X – Y) 100/Y. X was the PI fluorescence intensity of the slices under any experimental condition. Y is the fluorescence intensity of the slices with OGD only. The slices to provide X and Y were from the same mouse. Those results are presented in panels D to F. D: CA1 region. E: CA3 region. F: DG region. Results are mean ± S.E.M. (n = 5). * P < 0.05 compared with control; ^ P < 0.05 compared with OGD; # P < 0.05 for the comparison between RD- and HFD-fed mice by two-way repeated measures analysis of variance.
Fig. 3.
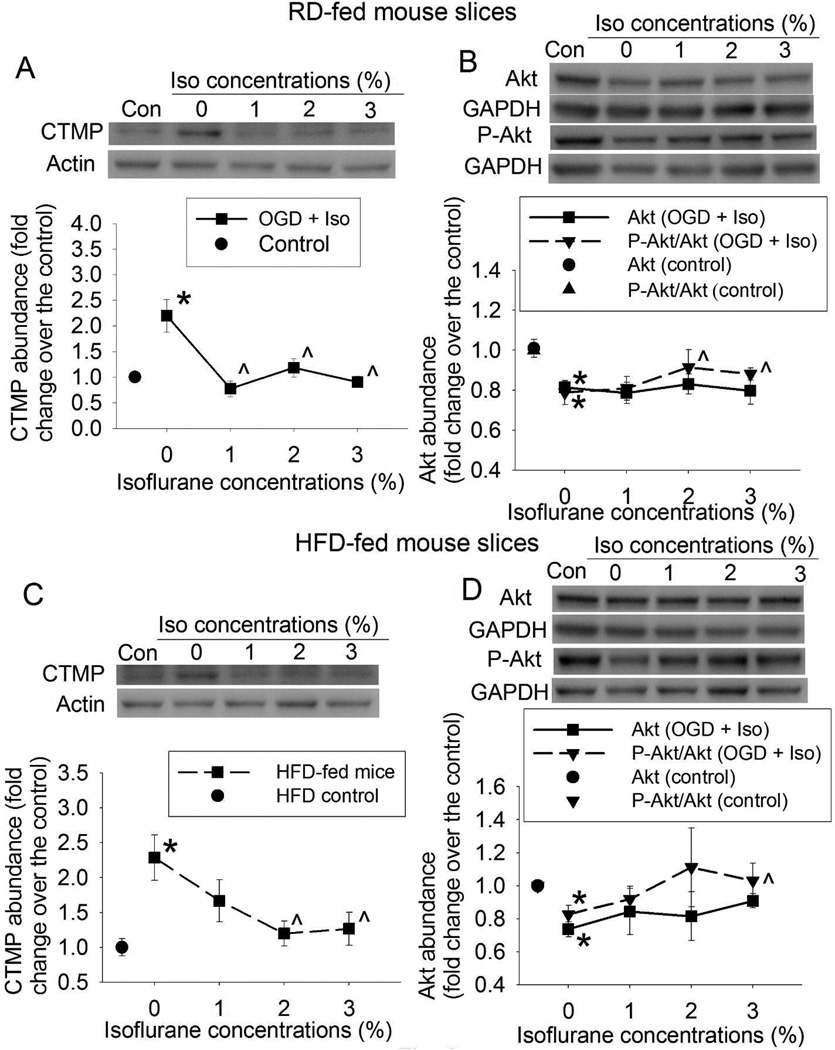
Attenuation of OGD-induced inhibition of Akt signaling by isoflurane. Slices were freshly prepared from 16-week old CD-1 mice that fed HFD or RD for 10 weeks. The slices were subjected to a 20-min OGD and then exposed to various concentrations of isoflurane for 30 min. Slices were harvested at 5 h after the OGD for Western blotting. A representative Western blotting image is presented at the top panel and the pooled results are presented in the bottom panel for each protein. A: CTMP from RD-fed mice. B: Akt and phospho-Akt (P-Akt) from RD-fed mice. C: CTMP from HFD-fed mice. D: Akt and P-Akt from HFD-fed mice. Results are mean ± S.E.M. (n = 6). * P < 0.05 compared with control; ^ P < 0.05 compared with OGD. Con: control, Iso: isoflurane.
Isoflurane post-treatment attenuated OGD-induced reduction of Akt signaling
We focused on Akt signaling, a prosurvival pathway (11), for our mechanism study. OGD significantly increased CTMP and decreased phospho-Akt, the active form of Akt, in the hippocampal slices of RD- and HFD-fed mice (Figs. 3A to 3D). These changes were dose-dependently attenuated by isoflurane. Similar to the protective effects, 1% or 2% isoflurane significantly attenuated the OGD-induced increase of CTMP and decrease of phospho-Akt in the RD-fed mouse slices (Figs. 3A and 3B) but 2% or 3% isoflurane was needed to achieve these effects in the HFD-fed mouse slices (Figs. 3C and 3D). OGD significantly decreased the expression of total Akt in the hippocampus of RD-fed mice and HFD-fed mice (Figs. 3B and 3D). This effect was not changed by isoflurane.
HFD feeding inhibited Akt signaling
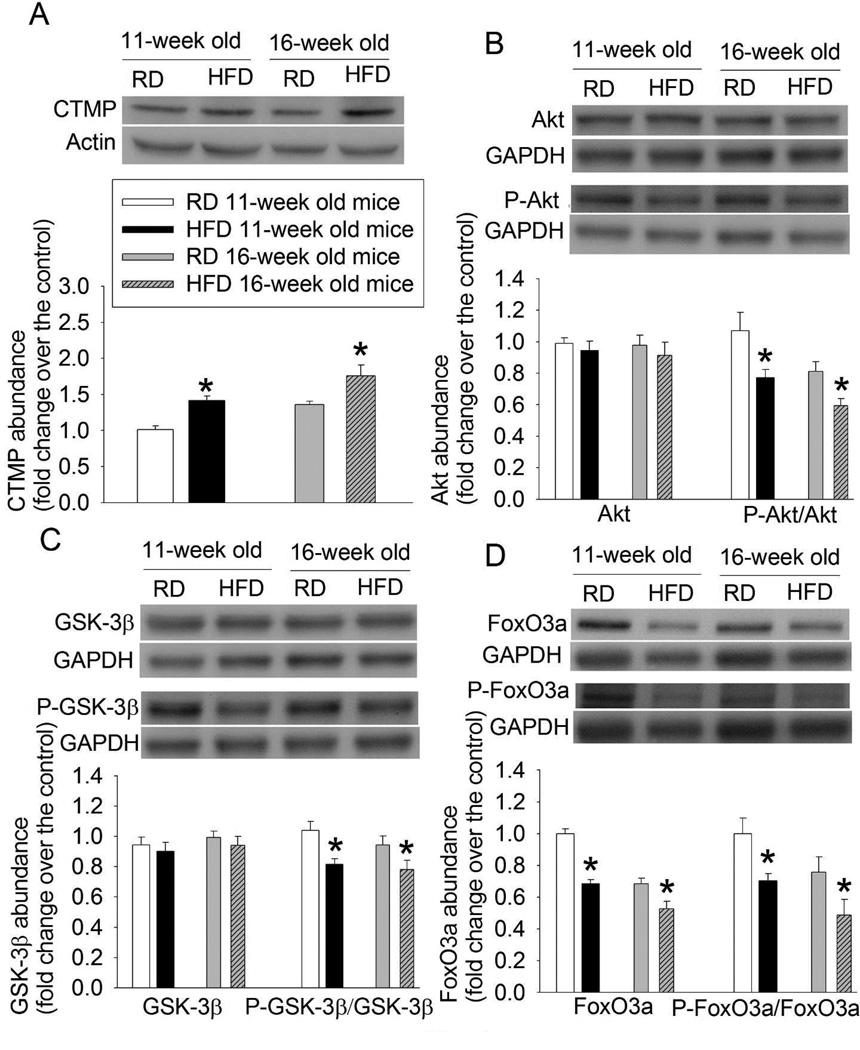
HFD feeding for 5 or 10 weeks significantly increased CTMP expression and decreased the amount of phospho-Akt (Figs. 4A and 4B). The amount of phospho-GSK-3β at Ser9 and phospho-FoxO3a, two Akt target proteins, was reduced by HFD feeding (Figs. 4C and 4D). HFD feeding did not affect Akt and GSK-3β expression but decreased FoxO3a. When the amount of phospho-FoxO3a was normalized by that of FoxO3a, 5- and 10-week HFD feeding still reduced the amount of phospho-FoxO3a (Fig. 4D).
Fig. 4.
HFD-induced reduction of Akt signaling. Hippocampus was harvested from 11- or 16-week old CD-1 mice that fed HFD or RD for 5 or 10 weeks, respectively. The hippocampus was used for Western blotting. A representative Western blotting image is presented at the top panel and the pooled results are presented in the bottom panel for each protein. A: CTMP. B: Akt and phospho-Akt (P-Akt). C: GSK-3β and phospho-GSK-3β (P-GSK-3β). D: FoxO3a and phospho-FoxO3a (P-FoxO3a). Results are mean ± S.E.M. (n = 6 – 8). * P < 0.05 compared with age-matched RD-fed mice.
Inhibition of Akt signaling reduced isoflurane post-treatment-induced neuroprotection
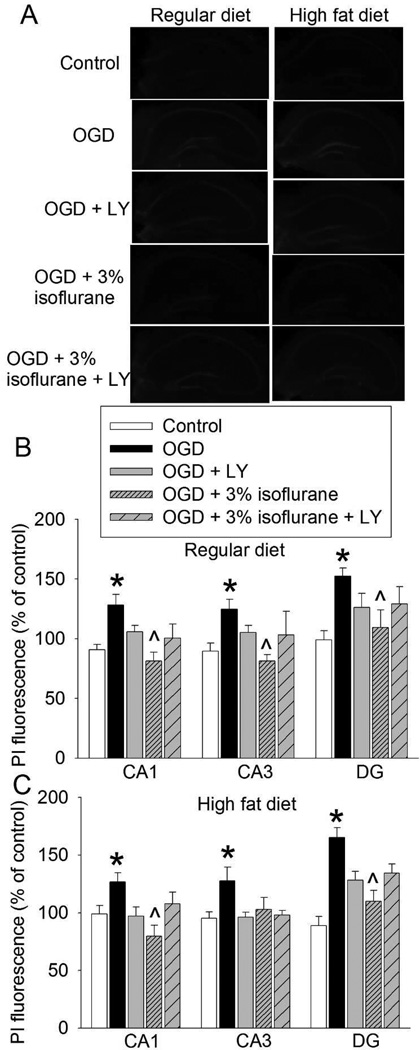
To determine the role of Akt in the isoflurane post-treatment-induced neuroprotection, the Akt activation inhibitor LY294002 was used. Similar to the above results, 3% isoflurane reduced OGD-induced cell injury in the CA1, CA3 and DG of the hippocampal slices from RD-fed mice. This reduction was attenuated by LY294002 (Figs. 5A and 5B). Also, 3% isoflurane decreased OGD-induced cell injury in the CA1 and DG of HFD-fed mice. This decrease was attenuated by LY294002 (Figs. 5A and 5C). The reduction of cell injury in the CA3 of HFD-fed mice by isoflurane did not reach statistical significance in this set of experiment. The effect of LY294002 on this reduction was also not apparent (Fig. 5C).
Fig. 5.
Attenuation of isoflurane post-treatment-induced neuroprotection by inhibition of Akt activation. Hippocampal slices were freshly prepared from 16-week old CD-1 mice that fed HFD or RD for 10 weeks. The slices were subjected to a 20-min OGD and then exposed to 3% isoflurane for 30 min. LY 294002 (LY) was presented from the beginning of OGD to the end of isoflurane exposure. PI staining was performed at 5 h after the OGD. A: representative images of PI staining. B: data of RD-fed mice. C: data of HFD-fed mice. Results are mean ± S.E.M. (n = 5). * P < 0.05 compared with control; ^ P < 0.05 compared with OGD.
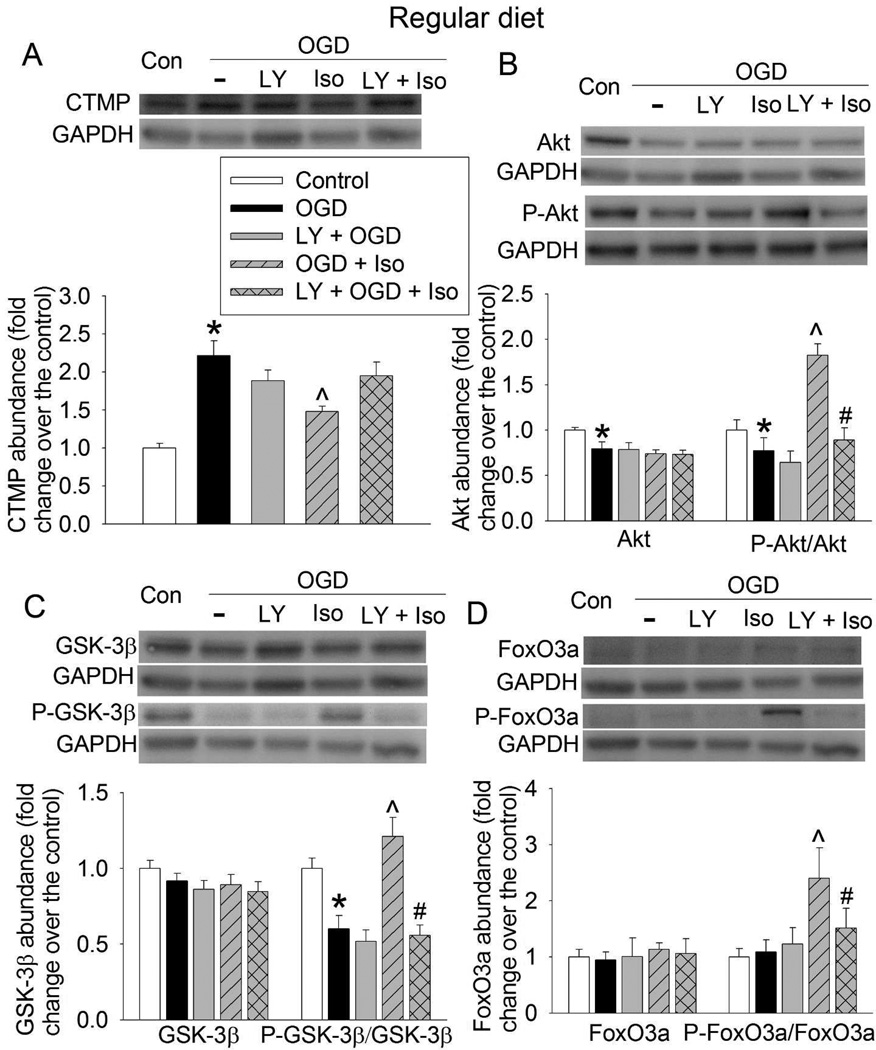
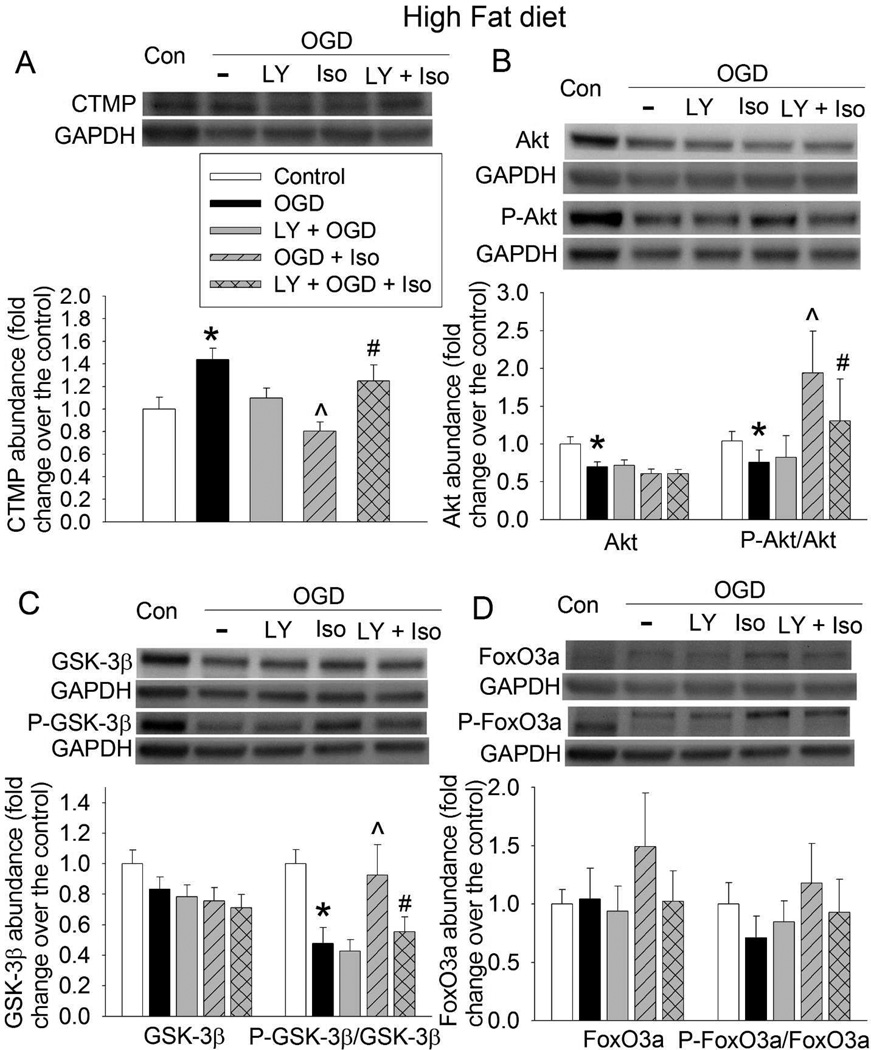
Consistent with cell injury results, OGD increased CTMP expression and decreased the amount of phospho-Akt and phospho-GSK-3β in the hippocampal slices of RD- and HFD-fed mice. These changes were reduced by 3% isoflurane post-treatment. These isoflurane effects were attenuated by LY294002 (Figs. 6 and 7). While OGD did not affect the amount of phospho-FoxO3a, isoflurane significantly increased phospho-FoxO3a in the hippocampus of RD-fed mice. Similar effect on phospho-FoxO3a was not shown in the HFD-fed mouse hippocampus (Figs. 6D and 7D). OGD significantly reduced Akt in the RD- and HFD-fed mouse hippocampus and this effect was not affected by isoflurane or LY294002 (Figs. 6B and 7B). Overall, these results suggest that LY294002 inhibits isoflurane post-treatment-preserved activation of Akt signaling after OGD in both RD- and HFD-fed mouse hippocampal slices.
Fig. 6.
Attenuation of isoflurane post-treatment-induced Akt signaling by inhibition of Akt activation in RD-fed mouse hippocampus. Hippocampal slices were freshly prepared from 16-week old CD-1 mice that fed RD. The slices were subjected to a 20-min OGD and then exposed to 3% isoflurane (Iso) for 30 min. LY 294002 (LY) was presented from the beginning of OGD to the end of isoflurane exposure. Slices were harvested at 5 h after the OGD for Western blotting. A representative Western blotting image is presented at the top panel and the pooled results are presented in the bottom panel for each protein. A: CTMP. B: Akt and phospho-Akt (P-Akt). C: GSK-3β and phospho-GSK-3β (P-GSK-3β). D: FoxO3a and phospho-FoxO3a (P-FoxO3a). Results are mean ± S.E.M. (n = 8). * P < 0.05 compared with control; ^ P < 0.05 compared with OGD; # P < 0.05 compared with OGD plus isoflurane post-treatment.
Fig. 7.
Attenuation of isoflurane post-treatment-induced Akt signaling by inhibition of Akt activation in HFD-fed mouse hippocampus. Hippocampal slices were freshly prepared from 16-week old CD-1 mice that fed HFD for 10 weeks. The slices were subjected to a 20-min OGD and then exposed to 3% isoflurane (Iso) for 30 min. LY 294002 (LY) was presented from the beginning of OGD to the end of isoflurane exposure. Slices were harvested at 5 h after the OGD for Western blotting. A representative Western blotting image is presented at the top panel and the pooled results are presented in the bottom panel for each protein. A: CTMP. B: Akt and phospho-Akt (P-Akt). C: GSK-3β and phospho-GSK-3β (P-GSK-3β). D: FoxO3a and phospho-FoxO3a (P-FoxO3a). Results are mean ± S.E.M. (n = 8). * P < 0.05 compared with control; ^ P < 0.05 compared with OGD; # P < 0.05 compared with OGD plus isoflurane post-treatment.
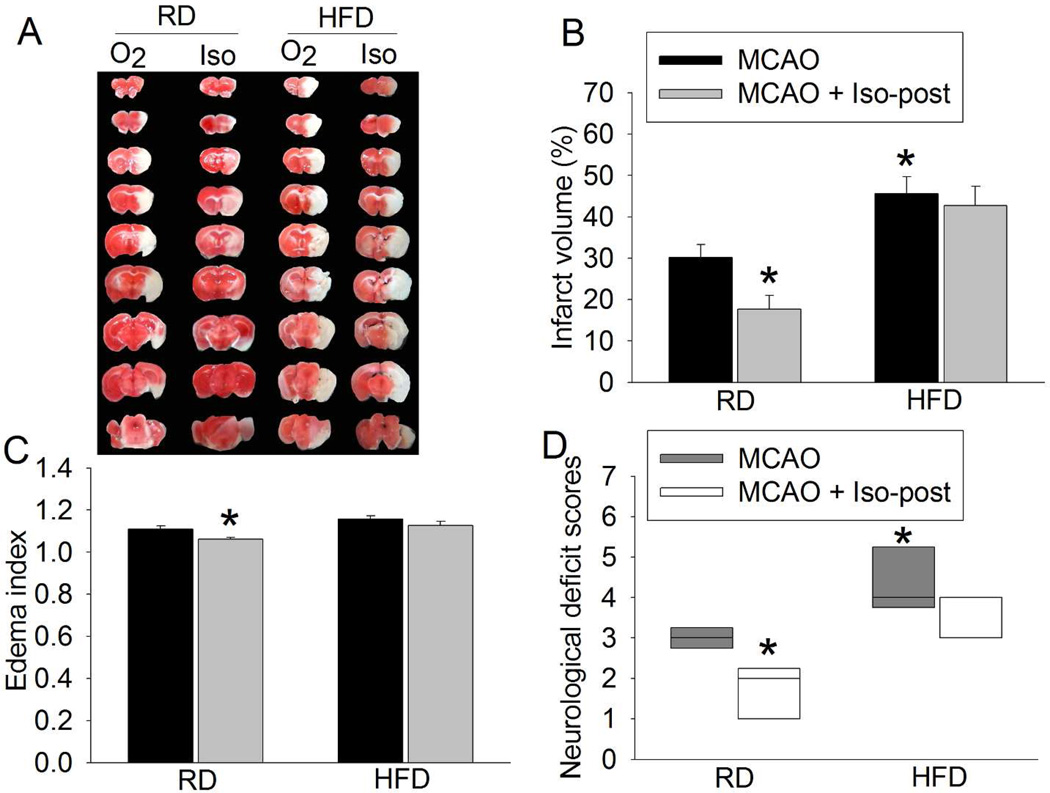
Isoflurane post-treatment induced neuroprotection after brain ischemia in RD-fed mice but not in HFD-fed mice
No animals had an episode of hypoxia (SpO2 < 90%) during the surgery to create MCAO and the isoflurane post-treatment period. Post-treatment with 2% isoflurane reduced brain infarct volume and edema and improved neurological deficit scores after MCAO in the RD-fed mice (Figs. 8A – 8D), suggesting that post-treatment with 2% isoflurane provides neuroprotection in the RD-fed mice. However, 2% isoflurane neither reduced infarct volume and brain edema nor improved the neurological deficit scores of HFD-fed mice (Figs. 8A – 8D). Consistent with our previous study (22), HFD-fed mice had a bigger brain infarct volume and worse neurological deficit scores than RD-fed mice (Figs. 8A – 8D).
Fig. 8.
Isoflurane post-treatment-induced neuroprotection under in vivo condition. Sixteen-week old CD-1 mice that fed HFD or RD for 10 weeks were subjected to a 90-min right MCAO. Mice in the isoflurane post-treatment group were exposed to 2% isoflurane for 30 min immediately after the MCAO (Iso-post). The neurological outcome was evaluated at 24 h after the MCAO. A: representative brain slices stained by 2,3,5-triphenyltet-razolium chloride. B: Infarct volume. C: edema index. D: neurological deficit score. Results are mean ± S.E.M. for infarct volume and edema index and in box plot for neurological deficit scores (n = 6 – 7). * P < 0.05 compared with MCAO group of the RD-fed mice.
Discussion
Consistent with our previous studies in rats (1, 3), this study showed that isoflurane post-treatment induced neuroprotection. Isoflurane at 1 or 2% provided neuroprotection in all three hippocampal brain regions of RD-fed mice. However, 3% isoflurane was needed to induce neuroprotection in the hippocampus of HFD-fed mice. Also, HFD was a significant factor to influence the isoflurane post-treatment effect in the DG region. In addition, post-treatment with 2% isoflurane improved neurological outcome after a 90-min MCAO in the RD-fed mice but not in the HFD-fed mice. These results suggest that isoflurane post-treatment-induced neuroprotection was attenuated in the HFD-fed obese mice.
Our previous study has suggested a role of Akt in the isoflurane post-treatment-induced neuroprotection (2). Consistently, we showed here that isoflurane post-treatment increased the phosphorylated/activated Akt and that LY294002, an inhibitor of Akt activation, attenuated isoflurane post-treatment-induced neuroprotection. Also, isoflurane inhibited OGD-induced CTMP increase, which extends the isoflurane effects on Akt signaling to events upstream of Akt. This effect on CTMP expression, together with the increased phosphorylated/activated Akt, lead to the increased phosphorylation of GSK-3β at Ser9 and FoxO3a, two proteins downstream of Akt (12, 23). Phosphorylation of GSK-3β at Ser9 inhibits GSK-3β, which reduces the formation of mitochondrial permeability transition pore and the mitochondrial membrane permeation (2, 24). Phosphorylation of FoxO3a induces its translocation away from nuclei, which reduces the capability of FoxO3a to induce proapoptotic protein expression (23). These effects after isoflurane post-treatment result in reduced cell injury/death. Thus, our current study provides evidence to suggest events/proteins upstream (CTMP) and downstream (GSK-3β and FoxO3a) of Akt for isoflurane post-treatment-induced neuroprotection.
The isoflurane concentrations to induce neuroprotection are similar to those to cause changes in the amount of CTMP and phospho-Akt in the RD- and HFD-fed mice, respectively. Also, we showed for the first time that mice fed with HFD for 5 or 10 weeks had higher CTMP levels and lower levels of phosphorylated Akt, GSK-3β at Ser9 and FoxO3a than the age-matched mice on RD. These novel results suggest that HFD decreases the prosurvival Akt signaling in the brain. These biochemical changes may explain the phenomenon that a higher isoflurane concentration is needed in the HFD-fed mice to increase the Akt signaling to a threshold for neuroprotection than that needed for RD-fed mice.
A previous study has shown that brain ischemia increases CTMP in rat hippocampus (12). We showed here that OGD increased CTMP in the hippocampal slices. However, phosphor-Akt was increased but the phosphorylation of GSK-3β at Ser9 and FoxO3a was not changed in the ischemic hippocampus in the previous study (12). The authors consider that this situation (increased Akt phosphorylation but no change in the phosphorylation of its target proteins) may be due to the inhibition of CTMP on Akt activity. Our study showed that OGD inhibited the phosphorylation of Akt. This decreased phosphorylated/activated Akt, together with increased CTMP, lead to decreased phosphorylation of GSK-3β at Ser9 and FoxO3a in our study. The reasons for the different findings on Akt signaling in the previous study and our study are not known. Different models (OGD vs. brain global ischemia) and species (mice vs. rats) may contribute to these different findings.
We used LY294002 to imply the involvement of Akt in the isoflurane post-treatment-induced neuroprotection. It is not surprised to see that LY294002 reduced the isoflurane post-treatment-induced increase of phosphorylated Akt, GSK-3β at Ser9 and FoxO3a because LY294002 is a known Akt activation inhibitor (2). Demonstration of these intended effects facilitates the interpretation of the effects of LY294002 on isoflurane post-treatment-induced neuroprotection. LY294002 also attenuated isoflurane post-treatment-induced inhibition of CTMP increase in the ischemic hippocampus. This effect, coupled with the inhibition of Akt activation, should significantly decrease Akt activity. Consistent with this possibility, isoflurane post-treatment-induced phosphorylation of GSK-3β at Ser9 and FoxO3a was significantly inhibited by LY294002.
The mechanisms for the effects of LY294002 on CTMP expression are not known. One possibility is that CTMP expression is regulated by Akt signaling. In supporting this possibility, isoflurane post-treatment increased Akt phosphorylation and also decreased CTMP expression. Similarly, OGD decreased Akt phosphorylation but increased CTMP expression. These three lines of evidence suggest a reciprocal relationship between CTMP and Akt phosphorylation/activation. Further studies are needed to determine the mechanisms for this relationship.
Hippocampal CA1 region is known to be very sensitive to ischemia (25). In our study, we measured cell injury in CA1, CA3 and DG to determine whether there was a difference in cell damage and isoflurane effects among these three regions. We showed that cell damage in the DG was as severe as in CA1 or might be more severe than that in CA1. Results similar to our findings have been reported in previous studies in which rat hippocampal slices were subjected to hypoxia or OGD (18, 19). The reasons for this different sensitivity among hippocampal regions are not known yet.
Despite variation in cell types and possible difference in sensitivity to ischemia among the regions of hippocampus, we used whole hippocampus for Western analysis. This practice was chosen because harvesting enough region-specific tissues with great accuracy in the hippocampal slices after incubation is very difficult. Dissecting specific hippocampal regions under microscope is time-consuming and may introduce selection bias. In addition, 3% isoflurane induced a postconditioning effect in all hippocampal regions. This concentration was used in the experiments to prepare tissues for Western blotting.
We used isoflurane to anesthetize mice for brain harvesting (~2 min exposure). This exposure time in the in vitro experiment may be too short to induce a protective effect (26).
In our in vivo study, we used 2% isoflurane that failed to induce neuroprotection in the HFD-fed mice. This in vivo result is consistent with our ex vivo hippocampal slices subjected to the OGD. Post-treatment with 3% isoflurane resulted in neuroprotection in the hippocampal slices from HFD-fed mice. However, it is difficult to use this high concentration of isoflurane to postcondition the mice due to the concerns of respiratory and circulatory inhibition.
In summary, we have shown that isoflurane post-treatment-induced neuroprotection is attenuated in the HFD-fed mice. Reducing CTMP expression and activating Akt signaling play an important role in the isoflurane post-treatment-induced neuroprotection in both RD-fed and HFD-fed mice. Decreased Akt signaling under baseline condition may contribute to the attenuated isoflurane post-treatment-induced neuroprotection.
What is already known about this subject?
Isoflurane post-treatment induces neuroprotection in healthy rodents
Isoflurane post-treatment-induced neuroprotection may be mediated by Akt.
Carboxyl-terminal modulator protein is an Akt inhibitor
What does this study add?
High fat diet (HFD) feeding decreases Akt signaling
Isoflurane post-treatment-induced neuroprotection is attenuated in HFD-fed mice
Attenuated Akt signaling contributes to decreased isoflurane post-treatment effects in the HFD-fed mice
Acknowledgements
This study was supported by grants (R01 GM065211 and R01 GM098308 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland, and the Robert M. Epstein Professorship endowment, University of Virginia.
ZZ conceived the idea of the study. HY, JD and ZZ designed the study. HY and JD carried out the experiments. HY, JD and ZZ analyzed the data. HY and JD drafted the methods and materials section. ZZ wrote the manuscript. All authors had final approval of the submitted manuscript.
Footnotes
Competing interests: the authors have no competing interests.
References
- 1.Lee JJ, Li L, Jung H-H, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–1062. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L, Zuo Z. Isoflurane postconditioning induces neuroprotection via Akt activation and attenuation of increased mitochondrial membrane permeability. Neurosci. 2011;199:44–50. doi: 10.1016/j.neuroscience.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Yin J, Li L, Deng J, Feng C, Zuo Z. Isoflurane postconditioning reduces ischemia-induced nuclear factor-kappaB activation and interleukin 1beta production to provide neuroprotection in rats and mice. Neurobiol Dis. 2013;54:216–224. doi: 10.1016/j.nbd.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Lekic T, Fathali N, Ostrowski RP, Martin RD, Tang J, et al. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke. 2010;41:1521–1527. doi: 10.1161/STROKEAHA.110.583757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 8.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Sarnowski B, Putaala J, Grittner U, Gaertner B, Schminke U, Curtze S, et al. Lifestyle Risk Factors for Ischemic Stroke and Transient Ischemic Attack in Young Adults in the Stroke in Young Fabry Patients Study. Stroke. 2013;44:119–125. doi: 10.1161/STROKEAHA.112.665190. [DOI] [PubMed] [Google Scholar]
- 10.George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann Neurol. 2011;70:713–721. doi: 10.1002/ana.22539. [DOI] [PubMed] [Google Scholar]
- 11.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 12.Miyawaki T, Ofengeim D, Noh KM, Latuszek-Barrantes A, Hemmings BA, Follenzi A, et al. The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci. 2009;12:618–626. doi: 10.1038/nn.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maira SM, Galetic I, Brazil DP, Kaech S, Ingley E, Thelen M, et al. Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science. 2001;294:374–380. doi: 10.1126/science.1062030. [DOI] [PubMed] [Google Scholar]
- 14.Zhao P, Huang Y, Zuo Z. Opioid preconditioning induces opioid receptor-dependent delayed neuroprotection against ischemia in rats. J Neuropathol Exp Neurol. 2006;65:945–952. doi: 10.1097/01.jnen.0000235123.05677.4b. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Lee J, Jung H, Zuo Z. Pretreatment with volatile anesthetics, but not with the nonimmobilizer 1,2-dichlorohexafluorocyclobutane, reduced cell injury in rat cerebellar slices after an in vitro simulated ischemia. Brain Res. 2007;1152:201–208. doi: 10.1016/j.brainres.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung HH, Lee JJ, Washington JM, Zuo Z. Inability of volatile anesthetics to inhibit oxygen-glucose deprivation-induced glutamate release via glutamate transporters and anion channels in rat corticostriatal slices. Brain Res. 2008;1227:234–239. doi: 10.1016/j.brainres.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo Z, Johns RA. Halothane, enflurane, and isoflurane do not affect the basal or agonist-stimulated activity of partially isolated soluble and particulate guanylyl cyclases of rat brain. Anesthesiology. 1995;83:395–404. doi: 10.1097/00000542-199508000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Gray JJ, Bickler PE, Fahlman CS, Zhan X, Schuyler JA. Isoflurane neuroprotection in hypoxic hippocampal slice cultures involves increases in intracellular Ca2+ and mitogen-activated protein kinases. Anesthesiology. 2005;102:606–615. doi: 10.1097/00000542-200503000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan BL, Leu D, Taylor DM, Fahlman CS, Bickler PE. Isoflurane prevents delayed cell death in an organotypic slice culture model of cerebral ischemia. Anesthesiology. 2002;96:189–195. doi: 10.1097/00000542-200201000-00033. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab. 2011;31:1283–1292. doi: 10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Wang Z, Zuo Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS One. 2013;8:e66069. doi: 10.1371/journal.pone.0066069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng J, Feng J, Feng C, Xiong L, Zuo Z. Critical role of matrix metalloprotease-9 in chronic high fat diet-induced cerebral vascular remodeling and increase of ischemic brain injury in mice. Cardiovasc Res. 2014 doi: 10.1093/cvr/cvu154. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 24.Lin D, Li G, Zuo Z. Volatile anesthetic post-treatment induces protection via inhibition of glycogen synthase kinase 3beta in human neuron-like cells. Neurosci. 2011;179:73–79. doi: 10.1016/j.neuroscience.2011.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 26.Zheng S, Zuo Z. Isoflurane preconditioning reduces Purkinje cell death in an in vitro model of rat cerebellar ischemia. Neurosci. 2003;118:99–106. doi: 10.1016/s0306-4522(02)00767-4. [DOI] [PubMed] [Google Scholar]