Abstract
Introduction
It is conceivable that overstimulation of chemo- and mechano-sensors in the Roux and common limbs by uncontrolled influx of undigested nutrients after Roux-en-Y gastric bypass surgery (RYGB) could lead to exaggerated satiety signaling via vagal afferents and contribute to body weight loss.
Purpose
Because previous clinical and preclinical studies using vagotomy came to different conclusions, the aim was to examine the effects of selective and histologically verified celiac branch vagotomy on reduced food intake and body weight loss induced by RYGB.
Materials and Methods
Male Sprague-Dawley rats underwent either RYGB + celiac branch vagotomy (RYGB/VgX, n = 13 ), RYGB + sham cel. branch vagotomy (RYGB/Sham VgX; n = 6), Sham RYGB + celiac branch vagotomy (Sham/VgX; n =6), or sham RYGB + sham celiac branch vagotomy (Sham/Sham; n = 6), and body weight, body composition and food choice were monitored for 3 months after intervention.
Results
In rats with RYGB, histologically confirmed celiac branch vagotomy significantly moderated weight loss during the first 40 days after surgery, compared to either sham or failed vagotomy (P<0.05). In contrast, celiac branch vagotomy slightly, but non-significantly, reduced body weight gain in sham RYGB rats compared to sham/sham rats. Furthermore, the significant food intake suppression during the first 32 days after RYGB (P<0.05) was also moderated in rats with verified celiac branch vagotomy.
Conclusions
The results suggest that signals carried by vagal afferents from the mid and lower intestines contribute to the early RYGB-induced body weight loss and reduction of food intake.
Keywords: Bariatric surgery, vagus nerve, rat, adiposity, fat preference
Introduction
Roux-en-Y gastric bypass surgery is significantly more effective in reducing excess body weight and reversing type-2 diabetes compared with intensive medical care [1, 2], but the mechanisms leading to these beneficial effects are not well understood. Besides directly signaling via circulating hormones and other factors [3–6], the gut communicates with the brain via vagal and spinal sensory pathways [7–12]. In addition to chemical signals, vagal afferent fibers also communicate gastrointestinal distension and pressure signals to the brain, controlling meal size and gastrointestinal transit time [13–17]. Therefore, vagal integrity may also play a role in RYGB-induced reduction of meal size and food intake, improved glucose homeostasis, and sustained body weight loss.
The paired anterior (accessory) and posterior celiac branches innervate the distal duodenum, jejunum, ileum, cecum, and colon and are in an ideal position to mediate chemical and mechanical changes induced by RYGB. Particularly the Roux limb, exposed to large quantities of undigested food, remains innervated by the vagal celiac branches. Among the sensory terminal structures produced by the celiac branches are the intraganglionic laminar endings (IGLEs), found abundantly in the myenteric plexus between the circular and longitudinal smooth muscle layers [18]. Similar structures in the stomach have been demonstrated to sense the degree of tension in the gastric wall [19], and it is conceivable that increased tension and stretch of the Roux limb activates IGLEs and their vagal afferent fibers travelling in the celiac branches. Thus, vagal tension sensors in the Roux limb could be responsible for the reduced meal size observed in rats [20, 21] and humans [22]. The finding that the threshold for eliciting Roux limb distension-induced sensations were strongly and negatively correlated to the preferred meal size in gastric bypass patients at 6 and 12 months after surgery [22] is consistent with such a role for vagal afferents, although dorsal root/spinal pathways may also participate [23].
Therefore, the aim of the present study was to test a role for the celiac branches in RYGB-induced hypophagia and body weight loss. We hypothesized that if the vagal sensory innervation of the Roux and common limbs carries signals critical for RYGB-induced early satiety and hypophagia, removal of the vagal celiac branches should attenuate or block these effects and result in higher body weight levels after surgery.
Material and Methods
Animals and housing
Male Sprague-Dawley rats, 3 months old and weighing ~200 g (Harlan Industries, Indianapolis, IN), were housed individually in wire-mesh cages at a constant temperature of 21–23° C with a 12hr light-dark cycle (lights on 07:00, off at 19:00). Food and water were provided ad libitum except before treatments or tests. For 12 weeks, rats were on a two-choice diet consisting of normal laboratory chow (Kcal%: Carb, 58; Fat, 13.5; Prot, 28.5, # 5001, Purina LabDiet, Richmond IN ) and high-fat high-sucrose diet (Kcal%: Carb, 35; Fat, 45; Prot, 20, D12451, Research Diets, New Brunswick, NJ), with each of the diets containing sufficient minerals and vitamins. They were then randomly assigned to one of the four surgical groups as indicated below. All protocols involved in this study were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center in accordance with guidelines established by the National Institutes of Health.
Selective celiac branch vagotomy and Roux-en-Y gastric bypass surgery
Overnight fasted animals were deeply anesthetized with isoflurane, and after a midline incision starting from the xephoid process, the perigastric ligaments were ligated and cut to release the stomach. Both the posterior celiac and the anterior (accessory) celiac branches were transected by removing the posterior vagal trunk. Because in these obese rats the dorsal trunk was typically embedded in a thick bundle of adipose tissue on the side of the esophagus, it was not possible to clearly visually identify the division of the posterior trunk into the posterior gastric and celiac branches, and the entire bundle was cut taking care not to damage the esophagus and left gastric artery. The subsequent RYGB surgery has been described in detail earlier [24]. The procedure resulted in a gastric pouch of <5% of the total gastric volume, connected to a 15–25 cm-long Roux limb, a 15–25 cm-long common limb, and a 65–75 cm-long biliopancreatic limb. For the sham operation, the perigastric ligaments were cut, and then a 3 mm incision was made in the stomach wall and closed with a titanium clip. The jejunum was transected 10 cm distal to the ligament of Treitz, and the two cut ends were anastomosed using 10-0 nylon suture in an interrupted fashion.
Three to five days before euthanasia, Fluorogold (1 mg in 1 ml of sterile saline) was injected intraperitoneally to retrogradely label intact vagal efferent fibers originating in the dorsal motor nucleus in the brainstem [25]. Thirty micron-thick sections of the caudal brainstem were mounted on glass slides and processed with an antibody against Fluorogold according to established protocols. Inventory of neurons in the medial and lateral longitudinal columns of the dorsal motor nucleus was taken to determine the integrity of the abdominal vagus nerve projections. Absence of retrogradely labeled neurons in the left and right lateral columns was taken as evidence for successful transection of the anterior and posterior celiac branches [25, 26].
Measurement of body weight, body composition, fecal output, food intake, and high-fat preference
Body weight was monitored daily before and for the first two weeks after surgery, and then was recorded weekly. Body composition was also measured before, and 8 – 10 weeks after surgery by using a Minispec LF 90 NMR Analyzer (Bruker Corporation, The Woodlands, TX). Feces were collected for 3 days at 60 days after surgery and both wet and dry weights were measured.
After withholding food and water for the first 24h after surgery, all rats were given access to water and Ensure for days 2–5, and a choice of powdered regular chow and high-fat pellets. For the remainder of the study a choice between regular chow and high-fat pellets was offered. Intake of chow and high-fat pellets was measured by weighing them separately at the beginning and end of an observation period, taking spillage into account. Percent fat preference was calculated as calories from high-fat diet/ calories from high-fat plus regular chow diets×100.
Statistical analyses
Body weight, food intake, food chice, and fecal output was analyzed by appropriate two-way ANOVA, with bypass surgery (RYGB or sham-surgery) and vagotomy (VgX or Sham VgX) as main factors, and time as repeated measure within subject factor if necessary. Bonferroni’s post-hoc multiple comparison tests were used for follow-up analysis of individual means .
Results
Body weight and body composition
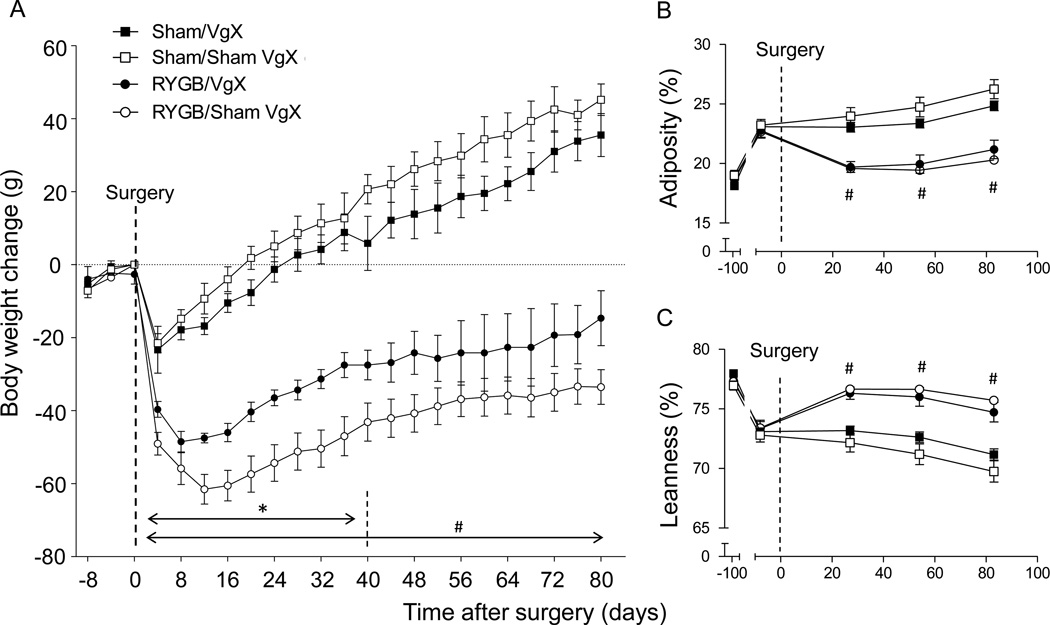
Exposure to the two-choice diet for 10 weeks before surgery increased body weight from 353g to 495g and adiposity from 18.6% to 23.0 %. As expected, RYGB surgery resulted in significant reversal of this obesity, while sham-operated rats continued to increase body weight and fat mass after an only transient decrease (Fig. 1). Compared to rats with RYGB and preserved celiac vagal branches, which lost about 61 g (−12%) body weight at the nadir around 2 weeks after surgery, rats with RYGB and verified celiac branch vagotomy lost only 48g at the nadir (− 9.7%). Applying a repeated measure (time) ANOVA to these two groups for the first 40 days post-surgery, yielded significant main effects of vagotomy (F[1,221] = 4.57; p = 0.031) and time (F[12,221] = 46.3; p < 0.0001) (Fig. 1A). However, between 40 and 80 days after surgery, body weight of the two groups tended to converge and were no longer significantly different. Also, despite the initial significantly smaller body weight loss in rats with RYGB and celiac vagotomy, their adiposity and leanness index was not significantly different from rats with RYGB and preserved celiac branches at any time point (Fig. 1B,C). In contrast to moderation of initial weight loss after celiac vagotomy, weight loss was slightly but not significantly exaggerated in rats with only sham RYGB surgery (Fig. 1A).
Fig. 1.
Effect of RYGB with or without celiac branch vagotomy on body weight and composition in rats on a two-choice high (45%) and low (10%) fat diet. Body weight (A), fat mass (B), and lean mass (C) of rats with Roux-en Y gastric bypass surgery plus celiac branch vagotomy (RYGB/VgX, n = 6, closed circles), RYGB plus sham or failed vagotomy (RYGB/Sham VgX, n = 15, open circles), sham operation plus celiac branch vagotomy (Sham/VgX, n = 6, closed squares), and sham operation plus sham vagotomy (Sham/Sham VgX, n = 6, open squares). * p < 0.05, RYGB/VgX vs. RYGB/Sham VgX. # p < 0.05, RYGB/VgX vs. Sham/VgX and RYGB/Sham VgX vs. Sham/Sham VgX.
Food intake
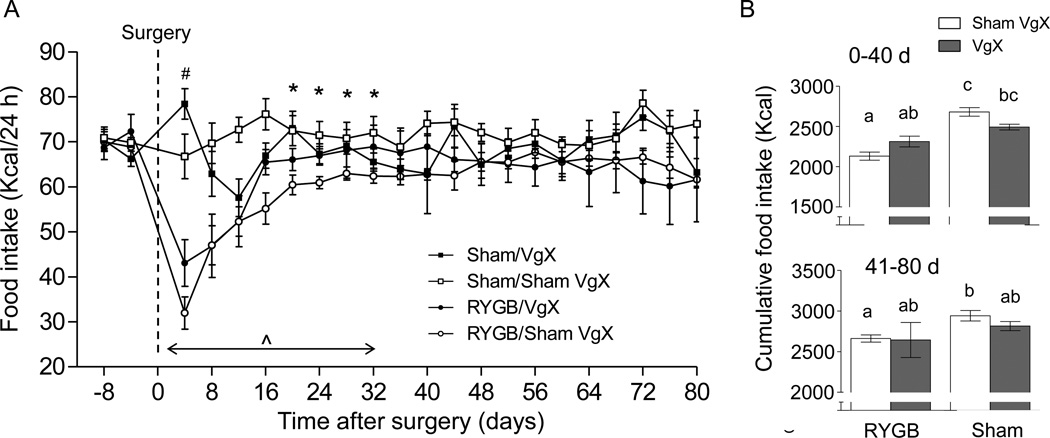
Food intake was similar for the 4 groups before surgery (Fig. 2). As previously reported, food intake was initially strongly suppressed by RYGB surgery in vagally intact rats and then gradually recovered (Fig. 2A), resulting in significantly reduced cumulative food intake over the first 40 postsurgical days (−20.5%) and non-significantly reduced food intake from day 40–80 (−10%) (Fig. 2B). There were time-dependent significant effects of vagotomy, with increased food intake of both RYGB and sham-operated rats on postoperative day 4 and differential effects (increase in RYGB and suppression in sham rats) on days 16–32 (Fig. 1A).
Fig. 2.
Effect of RYGB with or without celiac branch vagotomy on daily (A) and cumulative (B) food intake. A two-choice diet consisting of high-fat (45%) and low-fat (10%, regular chow was available throughout the experiment and intake of both diets was measured daily). Mean ± SEM of total calorie intake from both diets is shown. A: ^ p <0.05, significant suppression of food intake in RYGB/Sham VgX vs. Sham/Sham VgX; # p < 0.05, significantly higher food intake in both RYGB and sham-operated rats with VgX vs. Sham VgX. * p < 0.05, significant interaction between RYGB and VgX, indicating VgX-induced increase in food intake in RYGB rats, but suppression of food intake in sham-operated rats. B: Bars that do not share the same letters are significantly different from each other, p < 0.05.
Food choice
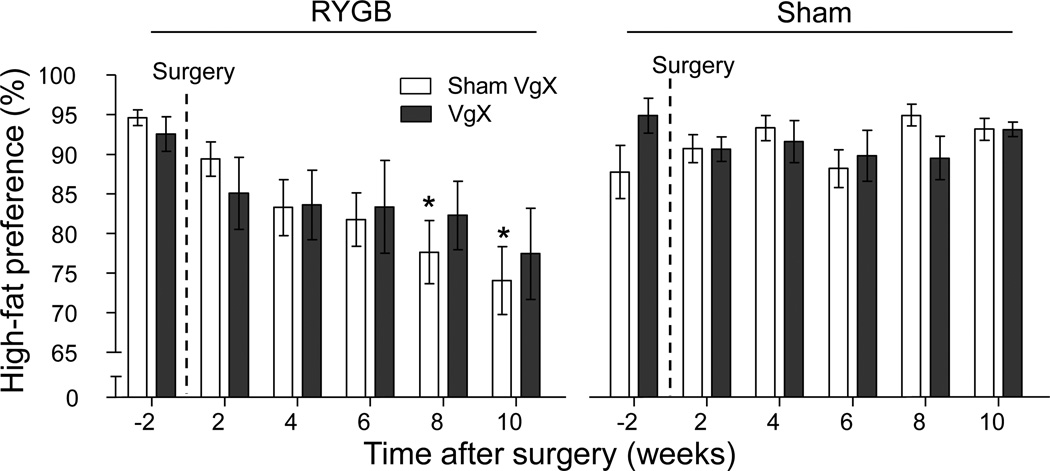
Before surgery, rats of all 4 groups had a similar high preference of over 90% for high-fat over low-fat diet (Fig. 3). This preference gradually decreased to below 80% in RYGB/sham and RYGB/VagX treated rats by 12 weeks after surgery, while it remained at over 90% in sham/sham and sham/VagX rats (Fig. 3).
Fig. 3.
Effect of RYGB with or without celiac branch vagotomy in rats on preference for high-fat food. A two-choice diet consisting of high-fat (45%) and low-fat (10%, regular chow was available throughout the experiment and intake of both diets was measured daily. Note that after RYGB but not sham operation, rats progressively reduced preference for the high-fat diet. Mean ± SEM of relative preference for the high-fat diet is shown. * p <0.05 vs. Sham/Sham VgX.
Fecal output
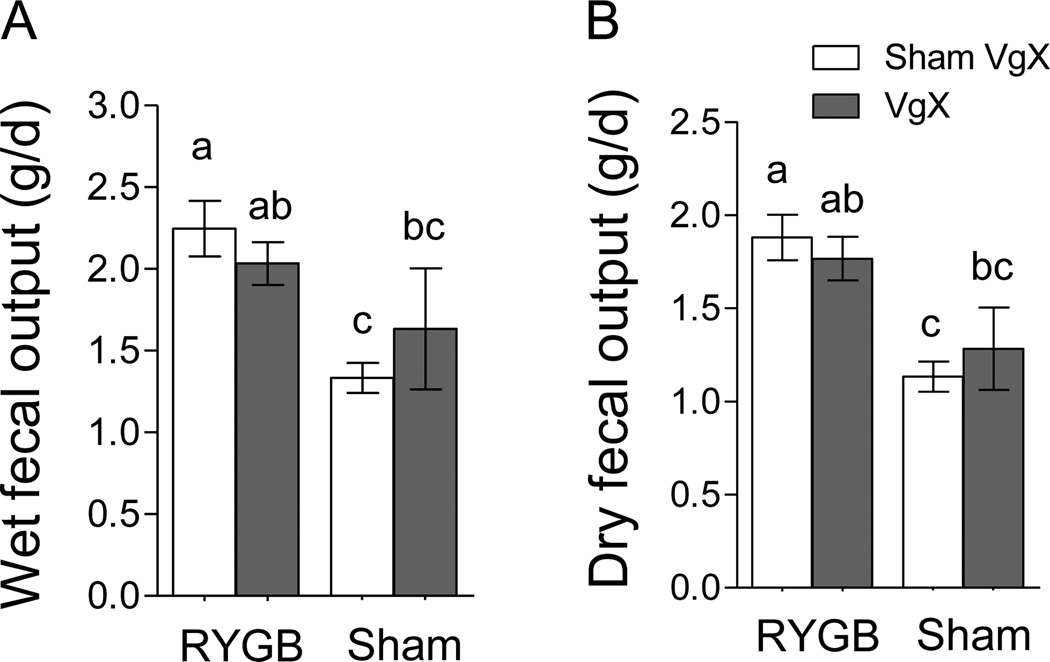
As revealed by ANOVA, both wet and dry fecal output was significantly affected by RYGB (wet: F[1,29] = 8.07, p < 0.01; dry: F[1,29] = 14.89, p < 0.001) but not celiac branch vagotomy (wet: F[1,29] = 0.04, p = 0.85; wet: F[1,29] = 0.02, p = 0.91). RYGB increased wet and dry fecal output by about 60 % and 50%, respectively, at 1 month post-surgery, similar to what we reported earlier in this rat model [27].
Vagotomy verification
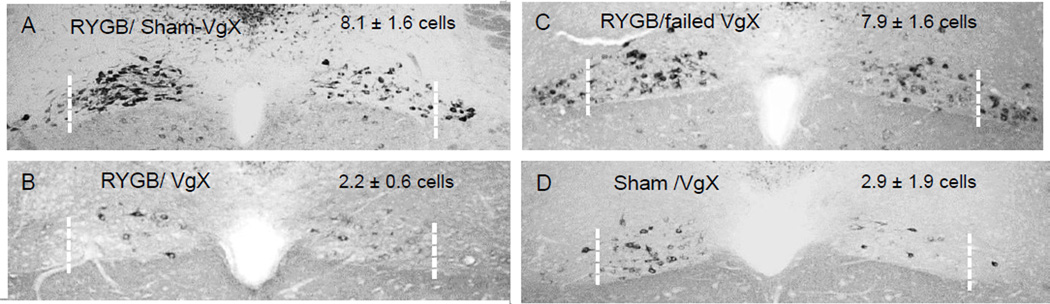
Near total elimination of vagal fibers in the two celiac branches was verified in only 6 of 13 RYGB animals with intended celiac branch vagotomy (Fig. 4). They had an average of 2.2 ± 0.6 retrogradely labeled neurons per section in the lateral columns of the dorsal motor nucleus (Fig. 4B), not different from the 2.9 ± 1.9 neurons in sham/VgX (Fig. 4D). RYGB animals with ‘failed’ vagotomy had 7.9 ±1.6 neurons (Fig. 4A), similar to the 8.1 + 1.6 neurons in RYGB/sham VgX animals (Fig. 4B).
Fig. 4.
Effect of RYGB with or without celiac branch vagotomy in rats on fecal output. Mean ± SEM of wet (A) and dry (B) daily fecal output measured 40 days post-surgery is shown. Note that RYGB, but not VgX, had a significant effect on both wet and dry fecal matter. Bars that do not share the same letters are significantly different from each other, p < 0.05.
Discussion
We find that RYGB with selective celiac and dorsal gastric branch vagotomy is less efficient in suppressing food intake and body weight compared to sparing vagal innervation. Rats with cut celiac vagal branches, as indicated by absence of retrogradely labeled neurons in the lateral columns of the dorsal vagal nucleus, lost significantly less body weight during the first 40 days after RYGB, compared to rats with intact celiac branches and lateral columns. This moderating effect on early body weight loss was accompanied by a similar moderating effect on food intake suppression by RYGB. In contrast, celiac branch vagotomy tended to suppress rather than moderate body weight and food intake in rats with sham gastric bypass surgery, except for the first few days after surgery, when it also increased food intake. These findings are consistent with the view that after RYGB, the celiac branches carry exaggerated satiation signals from the gut to the brain and that elimination of these signals leads to less satiation and less weight loss. The results confirm earlier observations with transection of the para-esophageal bundle as a means to remove vagal innervation in another model of rat RYGB [28]. However, there was a major difference in the time course of the effect. Whereas in our study the weight loss and anorexia-moderating effect of vagotomy was only seen for the first 40 days after surgery, the effect was mainly seen later in the postsurgical period, from 40 days onwards in the Bueter et al. study. The different time course in the two studies could be explained by a number of methodological details, including diet (figh-fat vs. regular chow), specific vagal branches cut, and RYGB model. In contrast to these two studies, subdiaphragmatic dissection of the vagus nerve had an exaggerated rather than moderating effect on body weight and food intake at 20 days but not 100 days after simultaneous gastric bypass surgery [29]. This opposite effect early after surgery might be explained by the very young age and low body weight (~ 190g) of the rats at the time of surgery [29], compared to much heavier rats (350–500g) used in the two other studies ([28] and present study). In the only clinical study, vagal dissection at the time of pouch formation did not appear to have any significant consequences on weight loss and esophageal functions 3 years after surgery [30].
One of the limitations of our study is the lack of specificity regarding abdominal vagal branches as well as sensory vs. motor fibers. Although we initially intended to carry out selective vagal de-afferentations of the celiac branches only, by local application of the neurotoxin capsaicin [31], this was not feasible considering that the celiac branches were embedded in large fat depots. We were unable to selectively eliminate the celiac branches and opted to cut the entire dorsal vagal bundle. This includes the dorsal (or posterior) celiac branch, the ventral (or accessory) celiac branch that wraps around the esophagus before joining the dorsal celiac branch, and the dorsal gastric branch innervating the remaining small gastric pouch. To verify these vagotomies, we used retrograde tracing of vagal motor fibres with corresponding neurons projecting through the various abdominal vagal branches located in more or less specific longitudinal columns of the dorsal motor nucleus [25]. Unexpectedly, intended celiac vagotomy failed in about half the cases, most likely due to missing the accessory celiac branch. We also cannot exclude the possibility that a few of the cut vagal fibers sprouted and were able to take up Fluorogold, even though they were functionally disconnected.
Because of these limitations, we cannot rule out that some of the moderating effects of vagotomy on body weight loss and anorexia are due to cutting the dorsal gastric branch, which could also contribute to exaggerated afferent signaling to the brain. Furthermore, it is possible that inadvertent loss of efferent vagal fibers masked the true effects of selective de-afferentation. We think this is unlikely, because loss of vagal motor innervation of the Roux and common limbs would be expected to result in hypo-motility and further aggravation of the effects of unhindered nutrient delivery. Thus, rather than preventing overstimulation, loss of vagal motor innervation would lead to even higher activity of vagal mechano- and chemo-sensors. Future studies using models of selective de-afferentation procedures such as systemic capsaicin-treatment [32] or unilateral vagal afferent rhizotomy combined with contralateral subdiaphragmatic vagotomy [33] will be necessary to corroborate the present findings.
Regarding potential mechanisms involved in exaggerated vagal satiation signaling after RYGB, It is plausible that the increased influx of undigested food, no longer controlled by the pyloric sphincter, leads to greater stimulation of vagal mechano- and chemo-sensors. Among the sensory terminal structures produced by the celiac branches are the intraganglionic laminar endings (IGLEs), found abundantly in the myenteric plexus between the circular and longitudinal smooth muscle layers [18]. Similar structures in the stomach have been demonstrated to sense the degree of tension in the gastric wall [19], and it is conceivable that increased tension and stretch of the Roux limb activates IGLEs and their vagal afferent fibers travelling in the celiac branches. Thus, vagal tension sensors in the Roux limb could be responsible for the reduced meal size observed in rats [20, 21] and humans [22]. Consistent with this idea, higher distension-induced sensations in the Roux limb predicted smaller preferred meals in gastric bypass patients at 6 and 12 months after surgery [22], and ingestion of a liquid meal produced much higher neural activation in brain areas controlling satiation and satiety ten days after RYGB compared with sham-operation in rats [34]. In addition, mucosal terminals of afferent fibers in the celiac branches [35, 36] are also in an excellent position to detect increased levels of gut hormones and other substances [7–12, 37].
In conclusion, our data suggest that intact vagal supply of the Roux and common limbs contributes by a small degree to the efficacy of RYGB to decrease body weight and food intake, at least during the early postsurgical period.
Fig. 5.
Verification of celiac branch vagotomies using the IP Fluorogold retrograde tracing technique [25]. Photomicrographs of representative frontal sections of the caudal brainstem showing retrogradely labeled vagal motor neurons (black) in the dorsal motor nucleus. Note the absence of retrogradely labeled neurons in the lateral pole (lateral to boken white line) of the dorsal motor nucleus in successfully celiac branch vagotomized rats (B,D) but not in sham and failed vagotomies (A,C). Means ± SEM (n = 3–10) of retrogradely labeled (intact) average cells per section in left and right lateral celiac branch columns is shown for each group.
Acknowledgments
We thank Katie Bailey for editorial help.
Support: This work was supported by National Institutes of Health Grants DK 47348 (HRB), DK 071082 (HRB), and DK 068036 (JY).
Footnotes
Except for grant support from the NIH, none of the authors declares a conflict of interest.
References
- 1.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 2.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 3-Year Outcomes. N Engl J Med. 2014 doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eatingstimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reidelberger RD, Hernandez J, Fritzsch B, Hulce M. Abdominal vagal mediation of the satiety effects of CCK in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1005–R1012. doi: 10.1152/ajpregu.00646.2003. [DOI] [PubMed] [Google Scholar]
- 5.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto H, Kishi T, Lee CE, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koda S, Date Y, Murakami N, et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 8.Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249:R638–R641. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- 9.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 10.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 11.Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil. 2008;20(>Suppl 1):64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang GJ, Tomasi D, Backus W, et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39:1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am J Physiol. 1996;271:R766–R769. doi: 10.1152/ajpregu.1996.271.3.R766. [DOI] [PubMed] [Google Scholar]
- 15.Kissileff HR, Carretta JC, Geliebter A, Pi-Sunyer FX. Cholecystokinin and stomach distension combine to reduce food intake in humans. Am J Physiol Regul Integr Comp Physiol. 2003;285:R992–R998. doi: 10.1152/ajpregu.00272.2003. [DOI] [PubMed] [Google Scholar]
- 16.Miranda A, Mickle A, Medda B, et al. Altered mechanosensitive properties of vagal afferent fibers innervating the stomach following gastric surgery in rats. Neuroscience. 2009;162:1299–1306. doi: 10.1016/j.neuroscience.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 18.Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 1997;195:183–191. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- 19.Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng H, Shin AC, Lenard NR, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1273–R1282. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furnes MW, Stenstrom B, Tommeras K, et al. Feeding behavior in rats subjected to gastrectomy or gastric bypass surgery. Eur Surg Res. 2008;40:279–288. doi: 10.1159/000114966. [DOI] [PubMed] [Google Scholar]
- 22.Bjorklund P, Laurenius A, Een E, Olbers T, Lonroth H, Fandriks L. Is the roux limb a determinant for meal size after gastric bypass surgery? Obes Surg. 2010;20:1408–1414. doi: 10.1007/s11695-010-0192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51(Suppl 1):i2–i5. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao Z, Zhao Z, Berthoud HR, Ye J. Development and verification of a mouse model for roux-en-Y gastric bypass surgery with a small gastric pouch. PLoS ONE. 2013;8:e52922. doi: 10.1371/journal.pone.0052922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol. 1987;253:R361–R370. doi: 10.1152/ajpregu.1987.253.2.R361. [DOI] [PubMed] [Google Scholar]
- 26.Berthoud HR, Powley TL. Identification of vagal preganglionics that mediate cephalic phase insulin response. Am J Physiol. 1990;258:R523–R530. doi: 10.1152/ajpregu.1990.258.2.R523. [DOI] [PubMed] [Google Scholar]
- 27.Shin AC, Zheng H, Townsend RL, Patterson LM, Holmes GM, Berthoud HR. Longitudinal Assessment of Food Intake, Fecal Energy Loss, and Energy Expenditure After Roux-en-Y Gastric Bypass Surgery in High-Fat-Fed Obese Rats. Obes Surg. 2013;23:531–540. doi: 10.1007/s11695-012-0846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bueter M, Lowenstein C, Ashrafian H, et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg. 2010;20:616–622. doi: 10.1007/s11695-010-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Liu J. Combination of bypassing stomach and vagus dissection in high-fat diet-induced obese rats-a long-term investigation. Obes Surg. 2010;20:375–379. doi: 10.1007/s11695-009-9862-2. [DOI] [PubMed] [Google Scholar]
- 30.Perathoner A, Weiss H, Santner W, et al. Vagal nerve dissection during pouch formation in laparoscopic Roux-Y-gastric bypass for technical simplification: does it matter? Obes Surg. 2009;19:412–417. doi: 10.1007/s11695-008-9657-x. [DOI] [PubMed] [Google Scholar]
- 31.Raybould HE, Tache Y. Capsaicin-sensitive vagal afferent fibers and stimulation of gastric acid secretion in anesthetized rats. Eur J Pharmacol. 1989;167:237–243. doi: 10.1016/0014-2999(89)90584-0. [DOI] [PubMed] [Google Scholar]
- 32.Kelly L, Morales S, Smith BK, Berthoud HR. Capsaicin-treated rats permanently overingest low- but not high-concentration sucrose solutions. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1805–R1812. doi: 10.1152/ajpregu.2000.279.5.R1805. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz GJ, Salorio CF, Skoglund C, Moran TH. Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am J Physiol. 1999;276:R1623–R1629. doi: 10.1152/ajpregu.1999.276.6.R1623. [DOI] [PubMed] [Google Scholar]
- 34.Berthoud HR, Shin AC, Zheng H. Obesity surgery and gut-brain communication. Physiol Behav. 2011 doi: 10.1016/j.physbeh.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995;191:203–212. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- 36.Powley TL, Spaulding RA, Haglof SA. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol. 2011;519:644–660. doi: 10.1002/cne.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox JE. Cholecystokinin satiety involves CCKA receptors perfused by the superior pancreaticoduodenal artery. Am J Physiol. 1998;274:R1390–R1396. doi: 10.1152/ajpregu.1998.274.5.R1390. [DOI] [PubMed] [Google Scholar]