Abstract
Nicotine increases the value of some reinforcing stimuli, and this effect may contribute to nicotine's widespread abuse. We aimed to quantify this effect using a behavioral economic analysis. Six Long- Evans rats were exposed to a modified observing response procedure. In this procedure, presses to one lever resulted either in food according to a variable-interval 15 second schedule or extinction; presses to a second, observing lever illuminated stimuli correlated with the schedule in effect on the food/extinction lever (i.e., conditioned reinforcers). The FR requirement on the observing lever increased across sessions. The number of presentations of the conditioned reinforcers was plotted as a function of FR value to generate a demand curve. Nicotine was then administered at a dose of 0.3 mg/kg. All demand curves were fitted to the exponential demand equation and a parameter reflecting reinforcer value was evaluated. Nicotine increased the value of the conditioned reinforcers as measured by this equation; nicotine also increased responding on the food/extinction lever. This analysis demonstrates that nicotine increases the value of conditioned reinforcers under certain conditions. The current procedure allows for a novel method of analyzing demand for conditioned reinforcers.
Keywords: rat, lever press, nicotine, observing, behavioral economics, behavioral pharmacology, conditioned reinforcement
Tobacco smoking is the leading cause of preventable death worldwide, leading to more than five million deaths each year (Hatsukami, Stead, & Gupta, 2008; WHO, 2011). Given the health risks associated with smoking, understanding why people smoke is an important goal. Nicotine, the primary psychoactive ingredient in tobacco, acts as a moderately potent primary reinforcer in people and animals (Rose and Corigall, 1997). However, there is growing evidence that the primary reinforcing effects of nicotine may not be sufficient to explain the persistence of tobacco addiction in humans. In addition to serving as a primary reinforcer, nicotine also enhances the value of nondrug reinforcers present in the environment when nicotine is administered (Caggiula et al., 2002; Donny et al., 2003). This value-enhancement effect of nicotine may contribute to the maintenance and reacquisition of smoking behavior, as nicotine may cause other stimuli in the smoker's environment to seem more valuable and pleasant (Chaudhri et al., 2006; Chaudhri et al., 2007; Perkins & Karelitz, 2013). Understanding the value-enhancing effects of nicotine may lead to improved treatment outcomes for smokers.
One source of evidence for the enhancement effect comes from rats responding to turn on a visual stimulus. Nicotine selectively increased rats’ responding for this visual stimulus, without increasing responding on an inactive lever (Donny et al., 2003; Palmatier, Liu, Caggiula, Donny, & Sved, 2007; Palmatier et al., 2009; Raiff & Dallery, 2009; Weaver at al., 2012). Daily injection of nicotine also increased rats’ responding for a primary-reinforcing audiovisual stimulus (Barrett & Odum, 2011). These findings led to the hypothesis that nicotine enhances responding for some primary reinforcers. Nicotine also appears to affect the value of conditioned reinforcers, which are stimuli that gain reinforcing value due to having been paired with or followed by the presentation of another reinforcer (Palmatier et al., 2007). For example, Olausson, Jentsch, and Taylor (2004) showed that nicotine increased responding for an auditory and visual stimulus as a compound conditioned stimulus that had previously predicted the availability of water in water-deprived rats.
Research has also shown that nicotine enhances responding for food-paired conditioned reinforcers using the observing response procedure. In this procedure, two levers are available concurrently (Wyckoff, 1952). One lever produces primary reinforcement according to a mixed schedule, one component of which is typically extinction (henceforth, the “active” lever). The other lever produces only stimuli correlated with the schedule in place on the active lever (henceforth, the “observing” lever). Thus, the animal can “observe” what schedule is in effect on the active lever by responding on the observing lever. Because responding on the observing lever never leads directly to primary reinforcement and does not affect the rate of primary reinforcement, this type of responding is considered to be maintained primarily by the conditioned reinforcing value of the food-predictive stimuli (Williams, 1994). Nicotine selectively increases observing responding; lending further support to the hypothesis that nicotine enhances the value of conditioned reinforcing stimuli (Jones, Raiff & Dallery, 2010; Raiff & Dallery, 2006, 2008).
Although nicotine increases the rate of responding maintained by conditioned reinforcers, it is not clear if a change in rate necessarily equates to a change in the “value” of the stimuli. The measurement of “value” is complicated, as the term is colloquial and not tied to any one dependent variable. For example, we speak of the declining value of a reinforcer with time in the context of delay discounting, and also of the value of a drug as it relates to the reinforcing efficacy of that drug as measured by a progressive ratio schedule (Bradshaw & Killeen, 2012; Mazur, 1987). Thus, in order to be used fruitfully, the concept of value must be clearly situated in an overarching theoretical framework that allows comparison of drug effects across commodities. Keeping this in mind, we have chosen to evaluate value in the context of behavioral economics. This framework is useful because the empirical measurement of value used in this framework is parametric in nature and sensitive to experimental manipulations, and provides a unitary measure that is relatively easy to interpret and compare across reinforcers, contexts, and species. Behavioral economics engages the concept of value by equating value with price elasticity of demand. In behavioral economics, the primary relationship of interest is between the price of a good and consumption. When plotted in graphical space, this relation describes a demand curve (Hursh, 1980, 1984). The point slope of the demand curve is the degree of elasticity of the commodity at a particular price.
The concept of elasticity forms one of the keystones of behavioral economic theory. The elasticity of a good can be thought of as synonymous with reinforcer efficacy and value. Hursh and Silberberg (2008) developed a new equation in an attempt to quantify reinforcer value (i.e., elasticity) using a single parameter. The model proposed by Hursh and Silberberg is the Exponential Demand Equation:
| (1) |
Equation 1 includes three parameters: Q0, k, and α. The variables Q and C represent reinforcer consumption and price, respectively. K is a constant (usually between the values of 1 and 4) that is set according to the observed range of the dependent variable in logarithmic units, and the parameters Q0 and α are free to accommodate the data. Q0 is the estimate of the level of consumption in units of the reinforcer at the lowest price possible (i.e., an estimate of demand level at theoretical price 0). The rate-constant parameter α of Equation 1 measures the rate of change in elasticity across the function.
Hursh and Silberberg (2008) propose that the single parameter α is a measure of “essential value”, because it should remain constant regardless of the size of the reinforcer. That is, the essential value of food pellets should remain constant regardless of whether the demand function is determined using a one-pellet, two-pellet, or four-pellet reinforcer, even if the demand function shifts up or down in absolute terms. In contrast, essential value may change as a function of a change in reinforcer efficacy. Manipulations that change the relevant motivating operations may alter the slope of the demand function, and thus may change the essential value of that reinforcer (Christensen, Silberberg, Hursh, Huntsberry & Riley, 2008).
Cassidy and Dallery (2012) used Equation 1 to determine the effects of nicotine on the essential value of food. Rats responded for food across increasing FR values during 23-hr, closed economy sessions. That is, food was only available during the session and not in the home cage. Then, the rats were exposed to chronic nicotine via osmotic minipumps at a dose of 3 mg/kg/day. Cassidy and Dallery found that nicotine did not appreciably alter demand for food as measured by this equation when the reinforcer was one pellet of food, and slightly increased the essential value of food when the reinforcer was two-pellets in some animals. In a similar protocol, Barrett and Bevins (2012) exposed rats to increasing FR schedules of primary reinforcing visual stimulus presentations and found that nicotine increased the essential value of these stimuli. Thus, we wished to determine if nicotine would also increase the essential value of conditioned reinforcers, in addition to primary reinforcing visual stimuli.
In the present experiment, we sought to determine the feasibility of using a novel, modified observing procedure to generate a demand curve for conditioned reinforcers and to test the effects of nicotine on demand for conditioned reinforcers. Furthermore, we wished to test the extent to which the resulting demand curves could be adequately described by Equation 1. To that end, we modified a typical observing-response procedure. Observing stimuli were presented after completion of a fixed ratio schedule, which varied across sessions—as in a typical demand procedure for primary reinforcers—and the number of stimulus presentations earned at each FR value was the main dependent variable.
Method
Subjects
Six male Long-Evans rats served as subjects, obtained from Harlan Laboratories, maintained at 85% of their free-feeding weight. The subjects were individually housed in a windowless colony room and had unrestricted access to water in their home cages. The colony room had a 12:12 hr light/dark cycle, and subjects received any extrasession feeding following the session in their home cages.
Apparatus
Three MED-PC aluminum and Plexiglas modular rodent operant chambers were used as experimental chambers. Each chamber was equipped with steel grid floors, measuring approximately 28 cm wide × 25.5 cm deep × 28 cm high and encased in light- and sound-attenuating outer cases and equipped with two standard levers. Each lever had an array of three LED lights above it, and the levers were situated approximately 3 cm from the bottom of the chamber. Each chamber also had a houselight that was used in the current experiments as a discriminative stimulus rather than for general illumination. Each chamber contained a pellet dispenser that dispensed 45-mg 50% sucrose pellets (TestDiet, AIN76A).
Discrimination Training
During discrimination training, components of fixed ratio (FR) 1 reinforcer availability alternated with components of extinction according to a VI 60-s schedule of component duration during each session. The schedule then increased to a variable interval (VI) 5-s schedule, and then a VI 15-s final schedule of food availability was reached. Throughout the session, one stimulus was paired with food components (either a solid or blinking house light, which was counterbalanced across rats; blinks were 0.25 s each) and the other stimulus was paired with extinction components, hereafter referred to as the S+ and S-, respectively. Either the right or left lever was designated the food/extinction lever, while the other lever had no programmed consequences. Sessions lasted 60 min, and continued until the following stability criteria were met: Discrimination indices (that is, presses in S+/Total presses) were over 0.75 on average for seven consecutive sessions; rate of responding in S+ for each session was within 20% of the 7-day average, and rates of responding in S+ for the first and last day of the last seven sessions were within 10% of the 7-day average. Following discrimination training, the animals began the observing response procedure.
Observing response procedure
Under the observing response procedure the S+ and S- were only presented after a right (or left, as stated above) lever press, the observing response; at all other times, the chamber was dark. One observing response illuminated the S+ or S- for 15 s, depending on the currently operative schedule component (VI 15 or extinction; Shahan, 2002). If the schedule was programmed to change, for instance from VI to extinction, during the 15 s of stimulus illumination, the current schedule remained in effect until the stimulus terminated. Observing responses during stimulus presentation had no effect but were recorded. The terminal schedule parameters were: a VI 15 s food schedule which alternated with extinction approximately every 60 s (VI 60 s component duration), and S+/S- stimuli presented for 15 s beginning on an FR 1 schedule. Each session lasted for 1hr.
Baseline
Following establishment of FR 1 observing lever responding, the schedule in place on the observing lever increased across days according to the following sequence: FR 1, FR 2, FR 3, FR 5, FR 7, FR 10. Each FR value was in place for one session. Observing response rates were redetermined at each FR value a minimum of three times; each time, Equation 1 was used to fit a demand curve of these rates. Redeterminations continued until the last two consecutive curves were similar enough that an extra sum-of-squares F-test determined that the curves were similar enough to warrant a shared α parameter.
Nicotine Administration
Nicotine (nicotine hydrogen tartrate salt; Sigma, St. Louis, MO) was dissolved in potassium phosphate to adjust pH to 7.4 and administered via subcutaneous injection at a dose of 0.3 mg/kg. Nicotine dose was calculated as the base form, and injection volume was based on body weight at the time of injection (1 ml/kg). Nicotine was administered immediately prior to the session for three curve determinations, with a minimum of 3 days between redeterminations. Vehicle administrations consisted of subcutaneous injections of potassium phosphate at the same volume as nicotine doses. Vehicle injections were administered for two curve determinations. Data from only the last two nicotine curves were included to equalize the number of data points across conditions.
Results
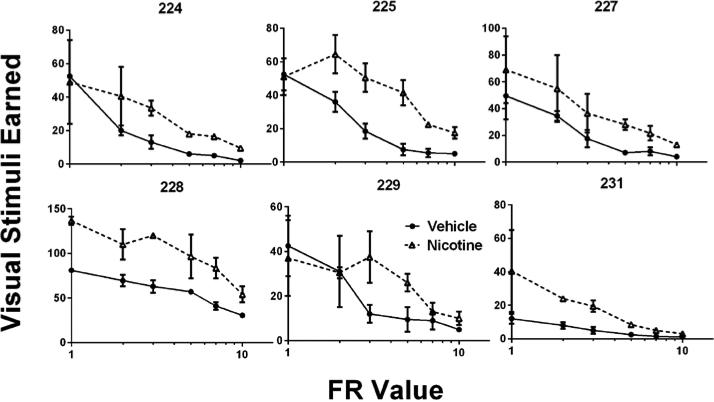
Figure 1 shows the average number of stimulus presentations earned by each subject across all FR values under vehicle and nicotine conditions. For all subjects, nicotine increased consumption of the conditioned reinforcers: Stimuli earned increased across FR values under nicotine conditions. This increase in consumption of the visual stimuli can be interpreted as a shift in the absolute height of the demand curve; however, note that not all shifts in the absolute height of the demand curve correlate with a change in the essential value of the relationship (Cassidy & Dallery, 2012; Hursh & Silberberg, 2008).
Fig. 1.
The absolute number of stimulus presentations earned for each subject, averaged across replications within subject at each schedule value, across vehicle and nicotine conditions. Error bars represent standard error of the mean. Note the different y-axis ranges across rats.
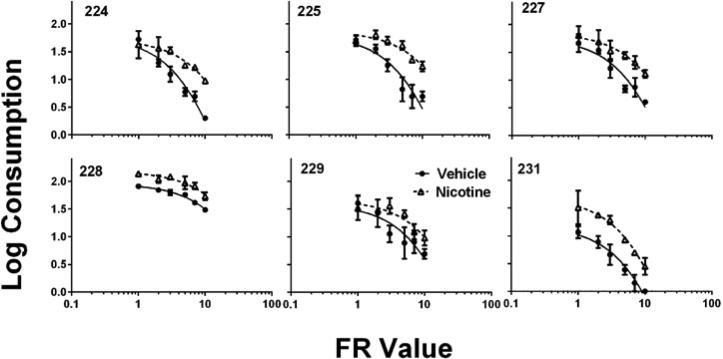
To determine whether nicotine changed the essential value of these conditioned reinforcers, Equation 1 was fitted via least-squares regression to the relation between FR value and number of stimulus presentations in log–log coordinates (GraphPad Prism version 5.0 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com). The results are shown in Figure 2. The k value was set at 3 and kept the same across all subjects and conditions. Equation 1 accounted for an average of 89% of the variance across the vehicle data sets (SD = .07), and 86% of the variance across the nicotine data sets (SD = .12). For all subjects, the nicotine curve does not significantly overlap the vehicle curve. This visual analysis is confirmed by an extra sum-of-squares F-test. The F-test suggests that for all six animals, the curves were significantly different and that distinct α values are needed to describe the nicotine and vehicle data sets. The F-test evaluates whether distinct α values are necessary, or whether one α value could describe the relation across both nicotine and vehicle data sets for each animal (Motulsky & Christopoulos, 2003).
Fig. 2.
The obtained consumption data, averaged across replication within subject, was fitted to Equation 1. Error bars represent standard error of the mean.
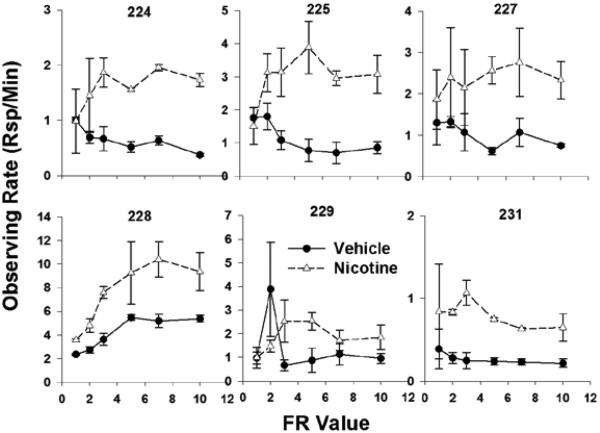
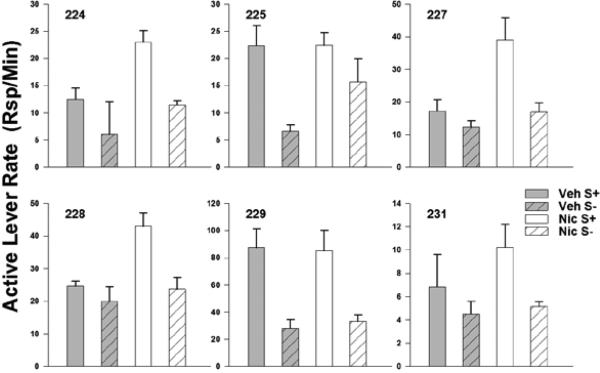
The increase in response rate on the observing lever as a function of nicotine administration can be seen in Figure 3. The data are from each subject and data points represent averages within each FR value across two replications. Keep in mind that animals could continue to respond on the observing lever during periods of S+/S- presentation; this occasionally occurred and accounts for the lack of total concurrence between the observing rates and demand output. Response rates for all animals show a marked increase when nicotine was administered compared to rates under vehicle administration. This effect is consistent with a value-enhancement interpretation. In this case, both the observing rate data and the essential value data agree across subjects.
Fig. 3.
The average rate of responses per min on the observing lever under both vehicle and nicotine conditions. Error bars represent the standard error of the mean.
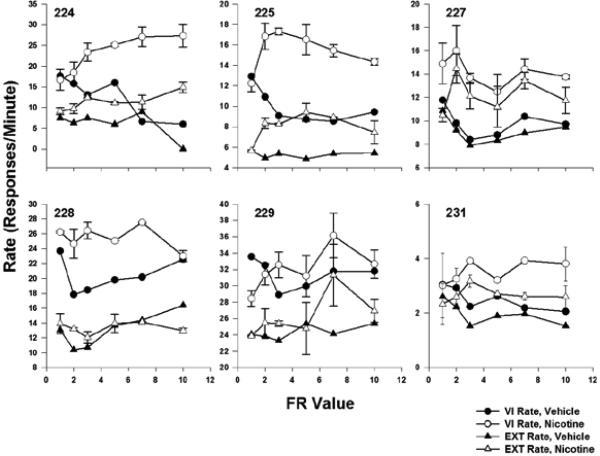
As shown in Figure 4, nicotine also tended to increase responding on the active lever regardless of the operative component, VI or extinction. Responding was higher under the VI component than in the extinction components and nicotine tended to increase both types of responding, though this effect was generally more pronounced for responding during the VI components.
Fig. 4.
The average rate of responses per min on the active lever during VI and extinction components under both vehicle and nicotine conditions. Error bars represent the standard error of the mean.
In light of the increased responding on the active lever under conditions of nicotine administration, we further analyzed responding on the active lever. Figure 5 presents rates of responding during periods of S+ and S- presentation, respectively, during both vehicle and nicotine administration. No systematic differences in rates were seen across observing-response FR values; therefore we averaged across these values to simplify presentation. Comparing rates of responding during S+ and S- presentations, there is evidence that the rats responded discriminatively in the presence of the stimuli when they were presented contingent upon a lever press. For most rats (225 and 229 being the exceptions), rates of responding on the active lever were highest during S+ presentations when nicotine was present. Similarly, it does not appear that nicotine increased S+ and S- rates equivalently. In other words, it does not seem to be the case that nicotine increased all types of responding indiscriminately.
Fig. 5.
The average rate of responses per min on the active lever during periods of S+ and S- presentation under both vehicle and nicotine conditions. Error bars represent the standard error of the mean.
Discussion
The present experiment demonstrated the feasibility and utility of using a behavioral economic approach to investigate the effects of nicotine on demand for conditioned reinforcers. Equation 1 provided good fits of demand for the S+ and S- stimuli. Alpha values derived from these demand curves revealed that nicotine increased the essential value of the S+ and S- stimuli in the context of alternating VI and extinction components. Nicotine also increased the rate of observing responding.
Nicotine's enhancement of active lever responding and observing responding raises several questions about the mechanism underlying this effect, other than direct enhancement of the value of the conditioned reinforcers. While there are many possible interpretations, we will discuss three possible explanations. First, nicotine could have produced a general locomotor increase that was not specific to observing behavior. However, experiments conducted by Barrett and Bevins (2013) suggest that a general increase in locomotion does not account for nicotine's reinforcement-enhancing effects. In their first experiment, rats completed sessions in activity-recording chambers equipped with two levers: One lever produced a visual stimulus, and the other had no programmed consequences. Nicotine increased responding on the active lever, on the inactive lever, and increased activity levels. However, in their second experiment, the same conditions were repeated with the exception that sucrose was the reinforcer, instead of a visual stimulus. Under these conditions, nicotine increased responding for sucrose but not responding on the inactive lever, and activity levels were not increased by nicotine. In other words, enhancement can and does occur without general activity increases, and general activity increases occur without enhancement. The authors concluded that general locomotor enhancement is therefore not a parsimonious mechanism for nicotine's enhancement effect. While such an account cannot be ruled out in the present experiment, the data presented in Figure 5 suggest that nicotine's effects on responding during the presence of the discriminative stimuli was more complex than a simple, equivalent increase in activity in all contexts. That is, for four out of six subjects, nicotine's enhancement effects were most evident during periods of S+ presentation, suggesting that nicotine's effect was due to the enhancement of the conditioned reinforcing effect of these stimuli and not to a general locomotor increase. However, future research using this procedure could incorporate simultaneous activity recording to further elucidate the influence of locomotor increase on enhancement.
Another question is whether nicotine's enhancement effect had a differential impact on sensory reinforcers (as distinct from food reinforcers which are discussed below). Previous research has shown that nicotine enhances the value of primary sensory reinforcers, particularly lights (e.g., Barrett & Bevins, 2012). In the current experiment, the conditioned reinforcers we used were also lights. Therefore, it is likely that the lights had primary reinforcing properties prior to their pairing with food (see Raiff & Dallery, 2008 for supporting evidence). A recent experiment investigating nicotine's enhancement effect demonstrated that nicotine did not increase responding for a light/tone stimulus when water was concurrently available; while when that same stimulus was paired with water to become a CS, nicotine enhanced responding for the stimulus; suggesting again that pairing with a primary reinforcer increases nicotine's enhancement effect beyond that which would be seen for the stimulus alone (Guy & Fletcher, 2014). In the present experiment, it is difficult to say whether the enhancement of the conditioned reinforcers was due to the sensory or conditioned reinforcing properties of the stimuli. Both sensory and conditioned reinforcing properties co-occur in the stimuli; in the present preparation it is not possible to dissociate them. A future experiment could include a lever that produces only a visual stimulus that has not been paired with food, and rates of responding could be compared to responding on the observing lever. Such a procedure could dissociate the effects of the primary reinforcing effects of lights per se from food-associated conditioned reinforcers.
Finally, it is possible that nicotine enhanced the value of food (reflected in increased active lever responding) and that this increase in value led to the increased value of the conditioned reinforcers. This interpretation is possible, as conditioned reinforcer value necessarily changes with the value of the primary reinforcers with which they are associated (Kelleher & Gollub, 1962). In previous experiments using the observing response procedure to study nicotine's enhancement effect, responding for food only increased after repeated, chronic nicotine administration (Raiff & Dallery, 2006). Similarly, in the present experiment, nicotine was administered repeatedly across sessions, and we observed increases in food- maintained responding. Therefore, the observed increases in food responding may be due to the dosing regimen.
Nicotine's effects on food responding have been explored in other contexts as well. As noted above, under closed economy conditions Cassidy and Dallery (2012) found that nicotine did not increase the essential value of one-pellet food reinforcers, but did produce some increases in two-pellet reinforcers. Donny, Caggiula, Weaver, Levin, & Sved (2011) extensively reviewed the complex relationship between nicotine's reinforcement-enhancing effects and food consumption. The authors noted that while nicotine does have anorectic effects under free-feeding conditions, most laboratory preparations involve some level of restriction. The authors concluded that under most laboratory conditions nicotine does enhance food reinforcement, but that these effects are masked under conditions approaching free-feeding. This may explain the results of Cassidy and Dallery, which failed to show that nicotine enhanced responding for food, as the animals were responding for food under 23-hr closed economy conditions. Similarly, Palmatier, O'Brien and Hall (2012) showed that nicotine enhanced responding on a progressive ratio for sucrose, and that this effect was increased as sucrose concentration increased; however, they also reported that under FR 3 conditions, nicotine did not increase responding for a high-concentration sucrose solution. However, nicotine's effect on food responding does not negate its effects on conditioned reinforcers; instead, it indicates that the enhancement was indirect via the enhancement of food. Barrett and Bevins (2013) also argue that the value of the food may be a critical determinant when examining nicotine's enhancement effect. Future experiments using the current procedure could manipulate the sucrose concentration of the food as in Barrett and Bevins (2013) to parametrically examine the impact of food value on nicotine's enhancement of conditioned reinforcers. Alternatively, the food reinforcer could be selectively devalued, perhaps by prefeeding, and nicotine's effects on responding for conditioned reinforcers could be tested under such circumstances.
In short, the current procedure represents a promising way to study demand for conditioned reinforcers. Behavioral economic demand analyses show promise for integrating experimental results across domains, and we hope that the present study can contribute to that overarching goal. Using a behavioral-economic approach to quantify nicotine's enhancement effect of conditioned reinforcers may increase the translational significance of laboratory work on this effect. While much remains to be explored, nicotine's enhancement effect may prove to be an important contributor to the widespread prevalence of smoking. Improving translational applicability of research on this topic may help contribute to better treatment outcomes for smokers in the future.
Acknowledgments
These data were collected as part of the first author's doctoral dissertation. We would like to thank Drake Morgan, Marc Branch, Philip Erb and Don Stehouwer for their invaluable comments and assistance. Manuscript preparation was supported by NIDA T32 DA016184.
Contributor Information
Rachel N. Cassidy, Brown University
Jesse Dallery, University of Florida.
References
- Barrett ST, Bevins RA. A quantitative analysis of the reward-enhancing effects of nicotine using reinforcer demand. Behavioural Pharmacology. 2012;23:781–789. doi: 10.1097/FBP.0b013e32835a38d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ST, Bevins RA. Nicotine enhances responding for qualitatively distinct reinforcers under maintenance and extinction conditions. Pharmacology, Biochemistry & Behavior. 2013;114:9–15. [PMC free article] [PubMed] [Google Scholar]
- Barrett ST, Odum AL. The effects of repeated exposure on the reward-enhancing effects of nicotine. Behavioural Pharmacology. 2011;22:283–289. doi: 10.1097/FBP.0b013e3283473c25. [DOI] [PubMed] [Google Scholar]
- Bradshaw CM, Killeen PR. A theory of behaviour on progressive ratio schedules, with applications in behavioural pharmacology. Psychopharmacology. 2012;222:549–564. doi: 10.1007/s00213-012-2771-4. [DOI] [PubMed] [Google Scholar]
- Cassidy RN, Dallery J. Effects of economy type and nicotine on the essential value of food in rats. Journal of the Experimental Analysis of Behavior. 2012;97:187–202. doi: 10.1901/jeab.2012.97-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Sved AF. Environmental stimuli promote acquisition of nicotine self-administration in rats. Psychopharmacology. 2002;163:515–530. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Sved AF. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: Impact of nicotine dose and reinforcement schedule. Psychopharmacology. 2007;190:353–362. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen CJ, Silberberg A, Hursh SR, Huntsberry M, Riley AL. Essential value of cocaine and food in rats: tests of the exponential model of demand. Psychopharmacology. 2008;198:221–229. doi: 10.1007/s00213-008-1120-0. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib S, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: Implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Weaver MT, Levin ME, Sved AF. The reinforcement-enhancing effects of nicotine: implications for the relationship between smoking, eating and weight. Physiology & Behavior. 2011;104:143–148. doi: 10.1016/j.physbeh.2011.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy EG, Fletcher PJ. The effects of nicotine exposure during Pavlovian conditioning in rats on several measures of incentive motivation for a conditioned stimulus paired with water. Psychopharmacology. 2014;231:2261–2271. doi: 10.1007/s00213-013-3375-3. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Stead LF, Gupta PC. Tobacco addiction. The Lancet. 2008;371:2027–2038. doi: 10.1016/S0140-6736(08)60871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Economic concepts for the analysis of behavior. Journal of the Experimental Analysis of Behavior. 1980;34:219–238. doi: 10.1901/jeab.1980.34-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics. Journal of the Experimental Analysis of Behavior. 1984;42:435–452. doi: 10.1901/jeab.1984.42-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychological Review. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Jones J, Raiff BR, Dallery J. Nicotine's enhancing effects on responding maintained by conditioned reinforcers are reduced by pretreatment with mecamylamine, but not hexamethonium, in rats. Experimental and Clinical Psychopharmacology. 2010;18:350–358. doi: 10.1037/a0020601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RT, Gollub LR. A review of positive conditioned reinforcement. Journal of the Experimental Analysis of Behavior. 1962;5:543–97. doi: 10.1901/jeab.1962.5-s543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analyses of Behavior: The Effect of Delay and of Intervening Events on Reinforcement Value. Vol. 5. Lawrence Erlbaum; Hillsdale, NJ: 1987. pp. 55–73. [Google Scholar]
- Motulsky HJ, Christopoulos A. Fitting models to biological data using linear and nonlinear regression: A practical guide to curve fitting. GraphPad Software, Inc.; San Diego, CA: 2003. [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology. 2004;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Levin ME, Mays KE, Donny EC, Caggiula AR, Sved AF. Bupropion and nicotine enhance responding for nondrug reinforcers via dissociable pharmacological mechanisms. Psychopharmacology. 2009;207:381–390. doi: 10.1007/s00213-009-1666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF. Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology. 2007;195:235–243. doi: 10.1007/s00213-007-0897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Caggiula AR, Craven L, Donny EC, Sved AF. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcer and increase with repeated drug injections. Drug and Alcohol Dependence. 2007;89:52–59. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, O'Brien LC, Hall MJ. The role of conditioning history and reinforcer strength in the reinforcement enhancing effects of nicotine in rats. Psychopharmacology. 2012;219:1119–1131. doi: 10.1007/s00213-011-2439-5. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology. 2013;228(3):479–486. doi: 10.1007/s00213-013-3054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Effects of acute and chronic nicotine on responses maintained by primary and conditioned reinforcers in rats. Experimental and Clinical Psychopharmacology. 2006;14:296–305. doi: 10.1037/1064-1297.14.3.296. [DOI] [PubMed] [Google Scholar]
- Raiff B, Dallery J. The generality of nicotine as a reinforcer enhancer in rats: Effects on responding maintained by primary and conditioned reinforcers and resistance to extinction. Psychopharmacology. 2008;201:305–314. doi: 10.1007/s00213-008-1282-9. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Responding maintained by primary reinforcing visual stimuli is increased by nicotine administration in rats. Behavioural Processes. 2009;82:95–99. doi: 10.1016/j.beproc.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology. 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Shahan TA. Observing behavior: Effects of rate and magnitude of primary reinforcement. Journal of the Experimental Analysis of Behavior. 2002;78:161–178. doi: 10.1901/jeab.2002.78-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver MT, Sweitzer M, Coddington S, Sheppard J, Verdecchia N, Caggiula AR, Donny EC. Precipitated withdrawal from nicotine reduces reinforcing effects of a visual stimulus for rats. Nicotine and Tobacco Research. 2012;14:824–832. doi: 10.1093/ntr/ntr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BA. Conditioned reinforcement: Experimental and theoretical issues. The Behavior Analyst. 1994;17:261–285. doi: 10.1007/BF03392675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO report on the global tobacco epidemic. World Health Organization; Geneva: 2011. [Google Scholar]
- Wyckoff LB. The role of observing responses in discrimination learning. Part I. Psychological Review. 1952;59:431–442. doi: 10.1037/h0053932. [DOI] [PubMed] [Google Scholar]