Abstract
Objective
Defining groups of individuals within a larger population with similar patterns of weight change over time may provide insight into influences of weight stability or gain.
Methods
Latent class growth modeling was used to define subgroups of weight change in adult members of the Gila River Indian Community participating in at least 4 non-diabetic health exams including OGTTs (N=1157, 762F/395M; 78.4±19.0 kg). In a separate study, 152 individuals had 24-hr EE measured in a respiratory chamber.
Results
Eight groups with baseline weights of 54.6±7.3 (n=124), 64.2±7.7 (n=267), 73.6±7.8 (n=298), 86.1±10.2 (n=194), 95.5±6.7 (n=90), 97.9±10.4 (n=92), 110.9±11.9 (n=61), and 122.1±13.6 (n=31) kg (P<0.001) were delineated. Group 5, (initial weight=95.5±6.7 kg) maintained a comparatively stable weight over time (+3.3±10.3 kg, +3.8±11.2% of initial weight; median follow-up time: 13.1 years). All other groups gained weight over time (+29.9±21.1% of initial weight; median follow-up time: 16.3 years). Higher starting weight defined weight gain in most groups, but higher 2hr glucose predicted membership in the lower weight trajectories. The weight stable group had higher rates of impaired glucose regulation at baseline and higher 24-hr EE.
Conclusions
Weight in young adulthood defined weight gain trajectory underscoring the importance of intervening early to prevent weight gain.
Keywords: weight gain, obesity, trajectory, type 2 diabetes, health
Introduction
Changes in weight may not occur uniformly across all members of a population and invoking a single pattern of change within a population may not be appropriate (1). Subgroups of individuals may follow distinct patterns of weight change over time (trajectories). Identifying such subgroups may help determine which trajectories predispose to worse metabolic outcomes and predictors of these unhealthy weight gain patterns. Trajectories are used to examine weight changes over time and identifying how they relate to a variety of risks. For example, BMI trajectories were predictive of mortality risk in older adults (2;3), Trajectory analysis has been used to determine pathways to childhood obesity (4-8) and characterizing differences between those children populating the varying trajectories. Furthermore, this tool has shown that lifestyle intervention in children is more effective at a younger age (9). There are reports of adolescent BMI trajectory predicting adult diabetes and coronary disease risk (10), socioeconomic position influencing weight trajectories (11-13), and physical activity affecting weight over time (14;15). Few of these studies, however, have physiological markers predicting which trajectory an individual may join.
Latent class growth modeling (LCGM) is a semi-parametric technique that can be used to identify unique subgroups in a study population that follow similar patterns of change over time(1;16). We used longitudinal data from a population based study in which body weight was measured as frequently as biennially to assess adult weight patterns. We hypothesized that weight change over time would separate into a number of distinct trajectories. We also hypothesized that metabolic measurements would define trajectory and that trajectory itself would inform diabetes risk. We conjectured that weight trajectories themselves might provide vital information that simple delta weight changes are not able to provide and that the route by which adult weight is achieved may be more notable than the weight itself.
Methods
Participants
Participants participated in a longitudinal study in which data was collected prospectively from members of the Gila River Indian (Pima) Community in southern Arizona from 1965 - 2007. Residents of the community regardless of health status were invited to participate in biennial health exams that included a 75g oral glucose tolerance test (OGTT); from 1975 on, participants were asked to fast overnight prior to the visit (17) (see supplementary materials). At each exam, review of the medical record was used to document other disease diagnoses and medication use. Exams at which the volunteers had a diagnosis of diabetes mellitus, as determined by the 2003 ADA criteria (18), were excluded from this analysis. Participants may have developed type 2 diabetes (T2DM) later, but only their visits prior to that diagnosis were used in the LCGM analysis. Participant were also classified as having impaired fasting glucose (IFG, fasting plasma glucose 100-125mg/dl), impaired glucose tolerance (IGT, 2 plasma glucose 140-199 mg/dl) and impaired glucose regulation (IGR, either fasting plasma glucose 100-125mg/dl or 2 hour plasma glucose 140-199). Blood pressure measurements were done in the supine position with a large cuff. From 1965-1992, total cholesterol was measured using ferric chloride/acetic acid-sulfuric acid on the Technicon Auto Analyzer and from 1992 to present using an enzymatic method (Ciba Corning Express). All participants with at least four complete, non-diabetic, non-pregnant adult visits (range 4-14 visits) with body weight recorded (in light clothing without shoes; fed or fasted) and at least one exam between the ages of 18 and 24, which was considered the baseline adult visit, were included in the LCGM analysis. Visits were only included through age 45 years as the number of participants older than 45 years with non-diabetic visits was small. Diabetes diagnosis, for analysis of relative risk of development of T2DM, could be after the required 4 visits allowing inclusion into LGCM, and prior to age 45 years. All participants gave written, informed consent prior to participation in study. The study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
A subsample of 152 individuals (age: 28.2±7.6 yrs) also participated in an inpatient study of risk factors for obesity and T2DM between 1987-2005. In this study, energy expenditure (24-h EE) was measured in a metabolic chamber as previously described (19). All these participants were healthy by physical examination, medical history, and laboratory test results and exclusion criteria included a diagnosis of type 2 diabetes mellitus by a 75-g oral glucose tolerance test and other medical conditions, or use of medications known to affect energy metabolism. On admission, participants were given a standard weight maintaining diet for three days before any test was performed. The weight maintaining energy needs were calculated as previously described (20). All participants were determined to be healthy by physical examination. Body composition was measured either by hydrodensitometry (n=132) or by DXA (n=20), and measures were made comparable using comparative equations as previously described (21). This study was also approved by the NIDDK IRB and all participants gave informed consent (see supplementary materials for details).
Data analysis and statistics
A P<0.05 was considered statistically significant. Data are presented as mean±SD or median with interquartile range (IQR). Statistical analyses were performed using SAS software (SAS E-guide 4.2 and SAS version 9.2, SAS Institute, Inc., Cary, NC) and SPSS (version 21, IBM Corp, Armonk, NY, USA). First, weight gain trajectory groups were determined with LCGM using the SAS procedure PROC TRAJ developed by Jones et al. (16;22),designed to identify homogeneous clusters of growth trajectories, whose degree and direction of change can vary freely. This procedure identified different categories of body weight trajectories over time with adjustment for sex and date of birth to account for temporal changes in weight. The number of groups in the model and the functional relationship of each group with time (linear or quadratic) were varied until the best possible log Bayes factor (BIC: Bayesian Information Criterion) and posterior probability were obtained for the model. The BIC is a fit index used to compare competing models with differing numbers or shapes of trajectories. Posterior probabilities are calculated post hoc to estimate the probability that each case is a member of each modeled trajectory and can be used to assign each individual membership to the trajectory that best matches the profile of change (1). Groups were required to have at least 2% of the population to be considered meaningful.
Following trajectory analysis, one-way analysis of variance (ANOVA) was used to assess the baseline differences in anthropometric and glycemic parameters for the identified weight trajectories. Insulin concentrations were log-transformed prior to ANOVA to approximate a Gaussian distribution. One-way ANOVA was also used to compare changes (defined as difference between follow-up and baseline values) in weight per year between different trajectories. ANOVA was adjusted for sex, age, and, where appropriate, baseline weight. Additionally, multinomial logistic regression analysis was used to determine if baseline parameters were predictive of the development of a particular weight gain trajectory. For each trajectory group, diabetes prevalence was calculated; this included development of diabetes after age 45. This was considered to be lifetime diabetes prevalence per trajectory group.
Results
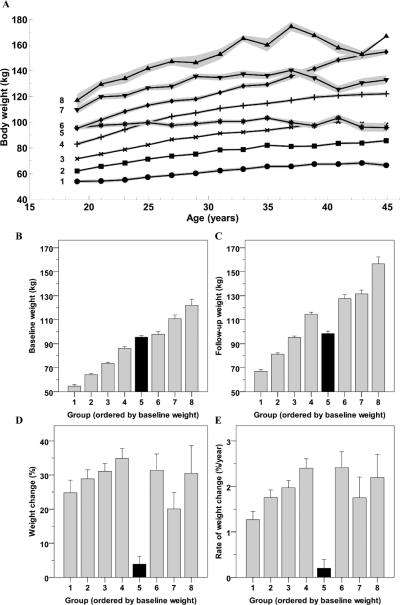
The baseline characteristics of the population are reported in Table 1. The best model for body weight change over time included 1157 individuals (395 males/762 females) separated into eight trajectory groups (Figure 1; posterior probability across groups = 0.900; BIC = -25,532.03). The groups were labeled Group 1 to 8 in ascending order according to the baseline body weight, which was different among groups (P<0.001). The trajectories were all parabolic (quadratic terms P<0.01), with the exception of Group 5 that showed a linear pattern of weight during time (linear term P<0.001, quadratic term P=0.96). The anthropometrics of the participants, by group, are shown in Table 2. Participants were young adults at baseline (20.3±1.8 years) and median follow up time was 16 years (IQR: 11 to 25 years). Results were not different when the analysis was restricted to full heritage Pima Indians. Weight change over time increased in proportion to starting weight, i.e. generally, groups with the lowest starting weight had the least total weight gain over time (P<0.001; Table 1). Percentage of weight change over time was significantly higher in all groups compared to Group 5 (range 20-35% total weight change among groups) and did not differ between other groups. Group 5, with an average starting weight of 95.5±6.7 kg (BMI: 33.6±3.5 kg/m2), gained the least amount of weight (3.3±10.3 kg and 3.8±11.2% of initial weight) over an average of 14.4 years (range: 5.7 to 25.5). When the analyses was separated by sex (Supplemental Figure 1), the trajectories looked similar. Both males and females segregated into 8 groups with 1 group remaining relatively weight stable over time and the others increasing weight.
Table 1.
Baseline characteristics of the study population.
| All (N=1157) | Men (N=395) | Women (N=762) | Sig. | |
|---|---|---|---|---|
| Age (years) | 20.3 ± 1.8 | 20.4 ± 1.8 | 20.2 ± 1.7 | 0.195 |
| BMI (kg/m2) | 29.1 ± 6.3 | 28.3 ± 5.9 | 29.6 ± 6.6 | 0.002* |
| Height (cm) | 163.8 ± 8.0 | 171.9 ± 5.7 | 159.6 ± 5.4 | <0.001* |
| Weight (kg) | 78.4 ± 19.0 | 84.1 ± 19.1 | 75.5 ± 18.2 | <0.001* |
| Fasting glucose (mg/dL) (N=803) | 90.0 ± 8.1 | 92.2 ± 9.0 | 88.8 ± 7.3 | <0.001* |
| 2-h glucose (mg/dL) (N=1140) | 100.7 ± 22.6 | 96.4 ± 22.3 | 103.0 ± 22.4 | <0.001* |
| Fasting insulin (mU/L) (N=791) | 23.9 ± 18.3 | 21.1 ± 14.6 | 25.4 ± 19.9 | 0.002* |
| Median and IQR | 19 (12-31) | 18 (11-28) | 20 (12-34) | 0.006*# |
| Logarithmic values | 1.5 ± 0.3 | 1.4 ± 0.3 | 1.5 ± 0.3 | 0.159 |
| 2-h insulin (mU/L) (N=811) | 108.3 ± 106.7 | 78.9 ± 88.0 | 123.9 ± 112.4 | <0.001* |
| Median and IQR | 84 (42-135) | 50 (27-100) | 95 (53-150) | <0.001*# |
| Logarithmic values | 2.0 ± 0.4 | 1.9 ± 0.4 | 2.1 ± 0.3 | <0.001* |
| Total cholesterol (mg/dL) (N=1134) | 160.9 ± 31.0 | 164.9 ± 32.8 | 158.8 ± 29.8 | 0.002* |
| Systolic BP (mmHg) (N=1117) | 115.5 ± 13.6 | 123.8 ± 13.2 | 111.2 ± 11.7 | <0.001* |
| Diastolic BP (mmHg) (N=1115) | 66.4 ± 10.5 | 69.8 ± 10.9 | 64.7 ± 9.8 | <0.001* |
P <0.05 for gender
Mann-Whitney U test P -value.
Figure 1.
Time courses of body weight at different biennial visits during adulthood (Panel A), body weight at the first (Panel B) and last (Panel C) visit of the observation period, percent weight change (Panel D) and rate of percent of weight change (Panel E) between the first and last visit in each of the eight trajectories as identified by latent class growth analysis. Trajectories in Panel A represent average body weight in each group while shaded areas indicate the 95% confidence interval of the mean. Error bars represent 95% confidence interval of the mean of each group.
Table 2.
Anthropometric characteristics of the eight trajectory-groups
| Group | N (%) |
Males (%) |
Birth year | Number of visits |
Baseline age(years) |
Baseline weight (kg) |
Baseline weight (kg) Odds ratio |
Baseline BMI (kg/m2) |
Weight change (kg) |
Weight change (%) |
BMI change (kg/m2) |
RoWC (kg/year) |
RoWC (%/year) |
Follow-up time (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 124 (10.7%) | 17.7* | 1957 ± 8* | 6.4 ± 1.9* | 20.3 ± 1.8 | 54.6 ± 7.3* | 0.50 (0.46-0.53)* | 21.8 ± 2.9* | 12.5 ± 10.3* | 24.8 ± 20.7* | 5.0 ± 4.2* | 0.6 ± 0.5* | 1.3 ± 1.0* | 19.4 ± 5.0* |
| 2 | 267 (23.1%) | 28.1* | 1959 ± 10* | 6.2 ± 1.9* | 20.6 ± 1.9 | 64.2 ± 7.7* | 0.61 (0.57-0.64)* | 24.7 ± 3.2* | 17.3 ± 12.2* | 28.9 ± 21.5* | 6.5 ± 4.7* | 1.1 ± 0.8* | 1.8 ± 1.3* | 17.3 ± 5.7* |
| 3 | 298 (25.8%) | 33.6* | 1961 ± 10* | 5.8 ± 1.8 | 20.4 ± 1.8 | 73.6 ± 7.8* | 0.72 (0.69-0.76)* | 27.8 ± 3.5* | 21.7 ± 12.7* | 31.1 ± 19.9* | 8.0 ± 5.0* | 1.4 ± 0.9* | 2.0 ± 1.4* | 16.9 ± 5.9* |
| 4 | 194 (16.8%) | 32.5* | 1962 ± 10* | 5.7 ± 1.9 | 20.0 ± 1.6 | 86.1 ± 10.2* | 0.88 (0.85-0.92)* | 32.1 ± 4.6* | 28.5 ± 14.5* | 34.9 ± 20.6* | 10.3 ± 5.6* | 2.0 ± 1.0* | 2.4 ± 1.5* | 15.5 ± 5.9 |
| 5¥ | 90 (7.8%) | 53.3 | 1966 ± 10 | 5.6 ± 1.5 | 20.1 ± 1.7 | 95.5 ± 6.7 | 1.00¥ | 33.6 ± 3.5 | 3.3 ± 10.3 | 3.8 ± 11.2 | 1.1 ± 3.6 | 0.2 ± 0.9 | 0.2 ± 0.9 | 14.4 ± 5.6 |
| 6 | 92 (8.0%) | 45.7 | 1966 ± 10 | 5.2 ± 1.5 | 19.9 ± 1.7 | 97.9 ± 10.4 | 1.04 (1.01-1.07)* | 35.0 ± 4.0 | 29.4 ± 19.3* | 31.4 ± 22.8* | 10.4 ± 7.4* | 2.3 ± 1.4* | 2.4 ± 1.7* | 13.3 ± 5.6 |
| 7 | 61 (5.3%) | 44.3 | 1968 ± 9 | 5.2 ± 1.6 | 19.7 ± 1.7 | 110.9 ± 11.9* | 1.18 (1.13-1.23)* | 39.1 ± 5.9* | 20.6 ± 17.3* | 20.1 ± 18.6* | 7.2 ± 6.2* | 1.8 ± 1.7* | 1.7 ± 1.8* | 12.4 ± 5.7 |
| 8 | 31 (2.7%) | 58.1 | 1967 ± 10 | 5.1 ± 1.2 | 20.0 ± 1.4 | 122.1 ± 13.6* | 1.27 (1.20-1.35)* | 41.5 ± 4.1* | 34.8 ± 22.8* | 30.5 ± 21.7* | 11.7 ± 8.0* | 2.5 ± 1.5* | 2.2 ± 1.4* | 14.1 ± 5.7 |
| Total | 1157 (100%) | 34.10% | 1962 ± 10 | 5.8 ± 1.8 | 20.3 ± 1.8 | 78.4 ± 19.0 | - | 29.1 ± 6.3 | 20.3 ± 15.6 | 27.9 ± 21.6 | 7.4 ± 5.8 | 1.4 ± 1.2 | 1.8 ± 1.5 | 16.2 ± 6.0 |
P <0.05 vs. Group 5 using Dunnet's test
#: P <0.05 vs. Group 5 using Wald test by logistic regression
Reference group.
Abbreviations: RoWC: rate of weight change
Odds Ratio calculations: Weight: per 1-kg in body weight
Weight analyses adjusted for age and sex
Medical diagnoses or use of prednisone (the only medication consistently classified over the duration of the study period), which may affect body weight in individuals, was not different between trajectory groups. No significant difference in prevalence of disease diagnoses including cancer, congestive heart failure, hypertension, hypotension, liver disease, and rheumatoid arthritis at any biennial visit existed between trajectories.
Predictors of weight gain trajectory
Using multinomial logistic regression analysis and setting Group 5 as reference, baseline weight, adjusted for age and sex, was a significant predictor of the trajectory group (P<0.001). For every 1-kg increase in baseline weight the odds of belonging to a specific trajectory differed significantly from Group 5 (Table 2). Results were similar when further adjusting for the fraction of Pima heritage (not shown).
Baseline 2-h glucose concentration adjusted for age (P<0.001), sex (P=0.19) and baseline weight (P<0.001) was a significant but negative predictor of the weight gain trajectory group (P=0.005), such that a 10-mg/dL higher 2-h glucose was associated with greater odds of developing particular weight gain trajectory compared to Group 5 (Table 3). Similar results were obtained for 2-h insulin (P=0.008, adjusted for age, sex, baseline weight and 2-h glucose), so that a 10-mU/L increase in 2-h insulin was overall negatively associated to the odds of the developing weight gain trajectory compared to Group 5 (table 3).
Table 3.
Glycemic characteristics of the eight trajectory-groups
| Group | IFG (%) |
IGT (%) |
IGR (%) |
T2D ever (%) |
T2D ever Odds ratio |
Fasting glucose (mg/dL) |
2-h glucose (mg/dL) |
2-h glucose Odds ratio |
Fasting glucose change (mg/dL) |
2-h glucose change (mg/dL) |
Fasting insulin (mU/L) |
2-h insulin (mU/L) |
2-h insulin Odds ratio |
Fasting insulin change (mU/L) |
2-h insulin change (mU/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.2* | 5.7 | 5.7* | 20.2 | 1.50 (0.72-3.11)* | 87.0 ± 6.7* | 99.5 ± 23.5 | 1.29 (1.06-1.58)* | 3.9 ± 10.1 | 20.2 ± 33.6* | 17 (9 - 23)* | 80 (45 - 123)* | 1.09 (1.02-1.15)* | –7 (–13 - –2) | –19 (–36 - 12) |
| 2 | 4.3* | 3.1 | 6.3* | 25.8# | 2.06 (1.08-3.95)* | 87.6 ± 7.2* | 97.8 ± 22.4* | 1.15 (0.97-1.37) | 6.1 ± 12.3* | 18.5 ± 35.2* | 15 (10 - 23)* | 71 (37 - 117)* | 1.06 (1.01-1.11)* | –6 (–11 - 1)* | –9 (–40 - 47) |
| 3 | 10.2* | 3.4 | 11.7* | 29.5# | 2.48 (1.31-4.70)* | 89.8 ± 7.7* | 99.4 ± 20.9* | 1.09 (0.94-1.27) | 6.2 ± 12.9* | 20.7 ± 37.3* | 18 (10 - 30)* | 72 (37 - 130)* | 1.04 (1.01-1.08)* | –2 (–15 - 5)* | 6 (–37 - 65)* |
| 4 | 10.5* | 6.2 | 14.5* | 34.5# | 3.12 (1.62-6.03)* | 90.3 ± 8.1 | 101.9 ± 23.5 | 0.95 (0.84-1.07) | 9.8 ± 13.2* | 25.1 ± 39.6* | 22 (13 - 30)* | 81 (38 - 135)* | 0.98 (0.94-1.01) | –1 (–13 - 9)* | 10 (–38 - 86)* |
| 5¥ | 26.7 | 6.9 | 31.9 | 14.4 | 1.00¥ | 93.2 ± 10.0 | 107.7 ± 22.7 | 1.00¥ | 1.0 ± 13.0 | 1.4 ± 30.1 | 28 (17 - 39) | 96 (65 - 170) | 1.00¥ | –15 (–25 - –3) | –38 (–87 - –8) |
| 6 | 19.5 | 5.6 | 24 | 32.6# | 2.87 (1.38-5.96)* | 91.7 ± 8.3 | 102.2 ± 25.3 | 0.86 (0.75-0.99)* | 8.5 ± 12.2* | 22.7 ± 35.0* | 23 (13 - 33) | 86 (49 - 134) | 0.97 (0.93-1.01) | 2 (–13 - 12)* | 7 (–51 - 84) |
| 7 | 16.3* | 4.9 | 16.3* | 27.9# | 2.29 (1.02-5.15)* | 92.6 ± 8.3 | 104.3 ± 22.4 | 0.83 (0.69-0.99)* | 6.8 ± 11.2 | 22.9 ± 36.5* | 30 (14 - 43) | 114 (51 - 160) | 0.99 (0.95-1.04) | –10 (–21 - 3) | –16 (–113 - 66) |
| 8 | 17.9* | 0 | 17.9* | 32.3# | 2.82 (1.09-7.33)* | 93.4 ± 6.8 | 104.3 ± 17.2 | 0.82 (0.63-1.06) | 8.8 ± 13.4* | 19.0 ± 27.6 | 34 (22 - 57) | 122 (102 - 200) | 0.99 (0.94-1.06) | –6 (–19 - 13) | –24 (–73 - 11) |
| Total | 11.6 | 4.5 | 14.1 | 27.6 | - | 90.0 ± 8.1 | 100.7 ± 22.6 | - | 6.4 ± 12.6 | 19.7 ± 36.2 | 19 (12 - 31) | 84 (42 - 135) | - | –5 (–15 - 4) | –5 (–48 - 53) |
P <0.05 vs. Group 5 using Dunnet's test
Reference group.
Glucose and insulin values given are baseline values at the first visit. Changes are defined as difference between follow-up and baseline visits values.
Legend:
IFG - Impaired Fasting Glucose (FPG: 100-125 mg/dL);
IGT - Impaired Glucose Tolerance (2hPG: 140-199 mg/dL).
IGR - Impaired Glucose Regulation (FPG: 100-125 mg/dL and/or 2hPG: 140-199 mg/dL)
T2D - Type 2 Diabetes
Odds Ratio calculations: 2-h glucose: per 10-mg/dL in 2-h glucose; 2-h insulin: per 10-mU/L in 2-h insulin
2-h glucose analyses adjusted for age, weight and sex; 2-h insulin analyses adjusted for age, weight, sex 2-h glucose
Fasting glucose (P=0.40), fasting insulin (P=0.06), total cholesterol (P=0.10) and systolic (P=0.57) and diastolic (P=0.93) blood pressure were not significantly associated with weight trajectory prediction in the adjusted logistic models. In the subgroup of 762 women, 215 (19%) had one pregnancy, 124 (11%) had two, and 118 (10%) had 3 or more pregnancies. Parity was a significant predictor of weight trajectory in women (P<0.001); Group 5 was not different from other groups (all P>0.05). The lower-rank trajectories (i.e., lower body weight) of female participants showed an increased prevalence of having more than one pregnancy as compared to higher-rank trajectories.
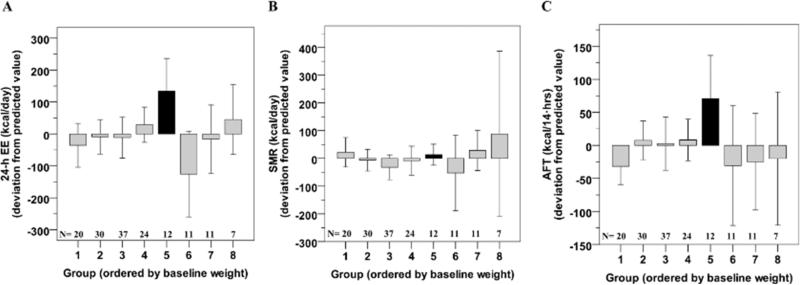
In the subset of 152 participants having a 24-h EE measurement within 8.1±7.3 years of their baseline visit, Group 5 showed on average the highest 24-h EE after adjustment for age, sex, fat mass and fat free mass (+134±161 kcal/day, N=12, ANOVA global P=0.02, Figure 2A), as well as the greatest adjusted AFT (+71±92 kcal/14 h, Figure 2C). No differences were found for adjusted SMR among groups (P=0.36, Figure 2B). Similar results were obtained by restricting the analysis to chambers within 5 years from the baseline visit (N=65, not shown).
Figure 2.
Twenty-four hour energy expenditure (EE, Panel A), sleeping EE (SMR, Panel B) and awake and fed thermogenesis (AFT, Panel C) in each group as identified by latent class growth analysis of body weight. 24-h EE and SMR measures are calculated after adjustment for age, gender, fat mass and fat free mass while AFT is calculated after adjustment for age, gender, % body fat, fat free mass and fasting glucose concentration. Error bars represent 95% confidence interval of the mean of each group.
Glycemic characteristics of the trajectory groups
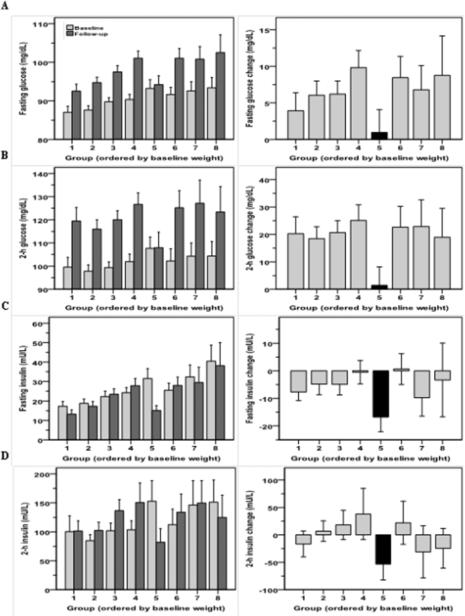
The glycemic characteristics of the groups are shown in Figure 3 and Table 3. Group 5 showed a higher proportion of IFG (27%) and IGR (32%) participants at baseline as compared to other groups, with the exception of Group 6 (Table 3). After adjustment for gender, age and body weight, neither baseline fasting nor 2 h glucose concentrations of Group 5 were different from the other groups. Results were similar when stratified analyses by sex. When calculating the change in glucose concentrations from baseline to the last non-diabetic visit, Group 5 showed no change on average in fasting and 2-h glucose concentrations (P=0.54 and P=0.68, respectively), however, all other groups showed significant increases (Figure 3). The greatest decreases in fasting and 2-h insulin levels were also observed in Group 5 (Table 3) (Δ=−17 mU/L and Δ=−53 mU/L, both P<0.001, respectively).
Figure 3.
Fasting (Panel A) and 2-h glucose levels (Panel B) during the oral glucose tolerance test and related changes between the baseline and the last visit in each group as identified by latent class growth analysis of body weight. Fasting (Panel C) and 2-h insulin levels (Panel D) during the oral glucose tolerance test and related changes between the baseline and the last visit. Error bars represent 95% confidence interval of the mean of each group.
Of note, some of the groups had weights at baseline or at the end of follow up similar to group 5 (the relatively weight stable group) but the trajectories they followed were quite different. For example, Groups 5 and 6 had similar mean baseline weight (95.5 vs. 97.9 kg, respectively, P=0.06), but Group 5 had on average higher 2-h insulin levels at baseline (P=0.03). At follow-up, Group 6 showed greater weight gain (P<0.001) and increases in glucose, insulin, total cholesterol concentrations (all P<0.05) but no difference for blood pressure (P>0.75) as compared to Group 5. On the other hand, Group 3 had a comparable average body weight at the last visit to that of Group 5 (95.2 vs. 98.5 kg, respectively, P=0.007) but a noticeably lower weight at baseline (73.6 vs. 95.5 kg, respectively, P<0.001). Group 3 showed a greater weight gain along with larger increases in glucose and total cholesterol concentrations (P<0.001) as compared to Group 5. At the last visit, Group 3 had higher glucose and insulin levels despite a similar body weight as compared to Group 5.
In total, T2DM developed in 319 of the participants. Only those visits prior to development of T2DM were included in the determination of the weight trajectories. Fourteen percent of the reference group (Group 5) developed T2DM. All other groups, with the exception of Group 1 (Wald statistic=1.16, P=0.28), had significantly greater percentages of individuals eventually develop T2DM than did Group 5 (all P<0.05, Table 3). By logistic regression analysis, the relative risk of developing T2DM as compared to Group 5 was higher for Groups 2 through 8, whereas no difference was found for Group 1 (odds ratios in Table 3).
Discussion
In this analysis using LCGM to analyze weight change in non-diabetic young adults, we found that generally higher initial weight at age 20-24 years associated with a greater weight gain trajectory, and often higher final weight. The population as a whole gained weight with the exception of one near weight stable trajectory. This group included 8% of the cohort and had higher baseline 2-h glucose levels and adjusted EE compared to the other groups. However, perhaps by maintaining weight stability, this group had stable glucose concentrations and a lower overall risk of T2DM.
Previous studies have addressed the issue of patterns of weight change using a variety of statistical techniques. In a study on the Pima Indians, Hanson et al (23) categorized weight fluctuation based on the root mean square error of the first four biennial exams after age 20 and associated these fluctuations with eventual mortality and vascular disease. Lee et al (24) used an age, period, & birth cohort analysis of NHANES data to evaluate obesity prevalence across cohorts in the US population. Mixed models have been used to determine whether socio-economic, psychosocial, or behavioral factors had a role in racial differences in weight gain over time (11) and to examine BMI trajectories in young adults followed from age 18-45 years(12). Additionally, several studies have used regression models to address patterns of weight change over time (15;25;26). However, use of a model which requires classification of individuals into defined groups by pre-specification of weight change may not provide a full picture of possible weight change trajectories as does using continuous data to define the trajectories.
LCGM has become more popular as a means to estimate longitudinal weight trajectories and then predict outcome or determine what anticipates trajectory placement (3;4;6;8;27). Primarily descriptive papers utilizing this method for assessing weight gain (6) identified different patterns in childhood obesity which might have implications for future research and treatment. Others have attempted to determine whether local environment of children differs by weight trajectory (4) and the effect of maternal risk factors on childhood weight (8). In adults, Østbye et al (27) related membership in a particular trajectory to birth year, education, gender, and years of marriage as well as to health outcomes in middle life. Likewise, Zheng et al (3) showed that those with changing weight trajectories in older adulthood had much different mortality outcomes that those with stable weight over time and that ~7% of deaths were attributable to an “obesity upward” trajectory after 51 years of age. All of these studies, however, are lacking in direct measures of anthropometrics and physiologic markers of health status. Our study provides these data in a longitudinal manner as well as data on energy expenditure in a subset of individuals.
Our finding that, in general, those with lower body weight early in adulthood were better able to maintain weight throughout adulthood indicates that interventions for weight management may need to occur prior to ages 18-24. This is supported by the retrospective study by Parsons et al (14) that found that females who were more active at 16 years of age gained BMI more slowly over future decades than less active females. Likewise, Viner et al (26) found that sedentary behaviors and fast food intake during adolescence influenced adult BMI, independent of baseline weight and socioeconomic status. Data from the Fels Longitudinal Study (28) found a tracking of BMI from about 20 years into adulthood, but changes in childhood BMI were also separately related to adult overweight and adiposity.
All but one of our weight gain groups (8% of the population) had a significant increase in weight over time. We analyzed the clinical characteristics of this weight-stable group as compared to other weight-gain groups in order to explain the difference in their future weight trajectory behaviors. We found that the weight-stable group had higher glucose and insulin levels at baseline compared with the other groups. However, this group maintained stable to improved glycemic measures over time likely due to the maintenance of body weight. Across all groups, relatively higher baseline 2-h glucose was associated with a lower starting trajectory weight. As previously shown, this indicates a role for increased insulin resistance in braking further weight gain (29). Whether this is due to increased energy expenditure possibly via increased hepatic glucose production or an effect on food intake (perhaps via increased glucose concentrations effect on satiety) is not clear. It is also possible that individuals, particularly in the weight stable trajectory, made life style changes in response to their OGTT results which prevented weight gain and T2DM; however it should be noted that this study started in 1965, in advance of the recognition that impaired glucose regulation was a clear and modifiable risk factor (via lifestyle interventions) for T2DM. The similar baseline weights of groups 5 and 6 but divergent outcomes may be indicative of this.
In addition to the glycemic status, the weight stable group also showed a higher-than-expected 24h EE, reflecting higher values of awake and fed thermogenesis (AFT). AFT is the component of daily EE that includes the cost of being awake and the thermic response to food, which we recently showed to be inversely associated with future weight gain in obese individuals (30). These results in the subset of participants with EE measurements indicate that a relatively higher EE may contribute to the ability of this group to maintain weight stability (31).
The timing of the visits included in this analysis span first visits that occurred between 1965-2003 to last visits between 1971-2007. This time period corresponds to the time of increasing prevalence of overweight and obesity in the United States in general (32). Birth year differed in groups 1-4 (all with lower starting weights), but was not a determinant of trajectory. Consistent with the overall US trend, all groups but one gained weight (33), and as an average percent weight change over the course of the analysis the range was 20.1-34.9% indicating substantial weight gain in all the groups except group 5. Again, this implies that beginning early adulthood with a lower body weight is one of the most desirable foundations for a healthy weight later in life.
Our data have some limitations that may affect the generalizability of the findings. The Pima Indian population has higher documented rates of obesity and T2DM than the US population in general. Secondly, we do not have any measurement of ad libitum food intake or eating behaviors, and even the 24hEE measurements may miss individual habitual physical activity. Additionally, we do not have socioeconomic data available on these volunteers.
We used LCGM to model weight change trajectories in a population with longitudinal data. We found that nearly all groups gained weight over time, and that those starting at heavier weights at young adulthood gained more and at a faster rate. Higher 2-hr glucose concentration predicted membership in lower baseline weight trajectory groups. We did indentify a near weight stable group characterized by higher rates of IGR and higher adjusted 24hEE. Overall young adult weight does indeed define subsequent weight change indicating that interventions to prevent weight gain prior to young adulthood would be beneficial. In adulthood, near weight stability can prevent hyperglycemia and T2DM.
Supplementary Material
What is already known about this subject?
The causes of weight gain are unknown but are likely multifactorial, including both genetic and environmental components
Changes in weight may not occur uniformly across all members of a population
Developing a better understanding of weight change over time in larger populations may be useful in determining appropriate intervention strategies
What does this study add?
Higher initial weight at age 20-24 years associated with a greater weight gain trajectory, and often higher final weight
Our study found one weight stable group (8% of the cohort) and had higher baseline 2-h glucose levels and adjusted EE compared to the other groups
The weight stable group had stable glucose concentrations and a lower overall risk of type 2 diabetes
Acknowledgements
We thank the volunteers for their participation in the study. This research was supported by the Intramural Research Program of the NIH, NIDDK.
Footnotes
Conflict of Interest
The authors do not have any competing financial interests.
Reference List
- 1.Andruff H, Carraro N, Thompson A, Gaudreau P. Latent class growth modelling: A tutorial. Tutorials in Quantitative Methods for Psychology. 2009;5:11–24. [Google Scholar]
- 2.Zajacova A, Ailshire J. Body Mass Trajectories and Mortality Among Older Adults: A Joint Growth Mixture-Discrete-Time Survival Analysis. Gerontologist. 2013 doi: 10.1093/geront/gns164. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng H, Tumin D, Qian Z. Obesity and Mortality Risk: New Findings From Body Mass Index Trajectories. Ann Epidemiol. 2013 doi: 10.1093/aje/kwt179. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter M, Dubois L, Tremblay M, Taljaard M, Jones B. Trajectories of childhood weight gain: The relative importance of local environment versus individual social and early life factors. PLoS One. 2012;7(10):e47065. doi: 10.1371/journal.pone.0047065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois L, Girard M. Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes. 2006;30:610–617. doi: 10.1038/sj.ijo.0803141. [DOI] [PubMed] [Google Scholar]
- 6.Hejazi S, Dahinten V, Marshall S, Ratner P. Developmental pathways leading to obesity in childhood. Health Rep. 2009;20(3):63–69. [PubMed] [Google Scholar]
- 7.O'Brien M, Nader P, Houts R, Bradley R, Friedman S, Belsky J, et al. The ecology of childhood overweight: a 12-year longitudinal analysis. Int J Obes. 2007;31:1469–1478. doi: 10.1038/sj.ijo.0803611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pryor L, Tremblay R, Boivin M, Touchette E, Dubois L, Genolini C, et al. Developmental trajectories of body mass index in early childhood and their risk factors: an 8-year longitudinal study. Arch Pediatr Adolesc Med. 2011;165(10):906–912. doi: 10.1001/archpediatrics.2011.153. [DOI] [PubMed] [Google Scholar]
- 9.Reinehr T, Kleber M, Lass N, Toschke A. Body mass index patterns over 5 y in obese children motivated to participate in a 1-y lifestyle intervention: age as a predictor of long-term success. Am J Clin Nutr. 2010;91:1165–1171. doi: 10.3945/ajcn.2009.28705. [DOI] [PubMed] [Google Scholar]
- 10.Tirosh A, Shai I, Afek A, Dubnov-Raz G, Ayalon N, Gordon B, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med;2011;364:1315–1325. doi: 10.1056/NEJMoa1006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baltrus P, Lynch J, Everson-Rose S, Raghunathan T, Kaplan G. Race/ethnicity, life-course socioeconomic position, and body weight trajectories over 34 years: the Alameda County Study. Am J Pub Health. 2013;95(9):1595–1601. doi: 10.2105/AJPH.2004.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke P, O'Malley P, Johnston L, Schulenberg J. Social disparities in BMI trajectories across adulthood by gender, race/ethnicity and lifetime socio-economic position: 1986-2004. Int J Epidem2009. 38(2):499–509. doi: 10.1093/ije/dyn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giskes K, van Lenthe F, Turrell G, Kamphuis C, Brug J, Mackenbach J. Socioeconomic inequalities in cardiovascular mortality and the role of childhood socioeconomic conditions and adulthood risk factors: a prospective cohort study with 17-years of follow up. Obesity. 2008;16:1377–1381. doi: 10.1186/1471-2458-12-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsons T, Manor O, Power C. Physical activity and change in body mass index from adolescence to mid-adulthood in the 1958 British cohort. Int J Epidem 2013. 35:197–204. doi: 10.1093/ije/dyi291. [DOI] [PubMed] [Google Scholar]
- 15.Tammelin T, Laitinen J, Nayha S. Change in the level of physical activity from adolescence into adulthood and obesity at the age of 31 years. Int J Obes. 2004;28:775–782. doi: 10.1038/sj.ijo.0802622. [DOI] [PubMed] [Google Scholar]
- 16.Jones B, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods Research. 2001;29:374–393. [Google Scholar]
- 17.Pavkov M, Hanson R, Knowler W, Bennett P, Krakoff J, Nelson R. Changing Patterns of Type 2 Diabetes Incidence Among Pima Indians. Diabetes Care. 2007;30(7):1758–1763. doi: 10.2337/dc06-2010. [DOI] [PubMed] [Google Scholar]
- 18.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;261(Supplement):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 19.Ravussin E, Lillioja S, Anderson T, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pannacciulli N, Salbe A, Ortega E, Venti C, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86(3):625–632. doi: 10.1093/ajcn/86.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tataranni P, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr. 1995;62:730–734. doi: 10.1093/ajcn/62.4.730. [DOI] [PubMed] [Google Scholar]
- 22.Jones B, Nagin D. Advances in group based trajectory modeling and an SAS procedure for estimating them. Sociological Methods Research. 2007;35:542–570. [Google Scholar]
- 23.Hanson R, Jacobsson L, McCance D, Narayan K, Pettitt D, Bennett P, et al. Weight fluctuation, mortality and vascular disease in Pima Indians. Int J Obes. 1996;20(5):463–471. [PubMed] [Google Scholar]
- 24.Lee J, Gebremariam A, Keirns C, Davis M, Vijan S, Freed G, et al. Getting heavier, younger: trajectories of obesity over the life course. Int J Obes. 2010;34:614–623. doi: 10.1038/ijo.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkins K, Fultz N, Fonda S, Wray L. Patterns of body weight in middle-aged and older Americans, by gender and race, 1993-2000. Soz - Praventivmed. 2003;48:257–268. doi: 10.1007/s00038-003-2053-3. [DOI] [PubMed] [Google Scholar]
- 26.Viner R, Cole T. Who changes body mass between adolescence and adulthood? Factors predicting change in BMI between 16 year and 30 years in the 1970 British Birth Cohort. Int J Obes. 2006;30:1368–1374. doi: 10.1038/sj.ijo.0803183. [DOI] [PubMed] [Google Scholar]
- 27.Ostbye T, Malhotra R, Landerman L. Body mass trajectories through adulthood: results from the National Longitudinal Survey of Youth 1979 Cohort (1981-2006). Int J Epidem 2011. 40:240–250. doi: 10.1093/ije/dyq142. [DOI] [PubMed] [Google Scholar]
- 28.Guo S, Huang C, Maynard L, Demerath E, Towne B, Chumlea W, et al. Body mass index during childhood, adolescence and young adulthood in relation to adult overweight and adiposity: the Fels Longitudinal Study. Int J Obes. 2000;24:1628–1635. doi: 10.1038/sj.ijo.0801461. [DOI] [PubMed] [Google Scholar]
- 29.Swinburn B, Nyomba B, Saad M, Zurlo F, Raz I, Knowler W, et al. Insulin resistance associated with lower rates of weight gain in Pima Indians. J Clin Invest. 1991;88:168–173. doi: 10.1172/JCI115274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piaggi P, Krakoff J, Bogardus C, Thearle M. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes. 2013;62(12):4043–4051. doi: 10.2337/db13-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piaggi P, Thearle M, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab. 2013;98:E703–E707. doi: 10.1210/jc.2012-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flegal K, Carroll M, Kuczmarski R, Johnson C. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 33.Ogden C, Carroll M, Kit B, Flegal K. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;82 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.