Abstract
Purpose
Inherited mutations in BRCA1 or BRCA2 (BRCA1/2) confer very high risk of breast and ovarian cancers. Genetic testing and counseling can reduce risk and death from these cancers if appropriate preventive strategies are applied, including risk-reducing salpingo-oophorectomy (RRSO) or risk-reducing mastectomy (RRM). However, some women who might benefit from these interventions do not take full advantage of them.
Methods
We evaluated RRSO and RRM use in a prospective cohort of 1,499 women with inherited BRCA1/2 mutations from 20 centers who enrolled in the study without prior cancer or RRSO or RRM, and were followed forward for the occurrence of these events. We estimated the age-specific usage of RRSO/RRM in this cohort using Kaplan-Meier analyses.
Results
Use of RRSO was 45% for BRCA1 and 34% for BRCA2 by age 40, and 86% for BRCA1 and 71% for BRCA2 by age 50. RRM usage was estimated to be 46% by age 70 in both BRCA1 and BRCA2 carriers. BRCA1 mutation carriers used RRSO more frequently than BRCA2 mutation carriers overall, but the uptake of RRSO in BRCA2 was similar after mutation testing and in women born since 1960. RRM uptake was similar for both BRCA1 and BRCA2. Childbearing influenced use of RRSO and RRM in both BRCA1 and BRCA2.
Conclusions
Uptake of RRSO is high, but some women are still diagnosed with ovarian cancer before undergoing RRSO. This suggests that research is needed to understand the optimal timing of RRSO to maximize risk reduction and limit potential adverse consequences of RRSO.
Keywords: Risk reduction, BRCA1, BRCA2, Surgical prevention, uptake
INTRODUCTION
BRCA1 and BRCA2 (BRCA1/2) mutations confer elevated risks of developing breast and ovarian cancer [3]. Genetic testing for BRCA1/2 mutations has value because medical decisions can be made using this information[20]. It has been well established that the use of preventive surgery can dramatically reduce cancer risks and mortality in women who carry these mutations [5–8, 13, 21]. Because there is no effective early detection for ovarian cancer that reduces mortality[15], and most ovarian tumors are detected at late (incurable) stages, it has been recommended that women undergo risk-reducing salpingo-oophorectomy (RRSO) by age 35–40 and once the completion of childbearing[1, 11, 19]. However, there are also long term risks and quality of life concerns associated with premature menopause, hence women might delay timing of oophorectomy (Ref Bradbury et al). Breast cancer early detection and preventive strategies are available for women with BRCA1/2 mutations and women can chose between these options and risk-reducing mastectomy (RRM).
The consequences for under-utilization of RRSO include elevated cancer incidence and mortality rates in women who do not undergo this surgery in a timely manner. Despite the proven effectiveness, uptake of these strategies still varies greatly and appears to be underutilized. Utilization of RRSO among BRCA1/2 mutation carriers has been reported to be no higher than 75% overall, 36% in unaffected women within 5 years of genetic testing, and 49% among breast cancer cases within 5 years of genetic testing[2, 4, 9, 10, 12, 14, 17, 18, 22, 23]. Utilization of RRM has been reported to be lower than RRSO in most studies.
While prior studies have demonstrated underutilization of RRSO and low rates RRM, they are limited in that they involved relatively small sizes, did not stratify utilization by BRCA1 and BRCA2 mutation carriers separately, were not prospective in nature, and do not account for concurrent events in the natural history of cancer or other forms of cancer prevention (e.g., screening mammography/MRI, SERMs, etc.). In this paper, we present the results of RRSO and RRM utilization in a large prospective cohort of women with BRCA1/2 mutations to obtain a better estimate of cancer preventive strategies, and to provide data that may help to increase appropriate utilization of these preventive options.
METHODS
Participants
Women with inherited, disease-associated BRCA1/2 mutations were identified from 20 centers of the PROSE consortium using research protocols as previously described[5, 6]. All participants underwent an informed consent process for participation in research. This protocol was approved by each institution’s IRB. Study participants were enrolled as a cohort with time of follow up starting from patient ascertainment into the research program. Genetic testing was performed per institutional guidelines and all patients received post-testing counseling to review medical management options. Women who declined RRSO or RRM were offered increased surveillance at all centers according to established guidelines. At US sites, this consisted of annual mammogram and annual MRI for those with breast tissue, and every 6–12 month transvaginal ultrasound and CA125 for those with ovaries in place (www.nccn.com). In the UK, women were offered yearly mammograms, as well as yearly MRI until age 50. Ovarian cancer screening consisted of trans-vaginal ultrasound (TVUS) and 4-monthly CA125, but only as part of the UKFOCSS screening trial which stopped recruiting in 201021. Participants were eligible for the study if they had no cancer diagnosis and no RRSO/RRM at the time of ascertainment
Prospective Follow-Up
Usage of RRSO or RRM was the primary end points of interest. Start of follow-up was from the age at study recruitment. For the probability of undergoing RRSO, age was right censored at the age of RRSO, RRM, diagnosis of ovarian or breast cancer, death, or the last follow-up. For the probability of using RRM, age was right centered at the time of undergoing RRM, diagnosis of ovarian or breast cancer, death, RRSO, or the last follow-up. Women were retained in the analyses if they were diagnosed with an occult ovarian cancer at RRSO.
Statistical Analysis
We used Kaplan-Meier analysis to estimate the cumulative probability of undergoing RRSO and/or RRM by age, stratified on BRCA1/BRCA2 carrier status, birth year (before and after year 1960), mutation testing status, or parity. A log-rank test was performed to assess the difference in the surgery uptake between strata. Similar analyses were performed to estimate rates of RRSO uptake among women who developed breast cancer. Follow-up time was accumulated from the time at breast cancer diagnosis until time at RRSO, RRM, death, or last follow-up. We also estimated RRSO uptake among women who used RRM. For this analysis, follow-up was accumulated from the age at RRM to the age of RRSO, ovarian cancer, death, or last follow-up. When missing data were encountered, the individual was dropped from the analysis that involved the missing data point, but the individual was included in other analyses where complete data were available. Inferences of statistical significance were made at the p=0.05 level based on two-sided hypotheses. All analyses were undertaken using software R (http://www.r-project.org/).
RESULTS
We studied a prospective cohort of 1,499 women with disease-associated BRCA1/2 mutations (Supplementary Table 1) born between 1899 and 1985 (Mean: 1960). 927 (62%) women had never undergone RRSO nor RRM, 444 (30%; mean age at RRSO: 43.6 years) had undergone RRSO only, 171 (11%; mean age at RRM: 37.4 years) had undergone RRM only, and 164 (11%) underwent both RRSO and RRM. Other commonly occurring events included RRSO after a breast cancer diagnosis (139; 9%), RRM after RRSO (74; 5%) and RRSO before RRM (74; 5%). Ttotals presented in Supplementary Table 1 and in this paragraph reflect the censoring of observations as described above. These figures reflect the number of observations actually used in analysis.
RRSO
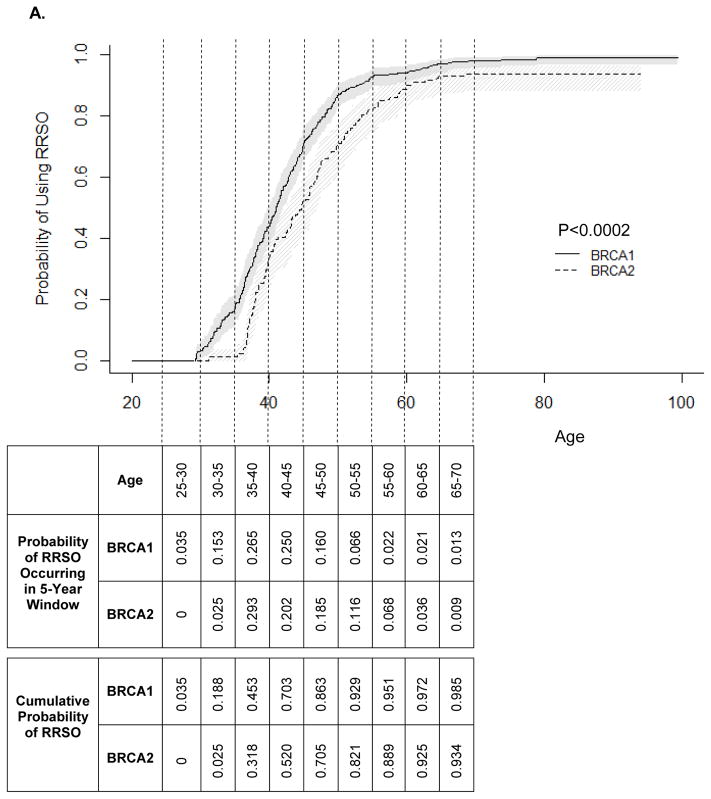
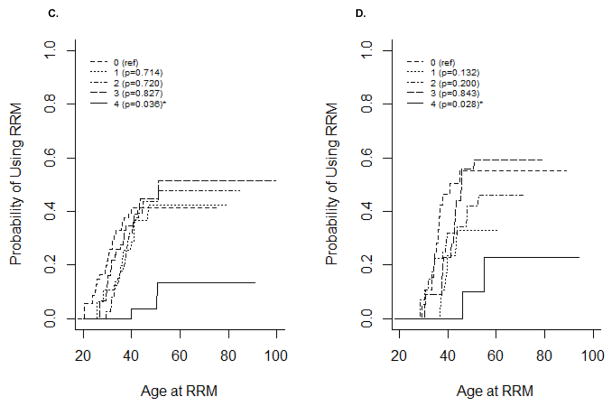
Age-specific utilization of RRSO is presented in Figure 1A. BRCA1 mutation carriers used RRSO more frequently than BRCA2 mutation carriers (P<0.0002). The cumulative probability estimates for RRSO indicate that most women with BRCA1 or BRCA2 mutation will undergo RRSO during their lifetimes (98.5% vs. 93.4% by age 70; Figure 1A). RRSO occurred between age 29.3 and age 79 years in BRCA1 mutation carriers (mean: 43.0 years) and age 31.2 and age 68.5 in BRCA2 mutation carriers (mean: 46.3 years). RRSO was most commonly used by women ages 35–40. Overall, RRSO usage decreased after age 40. No RRSO was observed in BRCA1 mutation carriers before age 25 and in BRCA2 mutation carriers before age 30. The age-specific rate of RRSO usage was significantly higher in BRCA1 compared with BRCA2 mutation carriers (P<0.001).
Figure 1.
Kaplan-Meier estimates for the cumulative probability of surgery. A) RRSO; B) RRM.
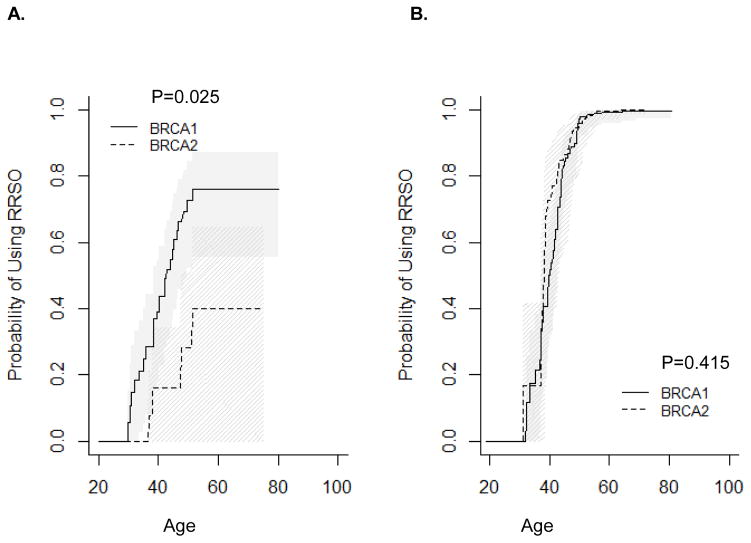
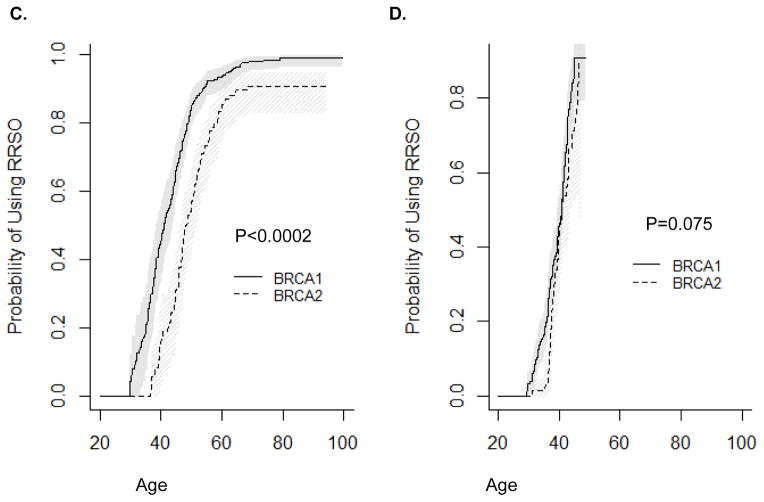
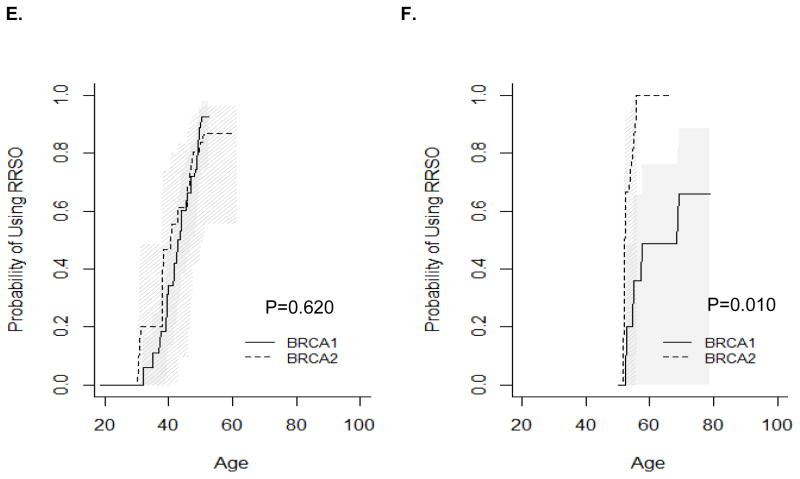
RRSO usage also differed by relative timing of mutation testing and by birth cohort. Before mutation testing, BRCA1 mutation carriers were significantly more likely to undergo RRSO than BRCA2 mutation carriers (Figure 2A, P=0.025), while there was no difference in use of RRSO after mutation testing in BRCA1 vs. BRCA2 mutation carriers (Figure 2B, P=0.415). When stratified by birth cohort, BRCA1 mutation carriers born in or before 1960 were significantly more likely to undergo RRSO than BRCA2 mutation carriers (Figure 2C, P<0.0002). Among women born after 1960 or tested for mutation before age 50 years, there was no difference in utilization of RRSO in BRCA1 vs. BRCA2 mutation carriers (Figure 2D, P=0.075; Figure 2E, P=0.62). Among women tested before age 50 years, the difference was significant (Figure 2F, P=0.01), but the p-value needs to be interpreted with caution due to small sample size.
Figure 2.
Timing of RRSO relative to mutation testing. A) RRSO before mutation testing; B) RRSO after mutation testing; C) Women born before or in 1960; D) Women born after 1960; E) Women tested before age 50 years (women excluded if follow up was less than 0.5 years); F) Women tested after age 50 years (women excluded if follow up was less than 0.5 years).
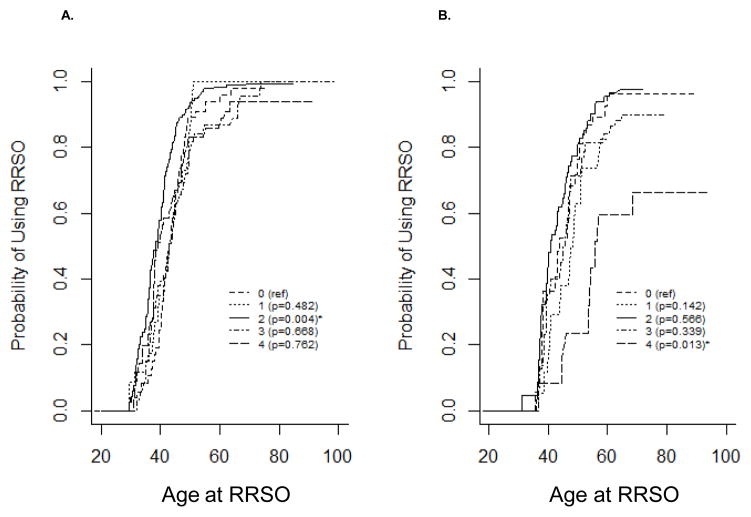
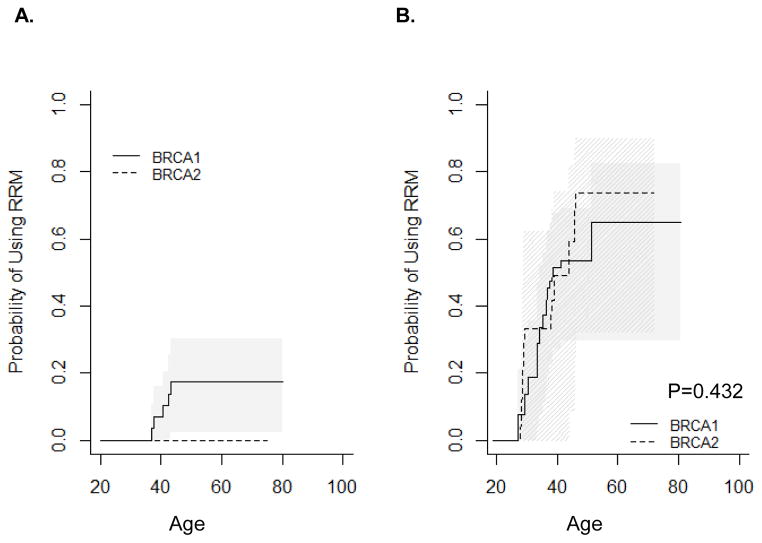
As most clinical recommendations regarding the use of RRSO refer to childbearing, family planning may also affect a woman’s decision about use of RRSO. Figure 3 presents Kaplan-Meier estimates for the probability of using RRSO stratified by number of live births. BRCA1 mutation carriers who had two children were most likely to use RRSO (Figure 3A, P=0.004) compared with nulliparous women. BRCA1 mutation carriers who had a history of 1, 3, or 4+ live births used RRSO similarly to nulliparous women. BRCA2 mutation carriers who had four or more children were less likely to use RRSO (Figure 3B, P=0.013) compared with nulliparous women. BRCA2 mutation carriers who had a history of 1–3 live births used RRSO similarly to nulliparous women. The estimated proportion of women who underwent RRSO by age is presented in Table 1.
Figure 3.
Kaplan-Meier estimates for the cumulative probability of using surgery stratified by the number of live births. A) RRSO-BRCA1; B) RRSO-BRCA2; C) RRM-BRCA1; D) RRM-BRCA2.
Table 1.
Estimated proportion of women using RRSO by age, birth cohort, and mutation testing.
| Age (Mean and 95% CI) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean Age (Range) | Total Sample | 35 | 45 | 50 | |||
|
|
|||||||
| BRCA1 | 43.0 (29.3–79.0) | 965 | 18.1 (12.5–23.4) | 71.4 (65.6–76.2) | 86.8 (82.5–90.0) | ||
| BRCA2 | 46.3 (31.2–68.5) | 534 | 1.3 (0–3.7) | 51.9 (42.1–60.1) | 70.9 (61.9–77.8) | ||
|
| |||||||
| Birth Cohort | Before/in 1960 | BRCA1 | 45.9 (29.9–79.0) | 460 | 21.0 (7.5–32.5) | 66.4 (57.1–73.7) | 84.7 (78.8–89.0) |
| BRCA2 | 50.2 (36.7–68.5) | 279 | 0 | 30.9 (17.5–42.0) | 56.9 (44.2–66.7) | ||
| After 1960 | BRCA1 | 37.7 (29.3–45.1) | 505 | 17.2 (10.8–23.1) | 90.8 (79.1–95.9) | 90.8 (79.1–95.9) | |
| BRCA2 | 39.2 (31.2–46.5) | 255 | 1.5 (0–4.5) | 71.1 (53.0–82.2) | 89.1 (47.6–97.8) | ||
|
| |||||||
| Mutation Testing | Before | BRCA1 | 40.3 (29.9–51.5) | 166 | 24.9 (5.3–40.4) | 57.9 (36.8–72.0) | 72.8 (52.2–84.5) |
| BRCA2 | 43.4 (36.7–51.4) | 95 | 0 | 16.1 (0–34.3) | 28.1 (0–51.2) | ||
| After | BRCA1 | 44.3 (32.1–69.3) | 175 | 17.3 (0–33.3) | 84.4 (70.7–91.7) | 97.3 (91.1–99.2) | |
| BRCA2 | 47.8 (31.2–64.8) | 118 | 16.7 (0–41.7) | 86.4 (59.3–95.5) | 96.0 (84.0–99.0) | ||
|
| |||||||
| Mutation Testing Age | Before 50 | BRCA1 | 42.6 (32.1–50.3) | 156 | 17.3 (0–33.3) | 84.4 (70.7–91.7) | 97.3 (91.1–99.2) |
| BRCA2 | 42.9 (31.2–51.0) | 91 | 16.7 (0–41.7) | 86.4 (59.3–95.5) | 96.0 (84.0–99.0) | ||
| After 50 | BRCA1 | 58.7 (52.6–69.3) | 19 | 48.8 (0–76.0)* | 79.5 (0–96.0)** | 86.3 (15.3–97.8)*** | |
| BRCA2 | 56.2 (50.9–64.8) | 27 | 90.0 (25.5–98.7)* | 100** | 100*** | ||
|
| |||||||
| Mutation Testing Age# | Before 50 | BRCA1 | 43.3 (32.1–50.3) | 112 | 5.9 (0–16.4) | 60.2 (38.3–74.4) | 88.8 (71.3–95.7) |
| BRCA2 | 43.6 (31.2–51.0) | 59 | 20.0 (0–48.4) | 61.1 (9.7–83.2) | 83.8 (48.9–94.9) | ||
| After 50 | BRCA1 | 58.6 (52.6–69.3) | 15 | 36.0 (0–65.6)* | 48.8 (0–76.0)** | 65.9 (0–88.7)*** | |
| BRCA2 | 55.9 (52.1–64.4) | 14 | 77.8 (0–96.3)* | 100** | 100*** | ||
|
| |||||||
| Live Births | Exactly 2 Not 2 | BRCA1 | 41.6 (29.4–69.3) | 318 | 26.2(14.6–36.2) | 84.4(76.3–89.7) | 93.5(88.3–96.4) |
| 44.1 (29.3–79.0) | 625 | 13.6(7.4–19.4) | 63.1(55.1–69.7) | 82.3(75.7–87.1) | |||
|
| |||||||
| Exactly 2 Not 2 | BRCA2 | 45.3 (31.2–64.4) | 180 | 4.6(0–12.9) | 64.7(48.9–75.5) | 81.4(68.6–89.0) | |
| 47.2 (35.4–68.5) | 345 | 0 | 43.2(30.1–53.9) | 62.4(49.2–72.1) | |||
Rates are for age 55 years;
Rates are for age 65 years;
Rates are for age 70 years;
Women who had less than 0.5 years of follow up were excluded.
The estimated proportion of women who underwent RRSO increased 1, 2, 5, and 10 years after being diagnosed with breast cancer (Table 2) to 66.2% in BRCA1 and 59.6% in BRCA2 10 years after diagnosis. The increases were similar in those women who had been diagnosed with breast cancer before age 50 and among BRCA2 mutation carriers diagnosed after 50, but the usage was lower among women who were diagnosed with breast cancer after age 50 in BRCA1 mutation carriers (26.7% 10 years after diagnosis).
Table 2.
Estimated proportion of women (95% CI) who used surgery 1, 2, 5, 10 years after being diagnosed with breast cancer or RRSO.
| Years Since Breast Cancer: | 1 | 2 | 5 | 10 | ||
|---|---|---|---|---|---|---|
| RRSO Use After Breast Cancer | Overall | BRCA1 | 22.4 (15.7–28.5) | 36.6 (28.4–43.8) | 51.2 (41.5–59.4) | 66.2 (53.2–75.6) |
| BRCA2 | 17.4 (10.0–24.3) | 27.3 (18.3–35.3) | 41.6 (30.4–51.1) | 59.6 (44.1–70.9) | ||
| Breast cancer before age 50 | BRCA1 | 23.5 (16.4–30.0) | 38.6 (29.8–46.3) | 53.5 (43.2–61.9) | 69.0 (55.6–78.3) | |
| BRCA2 | 19.8 (10.6–28.1) | 31.9 (20.7–41.5) | 46.9 (33.5–57.6) | 61.4 (43.5–73.6) | ||
| Breast cancer after age 50 | BRCA1 | 12.6 (0–27.6) | 26.7 (0.2–46.1) | 26.7 (0.2–46.1) | 26.7 (0.2–46.1) | |
| BRCA2 | 10.7 (0–21.5) | 14.4 (0.3–26.6) | 25.8 (4.7–42.3) | 57.6 (15.6–78.7) |
| Years Since RRSO: | 1 | 2 | 5 | 10 | ||
|---|---|---|---|---|---|---|
| RRM Use After | BRCA1 | 8.6 (5.2–11.9) | 13.8 (9.5–18.0) | 19.1 (13.7–24.1) | 29.1 (20.1–37.1) | |
| RRSO | BRCA2 | 9.7 (4.0–14.9) | 11.6 (5.5–17.4) | 17.9 (9.8–25.3) | 20.3 (11.0–28.6) |
RRM
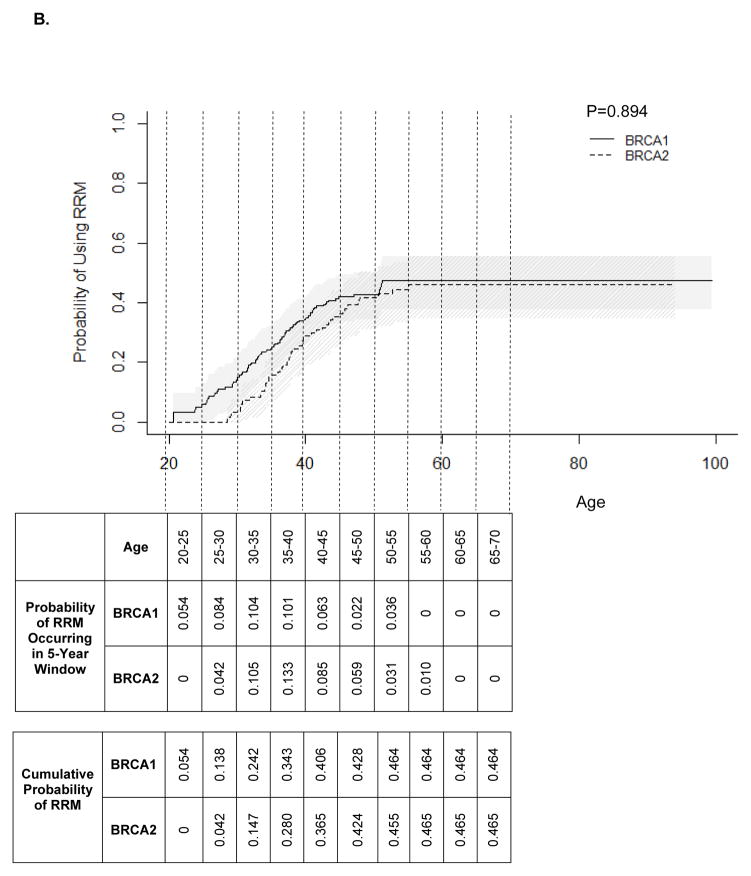
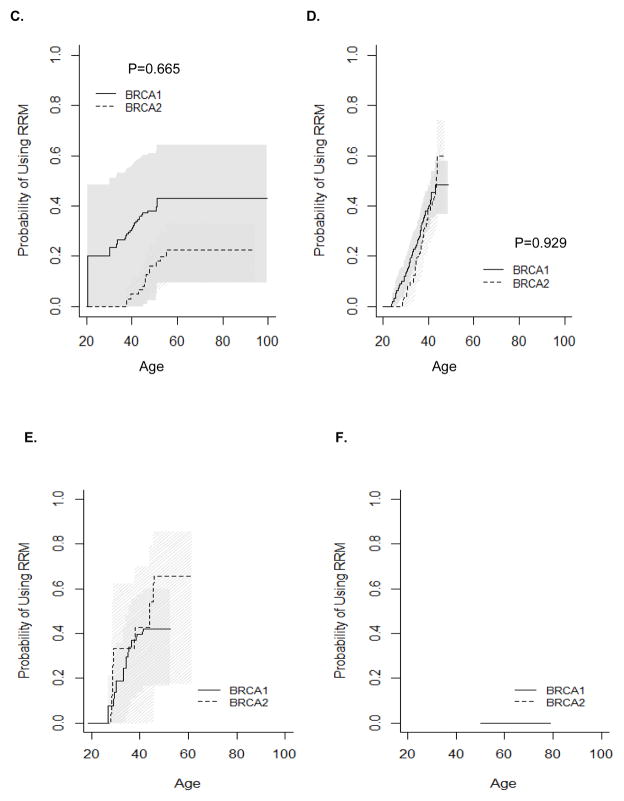
Age-specific utilization of RRM is presented in Figure 1B. BRCA1 mutation carriers were estimated to use RRM as frequently as BRCA2 mutation carriers by age 60, the age at the latest RRM (46%; P=0.894). The cumulative probability estimates for RRM indicate that about half of women with either BRCA1 or BRCA2 mutation will undergo RRM during their lifetimes (Figure 1B). The earliest RRM occurred at age 20.6 in BRCA1 mutation carriers and age 28.6 in BRCA2 mutation carriers. RRM was most commonly used by women ages 30–35 in BRCA1 mutation carriers and in ages 35–40 in BRCA2 mutation carriers. RRM usage decreased after age 40, with no RRM after age 55 in BRCA1 and none after age 60 in BRCA2 mutation carriers. RRM usage did not differ significantly by birth cohort, and data was insufficient to compare RRM usage by relative timing of mutation testing (Figure 4).
Figure 4.
Kaplan-Meier estimates for the cumulative probability of RRM. A) Before mutation testing (NB: No P-value could be computed as no BRCA2 carriers underwent RRM before mutation testing); B) After mutation testing; C) Women born before or during 1960; D) Women born after 1960; E) Women tested before age 50 years (women excluded if follow up was less than 0.5 years); F) Women tested after age 50 years (women excluded if follow up was less than 0.5 years); G) Women who had previously undergone RRSO.
Unlike oophorectomy, which is influenced by reproductive choices, there is no recommendation for RRM relative to childbearing. Figure 3C–D presents Kaplan-Meier estimates for the probability of using RRM stratified by number of live births. BRCA1 and BRCA2 mutation carriers who had four or more children were least likely to use RRM (P=0.036 and P=0.028, respectively) compared with nulliparous women. BRCA1 mutation carriers who had a history of 1, 2, or 3 live births used RRM similarly to nulliparous women. This likely reflects delay of RRM among women who choose to have more children.
RRM and RRSO
164 women underwent both RRSO and RRM. Of these, 74 underwent RRM before RRSO, 43 occurred simultaneously, and 74 underwent RRM after RRSO. None of the BRCA2 women whose mutation test happened after age 50 years used RRM, while there was only one BRCA1 woman whose mutation test happened after age 50 years used RRM.
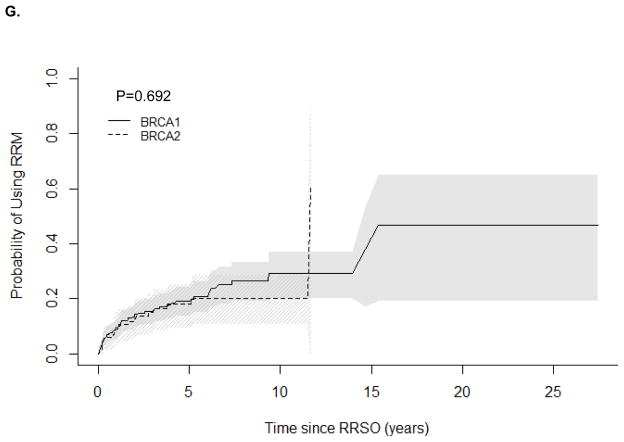
The correlation between age of RRM and age of RRSO was 0.90 (P<0.0001). We observed no difference in the cumulative probability of RRM after RRSO by BRCA1/2 status (P=0.692). The estimated proportion of women who underwent RRM increased 1, 2, 5, and 10 years after RRSO (Table 2 and Supplementary Figure 1) to 29.1% in BRCA1 and 20.3% in BRCA2 10 years after diagnosis.
DISCUSSION
We used a prospective cohort of BRCA1/2 mutation positive women to evaluate usage of RRSO and RRM. We modeled the lifetime utilization of RRSO and RRM, and showed that uptake of RRSO is improved in BRCA2 carriers after genetic testing. We also identify childbearing as having an influence on utilization of both RRSO and RRM. Finally, we estimate the usage of RRSO and RRM after a breast cancer diagnosis and the use of RRM after RRSO. Our results show that uptake of RRSO is high, but usage occurs later than recommended. BRCA2 mutation carriers do not use RRSO as often as BRCA1 mutation carriers until they have been genetically tested.
The present results represent an advance over prior studies of this type because we have used failure-time analyses in a prospective cohort to estimate usage of RRSO and RRM. Most previous studies have used retrospective cohorts, case-series, or cross-sectional studies to describe the usage of RRSO and/or RRM without accounting for follow-up or other events (e.g., other preventive surgeries or cancer diagnoses) [2, 4, 9, 10, 12, 14, 22, 23]. These previous studies have estimated wide ranges of RRSO or RRM usage from less than 30% to over 75% depending on the study sample or ascertainment strategy. Most concluded that RRSO is under-utilized. Similar variability in timing of surgery with respect to age or reproductive history has been reported.
Here, we demonstrate that RRSO is estimated to be used by most women in their lifetimes, as would be recommended by most professional bodies. It has been suggested that RRSO does not always occur during the period these organizations would recommend, usually by age 40 or after completion of childbearing. Our data suggest that about 86% of BRCA1 mutation carriers and 71% of BRCA2 mutation carriers undergo RRSO by age 50. In previous series, ovarian cancer is diagnosed in a non-trivial proportion of women with BRCA1/2 mutations before age 50. The earliest documented ovarian cancer in the PROSE data set was diagnosed at age 30.1 years, but it is rare for ovarian cancer to be diagnosed before age 40. In BRCA1 mutation carriers from the PROSE data, we observed 29 ovarian cancer cases before the age of 50 (3%), including 7 before the age of 40 (0.7%), and 2 before the age of 35 (0.2%) among 965 BRCA1 mutation carriers. In BRCA2 mutation carriers from the PROSE data, we observed 3 ovarian cancer cases before the age of 50 (0.06%), none before the age of 40, and none before the age of 35 among 534 BRCA2 mutation carriers. These values are similar to those reported previously in large retrospective cohorts20. Given the early age of some ovarian cancers, and the current inability to identify which women will develop these early cancers, under-utilization of RRSO is a major concern.
Because other prevention and early detection strategies are available for breast cancer, including mammography and MRI screening, use of selective estrogen receptor modifiers, and other interventions, RRM is an option rather than a mandate. In the present data, we estimate that less than half of BRCA1/2 mutation carriers undergo RRM in their lifetimes, with most surgeries occurring between the ages of 25–45.
While there are substantial benefits to RRSO and RRM, these surgical interventions are not without potentially negative psychosocial or medical consequences, and the use RRSO/RRM should be only undertaken in the context of genetic counseling that lays out the risks and benefits to ensure optimal decision-making by each patient. The body of literature regarding factors that influence decision-making suggests that decisions about RRSO and RRM are determined by psychosocial factors, mutation carriage, age, and prior cancer 22–28.
The present study has many advantages over prior studies, but it remains limited in a number of ways. First, we only consider the use of RRSO and RRM. Other preventive practices including secondary breast and ovarian screening occurred during the follow up period. As an observational study, as opposed to a randomized trial, we were not able to account completely for the use of other preventive strategies as has been done in other studies[16, 18]. These may have influenced some women’s use of RRSO or RRM. Second, while our prospectively ascertained sample is large, we have relatively small observations in some subgroups. Indeed, as shown in Table 1, some events were represented by only a handful of participants. It was therefore difficult to obtain statistically significant inferences about some groups of interest. Finally, the sample set studied here came from referral centers, which may not represent the entire population receiving genetic testing for BRCA1/2. However, these centers are representative of settings in which genetic testing and counseling practices would be performed today.
Our results support the knowledge that genetic testing and counseling increase the usage of RRSO in BRCA2 mutation carriers. Lifetime use of RRSO is estimated to be very high, but the timing of these surgeries remains suboptimal for cancer prevention in BRCA1/2 mutation carriers.
Supplementary Material
Supplementary Table 1. Description of RRSO and RRM utilization with respect to other preventive surgical events and cancer
Supplementary Figure 1: Kaplan-Meier estimates for the cumulative probability of RRSO for women who had developed breast cancer. A) All Women; B) Breast cancer diagnosed before age 50; C) Breast cancer diagnosed at or after age 50.
Acknowledgments
The PROSE Consortium acknowledges the following centers and individuals who contributed to the present study: University of Vienna, Austria (Christian Singer); Beth Israel, Boston, MA (Nadine Tung); Baylor-Charles A. Sammons Cancer Center (Joanne L. Blum, Becky Althaus, Gabrielle Ethington, Estelle Brothers); City of Hope, Duarte, CA (Jeffrey Weitzel); Creighton University, Omaha, NE (Carrie Snyder, Henry T. Lynch, Patrice Watson); Dana-Farber Cancer Institute, Boston, MA (Katherine Corso, Kathryn Stoeckert, Judy E. Garber); NorthShore University HealthSystem, Evanston, IL (Peter J. Hulick, Christina Selkirk, Scott M. Weissman); Fox Chase Cancer Center, Philadelphia, PA (Mary B. Daly); Guy’s Hospital and St. Thomas Foundation Trust, London, UK (Gabriella Pichert, Caroline Langman, Leena da Silva); Georgetown University, Washington, DC (Camille Jasper, Claudine Isaacs); University of California, Los Angeles (Patricia A. Ganz, Joyce L. Seldon); Mayo Clinic College of Medicine, Rochester, MN (Fergus Couch); Netherlands Cancer Institute, Amsterdam, Netherlands (Marc van Beurden, Laura van ‘t Veer); The Institute of Cancer Research & Royal Marsden NHS Foundation Trust, London & Sutton (Rosalind Eeles, Elizabeth Bancroft, Elizabeth Page, Lucia D’Mello, Susan Shanley, Audrey Ardern-Jones, Elena Castro, Anita Mitra, Kelly Kohut, Jennifer Wiggins, The Carrier Clinic Collaborators); St. Mary’s Hospital, Manchester, UK (Gareth Evans, Fiona Lalloo); University of Texas-Southwestern, Dallas (n=63, Gail Tomlinson); University of Chicago, Chicago, IL (Shelly Cummings, Olufunmilayo Olopade); University of Pennsylvania, Philadelphia, PA (Susan Domchek, Tara M Friebel, Timothy Rebbeck, Jill Stopfer, Jacquelyn Powers, Katherine Nathanson); University of Utah, Salt Lake City, UT and University of California, Irvine (Susan Neuhausen, Linda Steele); Women’s College Hospital, Toronto, CA (Steven A. Narod); Yale University, New Haven, CT (Ellen T. Matloff, Karina L. Brierly).
Research Support
This study was supported by grants from the Public Health Service (R01-CA164305 to XLC and JBC, R01-CA83855 and R01-CA102776 to TRR), The Basser Center for BRCA at the University of Pennsylvania (to TRR, SMD), Komen Foundation for the Cure (SMD), the Dana-Farber/Harvard Cancer Center SPORE in BC P50 CA-089393 (to JEG), the Department of Defense (DAMD-17-96-I-6088 to AKG; DAMD-17-94-J-4340 and DAMD-17-97-I-7112 to HTL), P30-CA51008-15 (to Georgetown University), The Utah Cancer registry (funded by Public Health Service Grant NO1-CN-6700) and the Utah State Department of Health, the Nebraska State Cancer and Smoking-Related Diseases Research Program (LB595 to HTL), P30-CA-16042 (to PAG), Cancer Research UK Grant Number C5047/A7357 (to RE), and NCI P30 CA51008-12 (to CI). OIO is Doris Duke Distinguished Clinical Scientist. RE acknowledges The Support of the NIHR to The Biomedical Research Centre at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust.
Footnotes
Conflict of Interest
None
References
- 1.Balmaña J, Díez O, Rubio IT, Cardoso F, Group EGW. BRCA in breast cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):vi31–34. doi: 10.1093/annonc/mdr373. [DOI] [PubMed] [Google Scholar]
- 2.Beattie MS, Crawford B, Lin F, Vittinghoff E, Ziegler J. Uptake, time course, and predictors of risk-reducing surgeries in BRCA carriers. Genet Test Mol Biomarkers. 2009;13:51–56. doi: 10.1089/gtmb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins IM, Milne RL, Weideman PC, McLachlan SA, Friedlander ML, Hopper JL, Phillips KA KCFCFRIFB Cancer. Preventing breast and ovarian cancers in high-risk BRCA1 and BRCA2 mutation carriers. Med J Aust. 2013;199:680–683. doi: 10.5694/mja13.10848. [DOI] [PubMed] [Google Scholar]
- 5.Domchek SM, Friebel TM, Neuhausen SL, Wagner T, vans GE, Isaacs C, Garber JE, Daly MB, Eeles R, Matloff E, Tomlinson GE, Van’t Veer L, Lynch HT, Olopade OI, Weber BL, Rebbeck TR. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol. 2006;7:223–229. doi: 10.1016/S1470-2045(06)70585-X. [DOI] [PubMed] [Google Scholar]
- 6.Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles R, Pichert G, Van t’veer L, Tung N, Weitzel JN, Couch FJ, Rubinstein WS, Ganz PA, Daly MB, Olopade OI, Tomlinson G, Schildkraut J, Blum JL, Rebbeck TR. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domchek SM, Stopfer JE, Rebbeck TR. Bilateral risk-reducing oophorectomy in BRCA1 and BRCA2 mutation carriers. J Natl Compr Canc Netw. 2006;4:177–182. doi: 10.6004/jnccn.2006.0017. [DOI] [PubMed] [Google Scholar]
- 8.Finch AP, Lubinski J, Møller P, Singer CF, Karlan B, Senter L, Rosen B, Maehle L, Ghadirian P, Cybulski C, Huzarski T, Eisen A, Foulkes WD, Kim-Sing C, Ainsworth P, Tung N, Lynch HT, Neuhausen S, Metcalfe KA, Thompson I, Murphy J, Sun P, Narod SA. Impact of Oophorectomy on Cancer Incidence and Mortality in Women With a BRCA1 or BRCA2 Mutation. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.53.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friebel TM, Domchek SM, Neuhausen SL, Wagner T, Evans DG, Isaacs C, Garber JE, Daly MB, Eeles R, Matloff E, Tomlinson G, Lynch HT, Tung N, Blum JL, Weitzel J, Rubinstein WS, Ganz PA, Couch F, Rebbeck TR. Bilateral prophylactic oophorectomy and bilateral prophylactic mastectomy in a prospective cohort of unaffected BRCA1 and BRCA2 mutation carriers. Clin Breast Cancer. 2007;7:875–882. doi: 10.3816/CBC.2007.n.053. [DOI] [PubMed] [Google Scholar]
- 10.Garcia C, Wendt J, Lyon L, Jones J, Littell RD, Armstrong MA, Raine-Bennett T, Powell CB. Risk management options elected by women after testing positive for a BRCA mutation. Gynecol Oncol. 2013 doi: 10.1016/j.ygyno.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 11.A.C.o.O.a. Gynecologists, A.C.o.P. Bulletins--Gynecology, A.C.o. Genetics, S.o.G. Oncologists. ACOG Practice Bulletin No. 103: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2009;113:957–966. doi: 10.1097/AOG.0b013e3181a106d4. [DOI] [PubMed] [Google Scholar]
- 12.Kauff N, Domchek S, Friebel T, Robson M, Lee J, Garber J, Isaacs C, Evans G, Lynch H, Eeles R, Neuhausen S, Daly M, Matloff E, Blum J, Sabbatini P, Hudis C, Norton L, Barakat R, Offit K, Rebbeck T. Risk-Reducing Salpingo-Oophorectomy for the Prevention of BRCA1 and BRCA2 Associated Breast and Gynecologic Cancer: A Multi-Center, Prospective Study. Journal of Clinical Oncology. 2008;26:1331–1337. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, Ellis NA, Boyd J, Borgen PI, Barakat RR, Norton L, Castiel M, Nafa K, Offit K. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609–1615. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 14.Mannis GN, Fehniger JE, Creasman JS, Jacoby VL, Beattie MS. Risk-reducing salpingo-oophorectomy and ovarian cancer screening in 1077 women after BRCA testing. JAMA Intern Med. 2013;173:96–103. doi: 10.1001/2013.jamainternmed.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menon U, Griffin M, Gentry-Maharaj A. Ovarian cancer screening-Current status, future directions. Gynecol Oncol. 2014;132:490–495. doi: 10.1016/j.ygyno.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metcalfe KA, Birenbaum-Carmeli D, Lubinski J, Gronwald J, Lynch H, Moller P, Ghadirian P, Foulkes WD, Klijn J, Friedman E, Kim-Sing C, Ainsworth P, Rosen B, Domchek S, Wagner T, Tung N, Manoukian S, Couch F, Sun P, Narod SA. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int J Cancer. 2008;122:2017–2022. doi: 10.1002/ijc.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metcalfe KA, Birenbaum-Carmeli D, Lubinski J, Gronwald J, Lynch H, Moller P, Ghadirian P, Foulkes WD, Klijn J, Friedman E, Kim-Sing C, Ainsworth P, Rosen B, Domchek S, Wagner T, Tung N, Manoukian S, Couch F, Sun P, Narod SAG Hereditary Breast Cancer Clinical Study. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int J Cancer. 2008;122:2017–2022. doi: 10.1002/ijc.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metcalfe KA, Snyder C, Seidel J, Hanna D, Lynch HT, Narod S. The use of preventive measures among healthy women who carry a BRCA1 or BRCA2 mutation. Fam Cancer. 2005;4:97–103. doi: 10.1007/s10689-005-4215-3. [DOI] [PubMed] [Google Scholar]
- 19.NCCN. NCCN guidelines for detection, prevention, and risk reduction: genetic/familial high risk assessment: breast and ovarian (v 2.2013) Cancer guidelines <span data-locatortype=“url”>. 2013 [Google Scholar]
- 20.Plon SE, Cooper HP, Parks B, Dhar SU, Kelly PA, Weinberg AD, Staggs S, Wang T, Hilsenbeck S. Genetic testing and cancer risk management recommendations by physicians for at-risk relatives. Genet Med. 2011;13:148–154. doi: 10.1097/GIM.0b013e318207f564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van’t Veer L, Garber JE, vans GE, Isaacs C, Daly MB, Matloff E, Olopade OI, Weber BL. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 22.Rhiem K, Foth D, Wappenschmidt B, Gevensleben H, Büttner R, Ulrich U, Schmutzler RK. Risk-reducing salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers. Arch Gynecol Obstet. 2011;283:623–627. doi: 10.1007/s00404-010-1476-3. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MD, Isaacs C, Graves KD, Poggi E, Peshkin BN, Gell C, Finch C, Kelly S, Taylor KL, Perley L. Long-term outcomes of BRCA1/BRCA2 testing: risk reduction and surveillance. Cancer. 2012;118:510–517. doi: 10.1002/cncr.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Description of RRSO and RRM utilization with respect to other preventive surgical events and cancer
Supplementary Figure 1: Kaplan-Meier estimates for the cumulative probability of RRSO for women who had developed breast cancer. A) All Women; B) Breast cancer diagnosed before age 50; C) Breast cancer diagnosed at or after age 50.