Abstract
Plants high in eugenol, a phenylpropanoid compound, are used as folk medicines to alleviate diseases including hypertension. Eugenol has been demonstrated to relax conduit and ear arteries and reduce systemic blood pressure, but mechanisms involved are unclear. Here, we studied eugenol regulation of resistance-size cerebral arteries that control regional brain blood pressure and flow and investigated mechanisms involved. We demonstrate that eugenol dilates arteries constricted by either pressure or membrane depolarization (60 mM K+) in a concentration-dependent manner. Experiments performed using patch-clamp electrophysiology demonstrated that eugenol inhibited voltage-dependent calcium (Ca2+) currents, when using Ba2+ as a charge carrier, in isolated cerebral artery smooth muscle cells. Eugenol inhibition of voltage-dependent Ca2+ currents involved pore block, a hyperpolarizing shift ( ~−10 mV) in voltage-dependent inactivation, an increase in the proportion of steady-state inactivating current, and acceleration of inactivaiton rate. In summary, our data indicate that eugenol dilates cerebral arteries via multi-modal inhibition of voltage-dependent Ca2+ channels.
Keywords: eugenol, voltage-dependent calcium channel, vasorelaxation, resistance-size artery
Introduction
Eugenol, a phenylpropanoid compound, is found in dietary plants, including clove (Syzygium aromaticum), african basil and basil (Ocimum gratissimum and O. basilicum), cinnamon (Cinnamomum zeylanicum) and nutmeg (Myristica fragrans). In addition to being used as food flavorings, these plants are also used as folk medicines to alleviate diseases, including hypertension 1-4. However, mechanisms by which these plants and, in particular, eugenol are clinically beneficial are unclear.
Eugenol has been proposed to possess several beneficial biological properties, including antimicrobial, antioxidant and anti-inflammatory actions 5. Eugenol has been shown to relax rabbit ear arteries 6 and rat and rabbit aorta 7, 8. Eugenol also reduced systemic blood pressure in both normotensive and hypertensive DOCA-salt rats 9, 10. However, eugenol regulation of contractility in small resistance-size arteries that control regional organ blood flow has not been studied.
Resistance-size cerebral arteries provide neurons and other brain cell types with a constant supply of oxygen and nutrients. A primary regulator of small artery contractility, including cerebral arteries, is intravascular pressure which activates mechanosensitive ion channels, leading to arterial smooth muscle cell depolarization 11-14. Subsequent activation of smooth muscle cell voltage-dependent calcium (Ca2+) channels, primarily CaV1.2, elevates intracellular calcium concentration, which stimulates vasoconstriction 12. This response, termed the “myogenic response”, maintains regional brain perfusion over a range of intravascular pressures and provides a baseline diameter from which vasoconstrictors and vasodilators can modify contractility 12. Pathological alterations in the myogenic response are associated with several cardiovascular diseases, including stroke and hypertension 12, 15. Therefore, identifying molecules that regulate membrane potential or plasma membrane Ca2+ influx may provide novel therapeutic approaches to control vascular physiology and dysfunctional contractility in diseases.
Here, we investigated the regulation of resistance-size cerebral artery contractility by eugenol. Our data obtained using a combination of pressurized artery myography and patch-clamp electrophysiology indicate that eugenol dilates rat cerebral arteries by multi-modal inhibition of smooth muscle cell voltage-dependent Ca2+ channels.
Methods
Tissue preparation
Animal protocols used were reviewed and approved by the Animal Care and Use Committee at the University of Tennessee Health Science Center. Male Sprague-Dawley rats ( ~250 g) were euthanized by intraperitoneal injection of sodium pentobarbital solution (150 mg/kg). The brain was removed and resistance size (<250 μm) cerebral (posterior cerebral, cerebellar and middle cerebral) arteries were harvested in cold (4° C) HEPES-buffered saline solution containing (in mM): 6 KCl, 134 NaCl, 2 CaCl2, 1 MgCl2, 10 HEPES and 10 glucose.
Pressurized artery myography
Experiments were performed similarly to those previously described 16. Briefly, middle cerebral artery segments 1-2 mm in length were cannulated at each end in a chamber (Living Systems Instrumentation; Burlington, VT). The chamber was continuously perfused with PSS (in mM): 6 KCl, 112 NaCl, 24 NaHCO3, 1.2 MgSO4, 1.2 KH2PO4, 1.8 CaCl2, and 10 glucose, pH 7.4 equilibrated with a mixture of 74% N2-21% O2-5% CO2, and maintained at 37 °C. Intravascular pressure was set using an attached reservoir and monitored using a pressure transducer. Pressurized arteries were observed with a charge-coupled device camera attached to an inverted microscope (Nikon TE 200,Tokyo, Japan). Arterial diameter was measured at 1 Hz using the automatic edge-detection function of IonWizard software (Ionoptix; Milton, MA). Intravascular pressure was increased to either 10 or 60 mmHg (measured with a perfusion pressure monitor; Living Systems Instrumentation, Burlington, VT) after which arteries were allowed to develop steady-state myogenic tone. In some experiments, an elevated K+ (60 mM K+) solution of composition (in mM): 58 NaCl, 60 KCl, 24 NaHCO3, 1.2 MgSO4, 1.2 KH2PO4, 1.8 CaCl2, and 10 glucose) was used to induce arterial depolarization at low pressure (10 mmHg). Eugenol (Sigma, St. Louis, MO) was applied via chamber superperfusion. Myogenic tone (%) was calculated as (1 – active diameter/passive diameter) × 100. Passive arterial diameter was measured by applying PSS solution that contained no CaCl2 and was supplemented with 5 mM EGTA.
Patch clamp electrophysiology
Smooth muscle cells were dissociated from posterior cerebral and cerebellar arteries using enzymes, as previously described 17, and placed in an experimental chamber. Whole-cell patch-clamp recordings were acquired at room temperature using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) and pCLAMP 8.2. Heat-polished borosilicate glass electrodes of resistance 3-6 MΩ were filled with pipette solution containing (in mM): 135 CsMeSO4, 5 CsCl, 5 EGTA, 4 MgATP, 0.25 NaGTP, 10 HEPES and 10 glucose (pH 7.2, with CsOH). The extracellular bath solution contained (in mM): 140 N-methyl-D-glucamine, 1 MgCl2, 10 HEPES, 20 BaCl2 and 10 glucose (pH 7.4, adjusted with L-aspartic acid).
Current-voltage (I-V) relationships were measured by holding cells at −80 mV and applying 300 ms voltage steps to between −60 and +60 mV in 10 mV increments every 5 s. To measure the time course of acute eugenol inhibition, voltage-dependent Ca2+ currents were elicited by repetitive 300 ms steps to +10 mV from −80 mV every 10 s. Steady-state inactivation was measured by evoking 500 ms conditioning pulses from a holding potential of −80 mV to between −80 and +60 mV in 10 mV increments before a 80 ms test pulse to +10 mV. Tail currents were generated by repolarization to −80 mV after a series of 20 ms test pulses to between −60 and +90 mV in 10 mV increments. Whole cell currents were filtered at 1 or 5 kHz and digitized at 5 or 20 kHz for the inactivation and activation protocols, respectively. Steady-state inactivation curves and tail currents were fit with a Boltzmann function: I/Imax = Rin + (Rmax – Rin)/(1 + exp((V – V1/2)/k)), where I/Imax is the normalized peak current, V is the conditioning pre-pulse voltage, V1/2 is the voltage for half-inactivation for steady-state inactivation or half-activation for tail currents, k is the slope factor, Rin is the proportion of non-inactivating current, Rmax is the maximal current.
Statistical Analysis
Data are expressed as means ± S.E. of the mean. Statistical significance was calculated by analysis of variance followed by Bonferroni post-hoc test for multiple comparisons and Student’s t test for paired data. P<0.05 was considered significant.
Results
Eugenol dilates cerebral arteries constricted by intravascular pressure or membrane depolarization
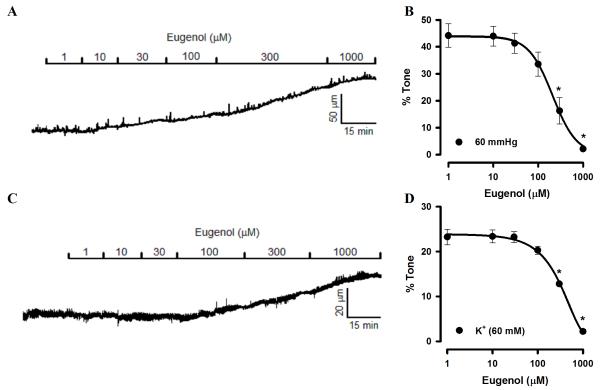
To investigate eugenol regulation of cerebral artery contractility, effects on pressurized arteries were measured. Arteries were pressurized to 60 mmHg and allowed to develop spontaneous steady-state myogenic tone. At 60 mmHg, mean arterial diameter was 116.3 ± 12.4 μm, which represented 47.7 ± 3.0% of passive diameter, as determined by applying a Ca2+-free bath solution. Bath application of eugenol caused concentration-dependent dilation of cerebral arteries, with an IC50 of 234.2 ± 11.3 μM (Fig. 1A, B).
Fig. 1.
Eugenol dilates cerebral arteries constricted by intravascular pressure or membrane depolarization. A, Exemplary recording of concentration-dependent eugenol-induced dilation of a pressurized (60 mmHg) artery with myogenic tone. B, Mean data for eugenol dilation of myogenic arteries at 60 mmHg (n=5). C, Representative recording of eugenol-induced dilation of an artery constricted with 60 mM K+ at low pressure (10 mmHg). D, Mean data for eugenol dilation of arteries constricted with 60 mM K+ (n=5). * P<0.05 vs control.
To investigate mechanisms by which eugenol dilates pressurized arteries, we tested the hypothesis that this molecule blocks voltage-dependent Ca2+ channels in arterial smooth muscle cells. This hypothesis seems reasonable, given that voltage-dependent Ca2+ channel activation is essential for the myogenic response. To test this hypothesis, arteries at low pressure (10 mmHg) were constricted with a bath solution containing 60 mM K+. This procedure constricted arterial diameter to 123.2 ± 14.6 μm, from a passive diameter of 138.6 ± 12.8 μm, or by 23.4 ± 2.2%. Increasing concentrations of eugenol dilated K+-constricted cerebral arteries with an IC50 of 323.3 ± 14.0 μM (Fig. 1C, D).
These data suggest that eugenol dilates cerebral arteries via a mechanism that involves voltage-dependent Ca2+ channel inhibition.
Eugenol inhibits voltage-dependent Ca2+ currents in cerebral artery smooth muscle cells
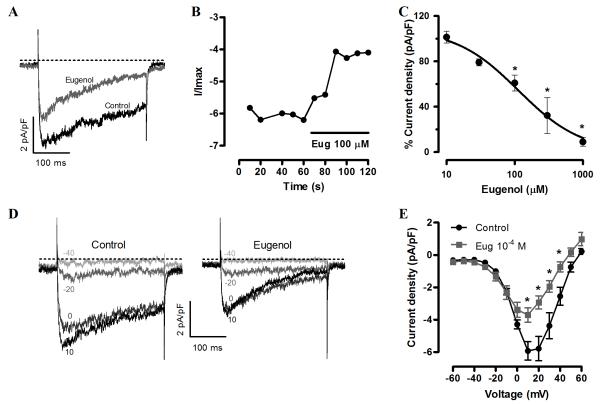
To directly test the hypothesis that eugenol inhibits arterial smooth muscle cell voltage-dependent Ca2+ channels, patch-clamp electrophysiology was performed on isolated cells. Voltage-dependent Ca2+ currents, measured using Ba2+ as a charge carrier, were stimulated by applying repetitive 300 ms voltage pulses to +10 mV from a holding potential of −80 mV. Eugenol inhibited voltage-dependent Ba2+ currents (IBa) immediately following application and in a concentration-dependent manner (Fig. 2A, B). For example, 30 μM eugenol reduced mean IBa to ~79% of control, whereas 100 μM reduced mean IBa to ~61% of control (Fig. 2C). Mean data were fit with a Hill function, which revealed a mean IC50 of ~113 μM and maximal inhibition to 3.2 × 10−11% of control, illustrating that eugenol can fully block voltage-dependent Ca2+ currents (Fig. 2C). Voltage steps to between −80 and +60 mV generated a current-voltage (IV) relationship of voltage-dependent Ca2+ currents with a peak at +10 mV. Eugenol (100 μM) reduced mean current at +10 mV from ~−5.9 to −3.7 pA/pF or to 63% of control, but did not shift the IV relationship peak voltage (Fig. 2D, E).
Fig. 2.
Eugenol inhibits voltage-dependent Ca2+ currents in isolated cerebral artery smooth muscle cells. A, Exemplary recording of IBa elcited by a voltage step from −80 to +10 mV in control and after steady-state inhibition by eugenol (100 μM). B, Time course of eugenol inhibition of IBa. C, Concentration-dependent IBa inhibition by eugenol (n: 10 μM, 5; 30 μM, 5; 100 μM, 5; 300 μM, 4; 1000 μM, 5). * P<0.05 vs control. D, representative recordings of IBa elicited by a series of depolarizing currents to voltages illustrated from a holding potential of −80 mV in control (left panel) and eugenol (100 μM, right panel). E, Mean data illustrating eugenol (100 μM) regulation of IBa IV relationship (n=7). * P<0.05 vs control.
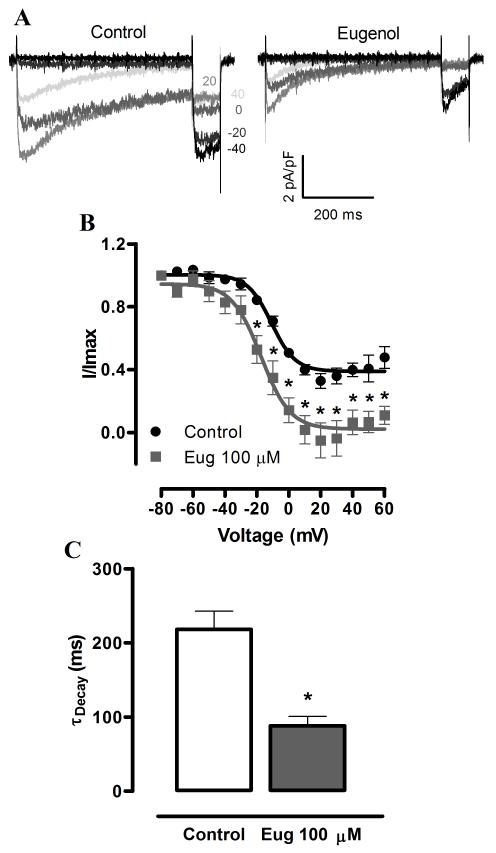
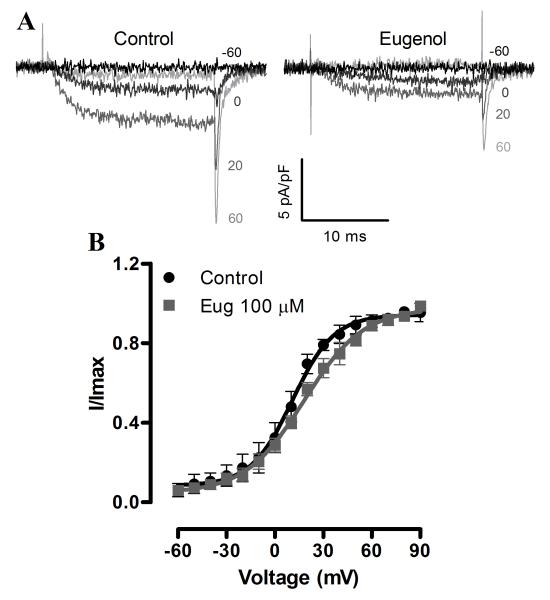
Next, we investigated the hypothesis that eugenol inhibits voltage-dependent Ca2+ currents, in part by modulating voltage-dependence. Eugenol induced a hyperpolarizing shift in the voltage-dependence of half-maximal inactivation (V1/2, inact) from ~−7.0 to −16.8 mV or by −9.8 mV, without altering the slope (Fig. 3B). Eugenol also increased the proportion of steady-state inactivating current from ~40% of maximal current in control to ~6% (at +40 mV, Fig. 3A, B). In addition, eugenol accelerated current inactivation rate (τ) from ~218.2 to 88.0 ms, or to ~40% of control (at +10 mV, Fig. 3C). In contrast, eugenol did not alter the voltage-dependence of half-maximal current activation (V1/2, act) or the slope (Fig. 4A, B). These data indicate that eugenol inhibits voltage-dependent Ca2+ currents in arterial smooth muscle cells via multiple mechanisms that include pore block, a hyperpolarizing shift in V1/2, inact and an increase in steady-state inactivation.
Fig. 3.
Eugenol modifies voltage-dependent Ca2+ current voltage-sensitivity and decay rate. A, Representative recordings of IBa steady-state inactivation in control and eugenol (100 μM). B, Mean IBa steady-state inactivation in control and eugenol (100 μM, n = 5). C, Eugenol (100 μM) regulation of the IBa rate of inactivation (n = 7). * P<0.05 vs control.
Fig. 4.
Eugenol does not alter the voltage-dependence of Ca2+ current activation. A, Representative recordings of IBa activation in control and eugenol (100 μM). B, Mean IBa activation in control and eugenol (100 μM, n = 5).
Discussion
Here, we demonstrate for the first time that eugenol, a phenylpropanoid compound found in medicinal and dietary plants, including clove, basil, cinnamon and nutmeg, dilates rat cerebral arteries. Eugenol dilated arteries constricted by both intravascular pressure and 60 mM K+, which both contract arteries via depolarization-induced voltage-dependent Ca2+ channel activation. Patch-clamp electrophysiology indicated that eugenol blocked arterial smooth muscle cell voltage-dependent Ca2+ currents by inducing pore block, promoting voltage-dependent inactivation and by increasing the proportion of inactivating current. We propose that eugenol dilates cerebral arteries by inducing multi-modal inhibition of voltage-dependent Ca2+ channels.
Resistance-size arteries regulate regional organ blood flow, including in the brain 18, 19. An elevation in myogenic tone occurs in cardiovascular diseases, including hypertension and diabetes 20, 21. This pathophysiological alteration in arterial contractility can contribute to increased blood pressure and restriction of regional organ blood flow. One hallmark of hypertension is an increase in voltage-dependent Ca2+ influx through voltage-dependent Ca2+ channels 22. The discovery and development of novel vasodilators could lead to innovative approaches to treat or alleviate these cardiovascular diseases and associated complications. Our data indicate that eugenol dilates cerebral arteries at a physiological intravascular pressure of 60 mmHg. Given that voltage-dependent Ca2+ channel activation is essential for pressure-induced vasoconstriction, we tested the hypothesis that eugenol blocks voltage-dependent Ca2+ channels. Consistent with this hypothesis, eugenol similarly relaxed arteries at low pressure that were contracted with 60 mM K+ to activate voltage-dependent Ca2+ channels. Our results are in agreement with previous studies which demonstrated that eugenol relaxed norepinephrine-and histamine-constricted rabbit ear arteries and K+-constricted rabbit aorta 6, 8. Eugenol relaxed rat aorta pre-contracted with phenylephrine and high K+ 7 and K+-depolarized rat mesentery bed contracted with bolus injections of CaCl223. Acute application of eugenol also reduced systemic blood pressure in normotensive and DOCA-salt hypertensive rats, supporting the concept that the vasodilation is functional in vivo 9, 10. Here, we show that eugenol dilates: 1) cerebral arteries and 2) arteries constricted by intravascular pressure to simulate physiological pressure, rather than receptor agonists or high K+.
Here, eugenol dilated cerebral arteries and inhibited voltage-dependent Ca2+ channels at similar concentrations, supporting the conclusion that this is a common mechanism of action. Results also indicate that eugenol inhibits voltage-dependent Ca2+ currents via multiple mechanisms, including pore block, a leftward-shift in V1/2,inact, increasing the proportion of current that undergoes steady-state inactivation and by acceleration of current decay. The conclusion that eugenol is a pore blocker is based on our data indicating that it reduced IV relationship current density, but did not shift the IV relationship peak voltage or alter V1/2,act. Smooth muscle cells in pressurized arteries undergo graded steady-state changes in membrane potential. Voltage-dependent Ca2+ channel activity at a steady voltage, and thus Ca2+ influx, is the equilibrium between voltage-dependent Ca2+ channel activation and inactivation. Therefore, data indicate that eugenol-induced vasodilation occurs due to a combination of Ca2+ channel pore block and augmented voltage-dependent inactivation. These mechanisms of voltage-dependent Ca2+ current inhibition may explain previously reported relaxant effects of eugenol in the vasculature 6, 7, 9.
Dihydropyridines, which are used in the clinic to alleviate a variety of diseases, including hypertension, bind with high affinity to the inactivated state of voltage-dependent Ca2+ channels to induce block 24. In contrast, phenylalkylamines are pore blockers that bind with high affinity to open channels 25, 26. Eugenol inhibition of voltage-dependent Ca2+ currents exhibited similarities and differences in mechanisms of action to both dihydropyridines and phenylalkylamines by modulating both inactivation and inducing pore block. Future studies will be required to identify whether binding sites for eugenol on voltge-dependent Ca2+ channels are distinct or similar to those of other better characterized blockers.
A previous study demonstrated that eugenol inhibited L-type Ca2+ currents in canine cardiomyocytes 27. However, cardiomyocyte and arterial smooth muscle cell voltage-dependent Ca2+ channels exhibit distinct properties and sensitivity to inhibitors, including dihydropyridines 28, 29. Vascular smooth muscle cell and cardiomyocyte CaV1.2 channels exhibit distinct splice variation that modifies properties including kinetics, sensitivity to inhibitors, and regulation by auxiliary subunits 17, 28, 30-32 Furthermore, vascular smooth muscle cells and cardiomyocytes can express some different auxiliary subunits 22, 33. Our data show that eugenol blocks arterial smooth muscle cell voltage-dependent Ca2+ currents and identifies mechanisms of this inhibition.
In conclusion, our study provides evidence that eugenol relaxes pressurized cerebral arteries by inhibiting voltage-dependent Ca2+ channels. Eugenol inhibition of voltage-dependent Ca2+ currents involved pore block and modulation of inactivation. Eugenol, given a dietary component or purified compound, may be a therapeutically useful vasodilator.
Acknowlegements
This work was supported by National Institutes of Health awards to J.H.J. and a CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) award to D. P-N.
Reference
- 1.Grover JK, Khandkar S, Vats V, Dhunnoo Y, Das D. Pharmacological studies on Myristica fragrans – antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 2002 Dec;24(10):675–80. doi: 10.1358/mf.2002.24.10.802317. [DOI] [PubMed] [Google Scholar]
- 2.Prabhu KS, Shirwaikar AA, Shirwaikar A. Ocimum gratissimum: A Review of its Chemical, Pharmacological and Ethnomedicinal Properties. The Open Complementary Medicine Journal. 2009:1–15. [Google Scholar]
- 3.Ranasinghe P, Pigera S, Premakumara GA, Galappaththy P, Constantine GR, Katulanda P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): a systematic review. BMC Complement Altern Med. 2013:13–275. doi: 10.1186/1472-6882-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umar A, Imam G, Yimin W, Kerim P, Tohti I, Berke B, Moore N. Antihypertensive effects of Ocimum basilicum L. (OBL) on blood pressure in renovascular hypertensive rats. Hypertens Res. 2010 Jul;33(7):727–30. doi: 10.1038/hr.2010.64. [DOI] [PubMed] [Google Scholar]
- 5.Kamatou GP, Vermaak I, Viljoen AM. Eugenol – from the remote Maluku Islands to the international market place: a review of a remarkable and versatile molecule. Molecules. 2012;17(6):6953–81. doi: 10.3390/molecules17066953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hume WR. Effect of eugenol on constrictor responses in blood vessels of the rabbit ear. J Dent Res. 1983 Sep;62(9):1013–5. doi: 10.1177/00220345830620090301. [DOI] [PubMed] [Google Scholar]
- 7.Damiani CE, Rossoni LV, Vassallo DV. Vasorelaxant effects of eugenol on rat thoracic aorta. Vascul Pharmacol. 2003 Jan;40(1):59–66. doi: 10.1016/s1537-1891(02)00311-7. [DOI] [PubMed] [Google Scholar]
- 8.Nishijima H, Uchida R, Kameyama K, Kawakami N, Ohkubo T, Kitamura K. Mechanisms mediating the vasorelaxing action of eugenol, a pungent oil, on rabbit arterial tissue. Jpn J Pharmacol. 1999 Mar;79(3):327–34. doi: 10.1254/jjp.79.327. [DOI] [PubMed] [Google Scholar]
- 9.Interaminense LF, Juca DM, Magalhaes PJ, Leal-Cardoso JH, Duarte GP, Lahlou S. Pharmacological evidence of calcium-channel blockade by essential oil of Ocimum gratissimum and its main constituent, eugenol, in isolated aortic rings from DOCA-salt hypertensive rats. Fundam Clin Pharmacol. 2007 Oct;21(5):497–506. doi: 10.1111/j.1472-8206.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- 10.Lahlou S, Interaminense LF, Magalhaes PJ, Leal-Cardoso JH, Duarte GP. Cardiovascular effects of eugenol, a phenolic compound present in many plant essential oils, in normotensive rats. J Cardiovasc Pharmacol. 2004 Feb;43(2):250–7. doi: 10.1097/00005344-200402000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, Retailleau K, Loufrani L, Patel A, Sachs F, Delmas P, Peters DJ, Honore E. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009 Oct 30;139(3):587–96. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 12.Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Invited review: arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J Appl Physiol. 2001 Aug;91(2):973–83. doi: 10.1152/jappl.2001.91.2.973. [DOI] [PubMed] [Google Scholar]
- 13.Earley S, Brayden JE. Transient receptor potential channels and vascular function. Clin Sci (Lond) 2010;119(1):19–36. doi: 10.1042/CS20090641. [DOI] [PubMed] [Google Scholar]
- 14.Bulley S, Neeb ZP, Burris SK, Bannister JP, Thomas-Gatewood CM, Jangsangthong W, Jaggar JH. TMEM16A/ANO1 channels contribute to the myogenic response in cerebral arteries. Circ Res. 2012 Sep 28;111(8):1027–36. doi: 10.1161/CIRCRESAHA.112.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schubert R, Mulvany MJ. The myogenic response: established facts and attractive hypotheses. Clin Sci. 1999 Apr;96(4):313–26. [PubMed] [Google Scholar]
- 16.Adebiyi A, McNally EM, Jaggar JH. Sulfonylurea receptor-dependent and – independent pathways mediate vasodilation induced by ATP-sensitive K+ channel openers. Mol Pharmacol. 2008 Sep;74(3):736–43. doi: 10.1124/mol.108.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng X, Liu J, Asuncion-Chin M, Blaskova E, Bannister JP, Dopico AM, Jaggar JH. A novel Cav1.2 N terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits. J Biol Chem. 2007 Oct 5;282(40):29211–21. doi: 10.1074/jbc.M610623200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902 May 28;28(3):220–31. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999 Apr;79(2):387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 20.Touyz RM. Molecular and cellular mechanisms regulating vascular function and structure – implications in the pathogenesis of hypertension. Can J Cardiol. 2000 Sep;16(9):1137–46. [PubMed] [Google Scholar]
- 21.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997 Dec;81(6):996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]
- 22.Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ. Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications. Vascul Pharmacol. 2006 Mar;44(3):131–42. doi: 10.1016/j.vph.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Criddle DN, Madeira SV, Soares de MR. Endothelium-dependent and -independent vasodilator effects of eugenol in the rat mesenteric vascular bed. J Pharm Pharmacol. 2003 Mar;55(3):359–65. doi: 10.1211/002235702694. [DOI] [PubMed] [Google Scholar]
- 24.Bean BP. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–92. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catterall WA, Striessnig J. Receptor sites for Ca2+ channel antagonists. Trends Pharmacol Sci. 1992 Jun;13(6):256–62. doi: 10.1016/0165-6147(92)90079-l. [DOI] [PubMed] [Google Scholar]
- 26.Lee KS, Tsien RW. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–4. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- 27.Magyar J, Szentandrassy N, Banyasz T, Fulop L, Varro A, Nanasi PP. Effects of terpenoid phenol derivatives on calcium current in canine and human ventricular cardiomyocytes. Eur J Pharmacol. 2004 Mar 8;487(1-3):29–36. doi: 10.1016/j.ejphar.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Tang ZZ, Hong X, Wang J, Soong TW. Signature combinatorial splicing profiles of rat cardiac- and smooth-muscle Cav1.2 channels with distinct biophysical properties. Cell Calcium. 2007 May;41(5):417–28. doi: 10.1016/j.ceca.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Welling A, Ludwig A, Zimmer S, Klugbauer N, Flockerzi V, Hofmann F. Alternatively spliced IS6 segments of the alpha 1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+ channels. Circ Res. 1997 Oct;81(4):526–32. doi: 10.1161/01.res.81.4.526. [DOI] [PubMed] [Google Scholar]
- 30.Liao P, Yu D, Lu S, Tang Z, Liang MC, Zeng S, Lin W, Soong TW. Smooth muscle-selective alternatively spliced exon generates functional variation in Cav1.2 calcium channels. J Biol Chem. 2004 Nov 26;279(48):50329–35. doi: 10.1074/jbc.M409436200. [DOI] [PubMed] [Google Scholar]
- 31.Liao P, Yu D, Li G, Yong TF, Soon JL, Chua YL, Soong TW. A smooth muscle Cav1.2 calcium channel splice variant underlies hyperpolarized window current and enhanced state-dependent inhibition by nifedipine. J Biol Chem. 2007 Nov 30;282(48):35133–42. doi: 10.1074/jbc.M705478200. [DOI] [PubMed] [Google Scholar]
- 32.Cheng X, Pachuau J, Blaskova E, Asuncion-Chin M, Liu J, Dopico AM, Jaggar JH. Alternative splicing of Cav1.2 channel exons in smooth muscle cells of resistance-size arteries generates currents with unique electrophysiological properties. Am J Physiol Heart Circ Physiol. 2009 Aug;297(2):H680–H688. doi: 10.1152/ajpheart.00109.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nargeot J, Lory P, Richard S. Molecular basis of the diversity of calcium channels in cardiovascular tissues. Eur Heart J. 1997 Jan;18(Suppl A):A15–A26. doi: 10.1093/eurheartj/18.suppl_a.15. [DOI] [PubMed] [Google Scholar]