Abstract
The cause of type I diabetes continues to be a focus of investigation. Studies have revealed that interferon (IFN)-α in pancreatic islets after viral infection or treatment with double-stranded RNA (dsRNA), a mimic of viral infection, is associated with the onset of type I diabetes. However, how IFN-α contributes to the onset of type I diabetes is obscure. In this study, we found that 2-5A dependent RNase L (RNase L), an IFN-α-inducible enzyme that functions in the antiviral and antiproliferative activities of IFN, played an important role in dsRNA-induced onset of type I diabetes. By using RNase L deficient, rat insulin promoter (RIP)-B7.1 transgenic mice which are more vulnerable to environmental harmful factors such as viral infection, we demonstrated that deficiency of RNase L in mice resulted in a significant delay of diabetes onset induced by polyinosinic:polycytidylic acid (poly I:C), a type of synthetic dsRNA, and streptozotocin (STZ), a drug which can artificially induce type I-like diabetes in experimental animals. Immunohistochemical staining showed that the population of infiltrated CD8+ T-cells was remarkably reduced in the islets of RNase L deficient mice, suggesting that RNase L may contribute to type I diabetes onset through regulating immune responses. Furthermore, RNase L was responsible for the expression of certain proinflammatory genes in the pancreas in induced conditions. Our findings provide new insight into the molecular mechanism underlying β-cells destruction and may suggest novel therapeutic strategies for treatment and prevention of the disease based on the selective regulation and inhibition of RNase L.
Keywords: RNase L, type I diabetes, interferon, poly I:C, immune cells
Introduction
The etiology of diabetes continues to be a focus of investigation. Both genetic and environmentaly factors such as toxins, viruses, and diets are believed to play an important role in its pathogenesis (Mathis, et al.2001). A reduction of insulin producing pancreatic β-cells has been considered as one of the key factors in the development of diabetes, particularly in type I diabetes (Yoon and Jun 2001; Mandrup-Poulsen 2003). In type I diabetes, autoimmune destruction of the pancreatic β-cells results in an absolute loss of insulin production. Investigation of the molecular mechanisms underlying β-cell destruction has revealed that microbial infection recruits immune effectors mediating β-cell apoptosis, which in turn triggers autoimmune responses (Anderson and Bluestone 2005). NOD mice are an ideal model of spontaneous type I diabetes, a T cell-mediated autoimmune disease. Histological studies have shown that infiltration of immune cells including macrophages and lymphocytes around the islets in NOD mice starts at 3 to 4 weeks of age, causing insulitis (Solomon and Sarvetnick 2004; Anderson and Bluestone 2005). It has been well demonstrated that CD4+ and CD8+ T-cells play an important role in the onset of type I diabetes. Clinical and animal studies have shown that CD8+ T cells take a central stage in the destruction of pancreatic β-cells and contribute to sustaining islet inflammation, leading to the onset of type I diabetes (Solomon and Sarvetnick 2004; Anderson and Bluestone 2005; Coppieters, et al. 2011; Coppieters, et al. 2012).
The expression of proinflammatory genes has been implicated in the pathogenesis of type I diabetes (Bergholdt, et al. 2004). In NOD mice, an increased expression of IFN-γ, TNF-α and IL-1β is associated with β-cell destructive insulitis (Solomon and Sarvetnick 2004; Anderson and Bluestone 2005). In recent years the role of IFN-α in autoimmune diseases including type I diabetes has been well established (Selmi, et al. 2006). Increased levels of IFN-α in the sera of type I diabetes patients have been documented (Chehadeh, et al. 2000). Transgenic mice expressing IFN-α in the β-cells develop hypoinsulinemic diabetes associated with a mixed inflammation centered on the islet, which can be prevented with a neutralizing antibody to IFN-α (Stewart, et al. 1993). It has been reported that the onset of type I diabetes in both BB rats and STZ-treated mice is IFN-α dependent (Huang, et al. 1994). In addition, IFN-α mediates induction of type I diabetes by poly I:C in the mice expressing the B7.1 costimulatory molecule driven by the rat insulin promoter (RIP) on β-cells in islets (Devendra, et al. 2005). Most recently, a study has revealed that blockade of IFN-α signaling by anti-IFNAR1 in 2- to 3-week old NOD mice remarkably delays the onset and decreases the incidence of diabetes, suggesting the involvement of IFN-stimulated genes in the pathogenesis of type I diabetes (Li, et al. 2008).
RNase L is an IFN inducible enzyme and plays an important role in the 2-5A system of IFN action against viral infection and cellular proliferation (Silverman, 1996). The 2-5A system consists of two enzymes: 2-5A synthetase and RNase L. IFNs induce a family of 2-5A synthetase genes (OAS). The 2-5A synthetases require double-stranded RNA (dsRNA) for their activities. DsRNA is frequently produced during viral infection. After activation by dsRNA, 2-5A synthetases convert ATP molecules to pyrophosphate (ppi) and a series of unique, 5’- phosphorylated, 2’-5’ linked oligoadenylates known as 2-5A with the general formula ppp(A2’p5’)nA (n≥2). 2-5A binds RNase L with high affinity, converting it from its inactive, monomeric state to a potent dimeric endoribonuclease, resulting in degradation of single- stranded viral and cellular RNAs [Zhou, et al. 1993]. It has been demonstrated that 2-5A accumulates and RNase L is activated in infected cells. Cells overexpressing RNase L overcome viral infection. In contrast, overexpression of a dominant negative mutant of RNase L results in increased susceptibility to certain viruses [Hassel, et al. 1993]. In vivo studies show that mice containing targeted disruption of RNase L gene succumb to encephalomyocarditis (EMCV) infection more rapidly than infected wild type mice [Zhou, et al. 1997]. Interestingly, OAS is persistently activated in patients with type I diabetes, suggesting its involvement in this disease (Bonnevie-Nielsen, et al. 2000). RNase L null mice show enlarged thymus glands and increased T cell numbers at their early age, suggesting that RNase L may be involved in T cell development, which likely results from reduced cell apoptosis. Furthermore, it has been demonstrated that overexpression of RNase L in the cells enhances cell apoptosis, whereas dominant negative RNase L suppresses cell apoptosis (Zhou, et al. 1997). These observations indicate that RNase L plays an important role in the immune system. Indeed, studies have revealed that skin allograft rejection is suppressed in mice lacking RNase L, suggesting the involvement of RNase L in T cell immunity, particularly CD4+ T cell mediated immunity (Silverman, et al. 2002). In addition, alphavirus-based DNA vaccination against a non-mutated tumor-associated self-antigen (tyrosinase-related protein-1, TRP-1) is severely impaired in RNase L null mice, indicating that RNase L plays an important role in the host immune system against cancer (Leitner, et al. 2003).
In this study, we present evidence showing that RNase L may be involved in the pathogenesis of type I diabetes. RNase L-deficient RIP-B7.1 mice displayed significantly delayed the onset of diabetes induced by STZ and poly I:C. Immunohistostaining revealed that the population of infiltrated CD8+ T cells was remarkably reduced in the islets of RNase L deficient mice, implicating RNase L in the onset of type I diabetes through mediating the infiltration of immune cells. Furthermore, RNase L regulated the expression of certain proinflammatory genes in the pancreas under these conditions. Our results suggest a novel role of RNase L in the development of type I diabetes.
Reagents and Methods
Tissue culture and animal treatment NIH 3T3 cells, NIT-1 (ATCC) and mouse embryonic fibroblasts (MEF) were grown in DMEM (Cleveland Clinic, OH) supplemented with 10% fetal bovine serum (PAA Laboratories, MA) and antibiotics in a humidified atmosphere of 5% CO2 at 37°C. BMMs were generated from the bone marrow cells of RNase L wild type and deficient C57BL/6 mice by using a modified method (Xin, et al. 2013). For RNase L induction in the pancreas of mice, each group (n=2) of C57BJ/6 mice were treated with or without poly I:C at a concentration of 5 μg/g body weight every other days for one week (three times). The pancreases were removed and tissue extracts were used for analysis of RNase L. For the treatment with a high fat diet, RNase L+/+ and −/− mice were fed with a high fat diet (21% milk fat, 1.25% cholesterol and 0.5% sodium cholate) (Harlan Teklad, WI) for 20 weeks. The body weight was monitored every two weeks and the blood from the eye corner was collected for analysis of glucose, cholesterol, triglyceride and insulin. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Cleveland State University (Permit Number: 21111-ZHO-AS). All efforts were made to reduce suffering. Mice were under CO2 for 5 min and then dislocated their neck at the termination of the experiments.
Generation of an RNase L deficient C57BL/6.RIP-B7.1 mouse An RNase L−/− mouse (C57BL/6) (a generous gift from Dr. Robert Silverman, Cleveland Clinic) was cross-bred with a C57BL/6.RIP-B7.1 mouse (Barbara Davis Center of Childhood Diabetes, University of Colorado Health Sciences Center, CO). Genotyping for RNase L and B7.1 was performed by using PCR with the condition: denaturing at 96°C for 4 min, for each cycle, denaturing at 96°C for 45 sec, annealing at 55°C for 45 sec, and extension at 72°C for 1.5 min, for 32 cycles, then an additional cycle at 72°C for 5 min. The pair of RNase L primers was: 5’GCA TTG AGG ACC ATG GAG AC3’ and 5’GGA GGA GAA GCT TTA CAA GGT3’; for B7.1, the primers were: TGA AGC CAT GGG CCA CAC3’ and 5’ GGC TCC TTG TCG GCG TTC TA3’.
Diabetes induction Six-week-old RNase L deficient and wild type C57BL/6 RIP-B7.1 mice were injected intraperitoneally (i.p.) with poly I:C (Sigma, St. Louis, MO) daily at a concentration of 5 μg/g body weight for 7 days, and then immunized (i.p.)with 25 μg/mouse for insulin ( Eli Lilly, Indianapolis, IN) on day 14 after poly I:C treatment. Blood glucose was measured weekly with a Glucose Oxidase Reagent Set (Pointe Scientific, Canton, MI). Briefly, 10 μl of plasma was mixed with 1ml of the working reagent provided by the manufacturer and incubated at 37°C for exactly 5 min. After incubation, the absorbance at 500 nm was read and recorded. The mice were considered diabetic after two consecutive blood glucose values ≥250 mg/dl.
Immunohistostaining The pancreases obtained from the mice were fixed in 10% formalin, paraffin embedded and sectioned at 5μm, subsequently stained with hematoxylin and eosin. Pancreatic sections were microscopically examined for infiltration of immune cells. The tissue sections were incubated with rat polyclonal antibodies against CD4, CD8, F4/80, CD11b (eBioscience, San Diego, CA) respectively and a monoclonal antibody to insulin (Abcam, Cambridge, MA), followed by incubating with a biotinylated secondary antibody, and color reaction was obtained by sequential incubation with avidin-peroxidase conjugate and diaminobenzidine–hydrogen peroxide (ICN, Costa Mesa, CA).
Flow Cytometry Single cell suspension from the spleens was stained with conjugated mAbs including FITC-CD4, PE-CD8, FITC-IgD, PE-B220, APC-Gr.1, FITC-CD11b, PE-cy7-CD25 (BD pharmingen, San Jose, CA). Cell-associated fluorescence was analyzed with a FACScan instrument and associated Winlist 5 software.
Western blot analysis After treatment, cells were washed twice with ice-cold PBS and collected with a scraper. Western blot analysis was performed as described in reference 23. Pancreatic tissue extracts obtained from mice (2/group) treated with or without poly I:C (Sigma, St. Louis, MO) every other day at a concentration of 5 μg/g body weight for one week (three times) were analyzed as described above.
Enzyme-linked immunosorbent assay (ELISA) The expressing level of proinflammatory genes in the extracts of the pancreases was measured by ELISA with commercial available kits (eBioscience, San Diego, CA and R&D Systems, Minneapolis, MN) according to the manufacture’s instruction. Plates were read at 450nm in a 96-well LD 400C microplate reader (Beckman Coulter, Fullerton, CA).
Results
Minimal impact of RNase L on high fat diet induced obesity Previously we observed that RNase L−/− mice gained significantly heavier body weight than RNase L+/+ mice and displayed hepatic steatosis and lipid droplets within renal tubular epithelial cell cytoplasm at their old age (24). To determine if RNase L plays any role in the metabolic pathways, we fed RNase L+/+ and −/− mice with a high fat diet for 20 weeks. Surprisingly, the growth rate of both mouse types was nearly identical under the same condition (Fig. 1A). However, the levels of glucose, cholesterol and triglyceride were 20.4%, 11.6% and 2.5 % higher in the serum of RNase L−/− mice compared to that from RNase L+/+ mice (Fig. 1B, C, D) although the level of insulin was also 18.7% higher (E) in the serum from RNase L−/− mice. These results suggest that RNase L may not directly be involved in the growth of body weight, but contribute, at least in part, to the metabolic pathways.
Fig.1.
Effect of RNase L on diet-induced obesity RNase L+/+ and −/− male mice (n=8/group) were fed with a high fat diet (Harlan Teklad) for 20 weeks. The body weight was measured every two weeks (A). Glucose (B), cholesterol (C) and triacylglycerol (D) in the plasma were analyzed using commercially available diagnostic kits from Sigma-Aldrich (St. Louis, MO), and insulin (E) was measured by using an ELISA kit (Fisher). Data are present as Mean ± SD for 8 mice of each strain. The samples were triplicates in every assay and two individual experiments were performed, * p< 0.05, **p<0.01.
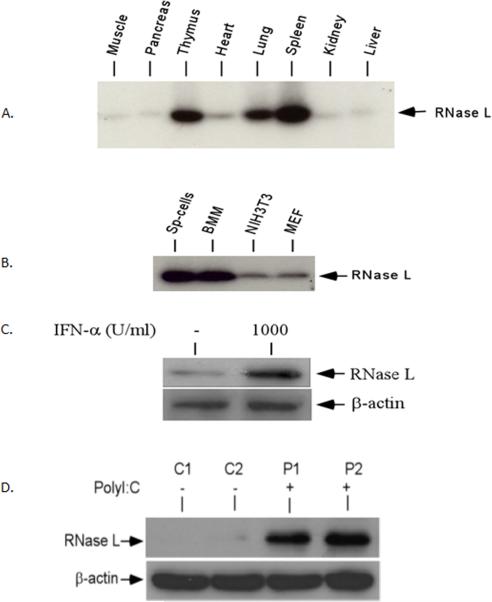
IFN-α and poly I:C induced the expression of RNase L in the tissues and cells RNase L is one of the key enzymes in the IFN- induced antiviral and antiproliferative functions.. RNase L is also found to be expressed in nearly all types of mammalian cells from mouse to man (Silverman 1996). To examine the expression of RNase L in the pancreas, the tissue extracts of eight different organs from a C57BL/6 mouse (Jackson Laboratory) were analyzed by the 2-5A binding assay (Nolan-Sorden, et al. 1990). As shown in Fig. 2A, RNase L is clearly expressed in the tissues. High levels of the RNase L expression were found in the lung, spleen and thymus. However, the basal level of RNase L in the pancreas was very low. Similar to our previous observation, RNase L was found to be highly expressed in primary immune cells (Fig. 2B), suggesting a possible role of RNase L in the immune system. To determine if IFN-α is able to induce the expression of RNase L in the pancreatic cells, NIT cells, a murine β-cell line, were treated with or without IFN-α and the induction of RNase L was examined by Western blot analysis. The expression of RNase L was highly induced in β-cells (Fig. 2C). Double stranded RNA such as poly I:C can be used to mimic viral infection in vitro and in vivo. To examine if poly I:C is able to up-regulate the RNase L expression in the pancreas, mice were treated with or without poly I:C and the expression of RNase L in the pancreatic tissue extracts was measured. Poly I:C was a potent inducer of the RNase L expression in the pancreas (Fig. 2D).
Fig.2.
Tissue distribution and expression of RNase L The expression of RNase L in the tissue extracts from eight organs (A) and several types of cells (B). Sp-cells: primary splenocytes; BMM: bone marrow-derived macrophages; NIH3T3: mouse fibroblasts; MEF: mouse embryonic fibroblasts. (C) IFN-α induces the expression of RNase L in NIT-1 cells NIT-1 cells were treated with 1,000 units/ml of IFN-α (R&D Systems) for 16 h and RNase L in the cells was determined by Western blot analysis. (D) Mice (2/group) were treated with or without poly I:C at a concentration of 5 μg/g body weight every other days for one week (three times) and the level of RNase L in the pancreatic tissue extract was determined by Western blot analysis with a polyclonal antibody to mouse RNase L.
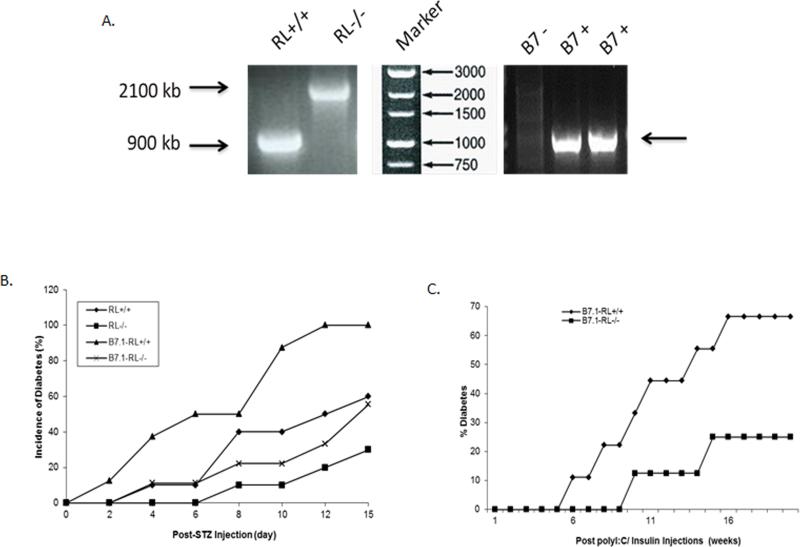
The onset of type I diabetes is delayed in RNase-L-deficient mice. Viral infection is believed to be a potent factor in triggering autoimmune responses in the pancreas, resulting in destruction of β-cells, leading to absolute deficiency of insulin. Indeed, poly I:C, a type of dsRNA commonly used to mimic viral infection, is able to effectively induce the onset of type I diabetes in C57BL/6 mice expressing B7.1 under the control of a rat insulin promoter on pancreatic β-cells islets (RIP-B7.1) in an IFN-α dependent manner (Devendra, et al. 2005). RNase L can be induced by both poly I:C and IFN-α in vitro and in vivo, and mediates the functions of IFN-α. To determine the role of RNase L in the onset of type I diabetes induced by poly I:C, RNase L−/− RIP-B7.1 mice were created by cross-breeding an RNase L−/− mouse (C57BL/6) with a C57BL/6.RIP-B7.1 mouse (RIP-B7.1 mouse). The genotype of these mice was determined by PCR as shown in Fig. 3A. The onset of type I diabetes was first induced by using STZ, a glucose analogue known to induce diabetes in experimental animals. Mice were interperitoneally (i.p.) injected with 40 mg/kg STZ for five consecutive days, the blood was collected from the saphenous vein and the level of glucose was measured as described in the Methods. As shown in Fig. 3B, mice with RIP-B7.1 were more sensitive to STZ-induced diabetic onset and RNase L deficiency markedly delayed the progress of this disease. To determine if RNase L mediates poly I:C induced onset of type I diabetes, RNase L+/+ and −/− RIP-B7.1 mice were injected (i.p.) with 5 μg/g body weight poly I:C daily for 7 days, and then injected (i.p.) with insulin at 25 μg/mouse on day 14 after poly I:C treatment. Diabetic progress in these mice was monitored every week and the mice were considered diabetic after two consecutive blood glucose values ≥250 mg/dl. As shown in Fig. 3C, the onset of diabetes was significantly delayed in the RNase L−/− RIP-B7.1 mice.
Fig. 3.
Effect of RNase L on STZ and poly I:C - induced Diabetes A. Genotyp ing of B7.1 - RL−/− mice. B. Incidence of diabetes in RNase L (RL)+/+ and −/− (n=10 for each group), B7.1-RL+/+ (n=8) and B7.1-RL−/− (n=9) mice after injection with 40mg/kg STZ for five consecutive days. C. Incidence of diabetes in B7.1-RL+/+ (n=9) and B7.1-RL−/− (n=8) mice after injected (i.p.) with 5 μg/g body weight poly I:C daily for 7 days, and then injected (i.p.) with 25 μg/mouse of insulin on day 14 after poly I:C treatment . The glucose levels in t he blood samples were measured by using a blood sugar test kit (Pointe Scientific). The mice were considered diabetic after two consecutive blood glucose values ≥ 250 mg/dl.
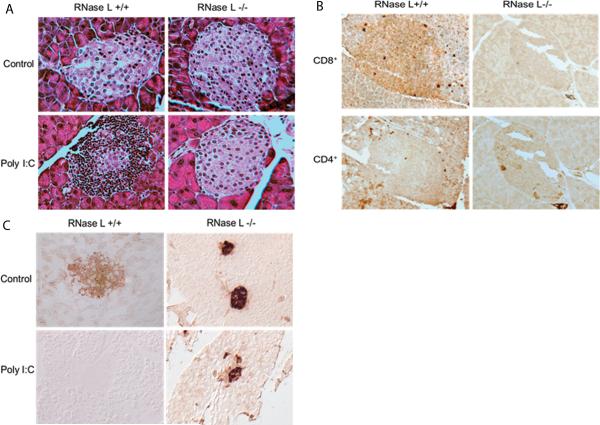
RNase L facilitates infiltration of immune cells RNase L is highly expressed in the thymus, spleen and all immune cells examined. Since the infiltration of immune cells initiates the progress of this disease, we investigated whether the delay of type I diabetes onset was caused by slowdown of immune responses in the pancreas of mice deficient RNase L. RNase L+/+ and −/− RIP-B7.1mice were treated with poly I:C for 40 days and then sacrificed and the pancreatic tissues were embedded and sectioned at 5 μm, followed by subjecting to hematoxylin and eosin (H&E) staining. Interestingly, the population of infiltrated immune cells was strikingly reduced in the islets of RNase L−/− mice, suggesting the involvement of RNase L in poly I:C-induced diabetes onset may be through regulating the infiltration of immune cells (Fig. 4A). To determine the identity of these infiltrated immune cells, immunohistostaining for macrophages, CD4+ and CD8+ T cells was performed. Apparently, the majority of the infiltrated immune cells in the islets of the pancreas from RNase L+/+ mice were CD8 positive T cells although some of CD4 positive T cells were found in the islets from both types of mice (Fig. 4B). However, macrophages were undetectable in the islets from both types of mice by using either CD11b or F4/80 as a biomarker (data not shown). The finding indicates that CD8 positive T cells may be the effector cells in the destruction of β-cells in the islets of the pancreas from RNase L+/+ RIP-B7.1 mice after poly I:C treatment. As expected, the insulin levels in the islets of RNase L−/− mice were significantly higher than that in RNase L+/+ before and after poly I:C treatment (Fig. 4C).
Fig.4.
RNase-L-dependent infiltration of immune cells in the islets in response to poly I:C A. Hematoxylin and eosin stained pancreatic islets from B7.1-RL+/+ and B7.1-RL−/− mice after treatment with or without poly I:C. B. Immunohistological staining of CD4+ and CD8+ T cells in the islets from both types of mice after poly I:C treatment. C. Immunohistological staining of insulin in the islets from both types of mice before and after poly I:C treatment. Magnification x 40.
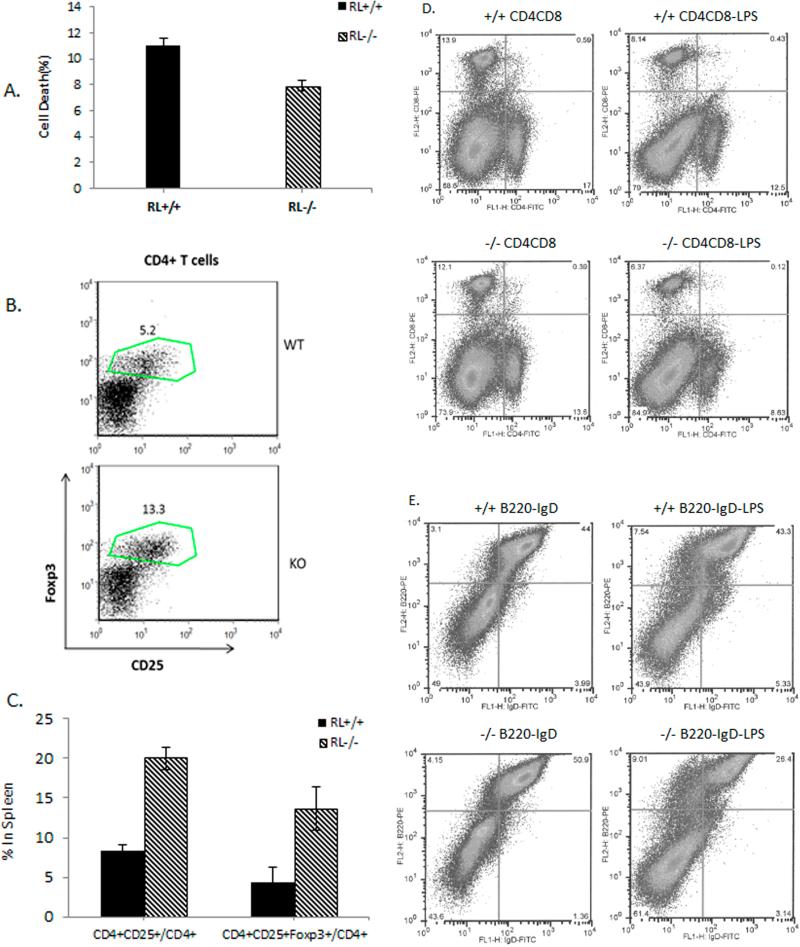
RNase L is associated with subclasses of immune cells in the spleen and T-cell function in the blood RNase L−/− mice display enlarged, hypercellular thymuses, suggesting that RNase L may be involved in the development of thymocytes and T-cell function. To determine if RNase L impacts T-cell function, lymphocytes isolated from the blood of RNase L+/+ and −/− mice were subjected to analysis of their cytotoxic activity against retinal endothelial cells. As shown in Fig. 5A, RNase L−/− lymphocytes exerted 46% lower cytotoxic activity than that from RNase L+/+ mice. CD4+CD25+ regulatory T cells (Tregs) have emerged as a dominant peripheral mechanism of immune suppression of self-reactive T cells and a master-switch factor controlling the balance between tolerance and immunity (Johnson, et al. 2013). Studies have demonstrated that CD4+CD25+ Tregs, in particular CD4+CD25+foxP3+ Tregs, are very important in controlling the development of diabetes in NOD mice (Manirarora, et al. 2008; Kaminitz, et al. 2014). To determine the effect of RNase L on CD4+CD25+ Tregs, we performed a flow cytometric assay to analyze the subtype of these cells in the spleen of RNase L+/+ and −/− mice. Interestingly, the percentage of CD4+CD25+ Tregs in CD4+ T cells in the spleen of RNase L−/− mice was significantly higher than that in RNase L+/+ mice. Particularly the percentage of CD4+CD25+foxP3+ Treg cells in CD4+ T-cells was 13.3% in the spleen of RNase L−/− mice in compared to 5.2% in RNase L+/+ mice (Fig.5 B and C). The population of other cell subclasses were also differentially represented in the spleens of RNase L+/+ and −/− mice, especially under the stimulation with LPS as shown in Fig.5D and E. The population of CD4+CD8+ T cells in the spleen of RNase L−/− mice was 37% less than that in RNase L+/+ mice, whereas the population of the double labeled T cells was 72% less in the spleen of RNase L−/− mice compared to that in RNase L+/+ mice after treatment with LPS. Similar to CD4+CD8+ T cells, the population of B220+IgD+ B cells in the spleen of RNase L−/− mice was 39% less than that in the spleen of RNase L+/+ mice after LPS stimulation although the population of B220+IgD+ B cells in the spleen of intact RNase L−/− mice was slightly higher than that in the spleen of RNase L+/+ mice under the same condition. Taken together, the results implicate RNase L in the immune system activities through mediating the context-specific modulation of immune cell subclasses in the spleen.
Fig.5.
Effect of RNase L on splenic immune cell subtypes and T-cell function A. Lymphocytes isolated from RNase L+/+ and −/− mice (n=4/group) were incubated with retinal endothelial cells at 1:5 at 37°C for 24 hr. Cell viability was measured by trypan blue exclusion. Samples were triplicates and the data are present as Mean ± SD, ***p<0.048. B and C. Treg cells in the spleens of RNase L+/+ (WT) and −/− (KO) mice were analyzed for the expression of CD4 and CD25 by flow cytometric analysis. The percentages of CD4+CD25+Treg cells and CD4+CD25+Foxp3 Terg cells in total CD4+T cells are present as Mean ± SD (n= 4). *P< 0.05; **p<0.025. D and E. Six-week-old male RNase L+/+ and −/− mice (3mice/group) were treated with or without 25 μg LPS for each mouse every other day for a week. The splenic cells were subjected to cell sorting after labeled with CD4, CD8, B220, and IgD alone or combined.
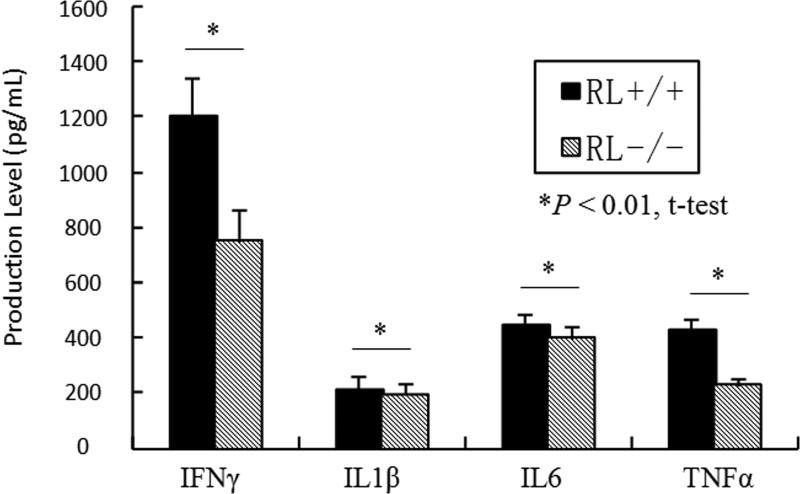
The expression of proinflammatory cytokines in the pancreatic tissues In addition to IFN-α, several proinflammatory genes such as TNF-α, IL-1β and IFN-γ are believed to contribute to the development of type I diabetes (Solomon and Sarvetnick 2004; Anderson and Bluestone 2005). To determine if deficiency of RNase L affects the expression of TNF-α, IL-1β, IL-6 and IFN-γ, RNase L+/+ and −/− RIP-B7.1mice were treated with poly I:C at a concentration of 5 μg/g body weight every other days for a week and the level of these gene products in the pancreatic extracts was determined by ELISA. As shown in Fig.6, RNase L-deficiency reduced IFN-γ and TNF-α production by 37.5% and 48.5% respectively in the pancreas after treatment with poly I:C. However, the expression of IL-1β and IL-6 was found to be slightly higher in the pancreas from RNase L+/+ as compared to RNase-L−/− mice.
Fig.6.
RNase L regulates the expression of proinflammatory genes RNase L deficient and wild type RIP-B7.1 mice were treated with poly I:C at a concentration of 5 μg/g body weight every other days for a week and the level of these factors in the pancreatic extracts was determined by ELISA using an ELISA kit for each of the analyzers. Experiments were performed two times in triplicates. Data are presented as Mean ±SD, p<0.01.
Discussion
B7.1 and B7.2 are homologous costimulatory ligands expressed on the surface of antigen presenting cells (APCs). Binding of these molecules to the T cell costimulatory receptors, CD28 and CTLA-4, is essential for the activation and regulation of T cell immunity (Greenwald, et al. 2005). Although expression of the B7.1 costimulator alone is not sufficient to induce diabetes in mice, β-cells expressing the molecule are more vulnerable to environmental harmful factors such as viral infection, resulting in destruction of β-cells and leading to disease (Wong, et al. 1995). As a mimic of viral infection, poly I:C treatment alone can induce insulitis, but not lead to diabetes in C57BL/6 mice. However, it can effectively induce diabetes in C57BL/6 mice expressing B7.1 on β-cells in the islets under a rat insulin promoter (Devendra, et al. 2005). In this study, we present evidence showing that RNase L −/− RIP-B7.1 mice display a significant delay of type I diabetes onset induced by poly I:C, suggesting that RNase L is associated with the pathogenesis of this disease.
The role of RNase L in mediating IFN-induced functions against viral infection and cell proliferation has been well established. A line of evidence has shown that IFN-α plays a vital role in initiating autoimmune responses and the molecular mechanism is a continuing focus of investigation. Studies have revealed that type I diabetes onset in animal models induced by STZ and poly I:C is IFN-α dependent (Huang, et al. 1994; Devendra, et al. 2005). In humans, the development of type I diabetes have been widely documented during IFN-α therapy for viral infection such as hepatitis B and C (Di, et al. 1996; Kose, et al. 2012). How IFN-α promotes the onset of type I diabetes remains to be further elucidated. RNase L is one of the key enzymes in the 2-5A system of IFN-α function. 2-5A activation of RNase L results in apoptosis in cells. It is believed that apoptosis is one of the key factors in triggering autoimmune responses in islets (Kawazoe, et al. 2012). Thus, our results suggest that poly I:C treatment may induce an increase of IFN-α production in islets and subsequently activate the 2-5A system. Activation of the 2-5A system, in turn, leads to β-cell apoptosis which further promotes the autoimmune responses resulting in destruction of β-cells and diabetes onset. Deficiency of RNase L in mice delays the onset of type I diabetes. Actually, this hypothesis is supported by a previous study which poly I:C induces cell apoptosis in pancreatic islets, resulting in release of islet autoantigens, triggering the autoimmune responses. In contrast, none of the bacterial products such as LPS and CpG are able to induce diabetes in these mice because they cannot induce islet cells to undergo overt apoptosis (Maniati, et al. 2008).
RNase L may directly be involved in the function of immune cells to impact the development of type I diabetes. Although T lymphocytes are primarily the effectors, studies have indicated that B cells may play a vital role in the pathogenesis of autoimmune diseases. In NOD mice, the contribution of B cells was clearly demonstrated by the remarkable reduction in insulitis and diabetes incidence after B cell depletion using anti-IgM antibodies at birth or in NOD mice genetically deficient in B cells (Silveira and Grey 2006; Mariño, et al. 2011). By using the same animal model, studies have shown that B cells function as islet antigen–presenting cells for autoreactive T cells and produce antibodies which are directly pathogenic. In clinical trials, patients with type I diabetes treated with rituximab, a chimeric monoclonal antibody against the protein CD20 that is primarily found on the surface of B cells, decreased C-peptide and reduced insulin requirements, suggesting an essential role of B cells in the development of this disease (Pescovitz, et al. 2009). Interestingly, the population of B220+IgD+ double labeled B cells and CD4+/CD8+ double labeled T cells was 39% and 72% down after LPS treatment (Fig. 2A) or 46% and 45% down after poly I:C treatment (data not shown) in the spleen of RNase L−/− mice, implicating that delayed onset of type I diabetes may be due to an attenuated immune responses as a result of decreases the population of certain immune cells, such as B cells, induced by LPS or poly I:C . The effect of RNase L on immunity is a focus of our current investigations.
The production of proinflammatory genes in the islets plays a critical role in the pathogenesis of type I diabetes. It has been demonstrated that disruption of IFN-γ function by using either IFN-γ specific Abs or soluble IFN-γ receptors (IFNγR) significantly reduces the incidence of spontaneous diabetes in NOD mice, indicating an important role of IFN-γ in the development of this disease (Campbell, et al. 1991; Nicoletti, et al. 1996). Complete protection from diabetes was observed when TNFR1, but not TNFR2 was knocked out in NOD mice although the direct effect of TNFR1 on β-cell death in vivo has not been defined clearly (Kägi, et al. 1999). It has been reported that RNase L mediates the expression of certain genes through regulating RNA turnover (Bisbal, et al 2000; Chandrasekaran, et al. 2004). Our observation suggests that RNase L specifically regulating the expression of proinflammatory genes in the pancreas may contribute to type I diabetes onset induced by poly I:C. Interestingly, RNase L seemed not to influence the growth rate of mice fed with a high fat diet, but the level of blood sugar in RNase L−/− mice was significantly higher although the insulin level was markedly increased, suggesting that insulin sensitivity in RNase L−/− cells may be impaired. Indeed, we have observed that phosphorylation of Akt, a key component of the insulin signaling pathway, in RNase L−/− mouse embryonic fibroblasts (MEFs) and primary hepatocytes stimulated by insulin was significantly reduced in compared to that in the same type of RNase L+/+ cells (our unpublished data). Thus, this study highlights the potential benefits of targeting RNase L in type I diabetes treatment.
Acknowledgement
This work was supported by Marousch Grant-in-Aid Award, the Diabetes Association of Great Cleveland to AZ and partially by grants from the National Institutes of Health (R15DK084460- 01A2) to AZ and (AI089518) to KM. We thank Dr. Bret A. Hassel (University of Maryland School of Medicine) for helpful comments on the manuscript.
References
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- Bergholdt R, Heding P, Nielsen K, Nolsøe R, Sparre T, Størling J, et al. Type 1 database mellitus: an inflammatory disease of the islet. Adv Exp Med Biol. 2004;552:129–153. [PubMed] [Google Scholar]
- Bisbal C, Silhol M, Laubenthal H, Kaluza T, Carnac G, Milligan L, et al. The 2’-5’ oligoadenylate/RNase L/RNase L inhibitor pathway regulates both MyoD mRNA stability and muscle cell differentiation. Mol Cell Biol. 2000;20:4959–4969. doi: 10.1128/mcb.20.14.4959-4969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnevie-Nielsen V, Martensen PM, Justesen J, Kyvik KO, Kristensen B, Levin K, et al. The antiviral 2’,5’-oligoadenylate synthetase is persistently activated in type 1 diabetes. Clin. Immunol. 2000;96:11–18. doi: 10.1006/clim.2000.4874. [DOI] [PubMed] [Google Scholar]
- Campbell IL, Kay TW, Oxbrow L, Harrison LC. Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest. 1991;87:739–742. doi: 10.1172/JCI115055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehadeh W, Weill J, Vantyghem MC, Alm G, Lefèbvre J, Wattré P, et al. Increased level of interferon-alpha in blood of patients with insulin-dependent diabetes mellitus: relationship with coxsackievirus B infection. J Infect Dis. 2000;181:1929–1939. doi: 10.1086/315516. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran K, Mehrabian Z, Li X-L, Hassel B. RNase-L regulates the stability of mitochondrial DNA-encoded mRNAs in mouse embryo fibroblasts. Biochem. Biophys. Res. Commun. 2004;325:18–23. doi: 10.1016/j.bbrc.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TWH, Atkinson MA, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters KT, von Herrath MG. Viruses and cytotoxic T lymphocytes in type 1 diabetes. Clin Rev Allergy Immunol. 2011;41:169–178. doi: 10.1007/s12016-010-8220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devendra D, Jasinski J, Melanitou E, Nakayama M, Li M, Hensley B, et al. Interferon-alpha as a mediator of polyinosinic:polycytidylic acid-induced type 1 diabetes. Diabetes. 2005;54:2549–2556. doi: 10.2337/diabetes.54.9.2549. [DOI] [PubMed] [Google Scholar]
- Di Cesare E, Previti M, Russo F, Brancatelli S, Ingemi MC, Scoglio R, et al. Interferon-alpha therapy may induce insulin autoantibody development in patients with chronic viral hepatitis. Dig Dis Sci. 1996;41:1672–1677. doi: 10.1007/BF02087923. [DOI] [PubMed] [Google Scholar]
- Fabre O, Salehzada T, Lambert K, Boo Seok Y, Zhou A, Mercier J, Bisbal C. RNase L controls terminal adipocyte differentiation, lipids storage and insulin sensitivity via CHOP10 mRNA regulation. Cell Death Differ. 2012;19(9):1470–1481. doi: 10.1038/cdd.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Hassel BA, Zhou A, Sotomayor C, Maran A, Silverman RH. A dominant negative mutant of 2-5A-dependent RNase suppresses antiproliferative and antiviral effects of interferon. EMBO J. 1993;12:3297–3304. doi: 10.1002/j.1460-2075.1993.tb05999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Hultgren B, Dybdal N, Stewart TA. Islet expression of interferon-alpha precedes diabetes in both the BB rat and streptozotocin-treated mice. Immunity. 1994;1:469–478. doi: 10.1016/1074-7613(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Johnson MC1, Garland AL, Nicolson SC, Li C, Samulski RJ, Wang B, Tisch R. β-cell-specific IL-2 therapy increases islet Foxp3+Treg and suppresses type 1 diabetes in NOD mice. Diabetes. 2013;62(11):3775–3784. doi: 10.2337/db13-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kägi D, Ho A, Odermatt B, Zakarian A, Ohashi PS, Mak TW. TNF receptor 1-dependent beta cell toxicity as an effector pathway in autoimmune diabetes. J Immunol. 1999;162:4598–4605. [PubMed] [Google Scholar]
- Kaminitz A1, Mizrahi K, Ash S, Ben-Nun A, Askenasy N. Stable activity of diabetogenic cells with age in NOD mice: dynamics of reconstitution and adoptive diabetes transfer in immunocompromised mice. Immunology. 2014;142(3):465–73. doi: 10.1111/imm.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawazoe T, Araki M, Lin Y, Ogawa M, Okamoto T, Yamamura T, et al. New-onset type 1 diabetes mellitus and anti-aquaporin-4 antibody positive optic neuritis associated with type 1 interferon therapy for chronic hepatitis C. Intern Med. 2012;51:2625–2629. doi: 10.2169/internalmedicine.51.7771. [DOI] [PubMed] [Google Scholar]
- Kose S, Gozaydin A, Akkoclu G, Ece G. Chronic hepatitis B with type I diabetes mellitus and autoimmune thyroiditis development during interferon alpha therapy. J Infect Dev Ctries. 2012;6:364–368. doi: 10.3855/jidc.1632. [DOI] [PubMed] [Google Scholar]
- Leitner WW, Hwang LN, deVeer MJ, Zhou A, Silverman RH, Williams BRG, et al. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat Med. 2003;9:33–39. doi: 10.1038/nmxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA. 2008;105:12439–12444. doi: 10.1073/pnas.0806439105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem. Pharmacol. 2003;66:1433–1440. doi: 10.1016/s0006-2952(03)00494-5. [DOI] [PubMed] [Google Scholar]
- Maniati E, Potter P, Rogers NJ, Morley BJ. Control of apoptosis in autoimmunity. J Pathol. 2008;214:190–198. doi: 10.1002/path.2270. [DOI] [PubMed] [Google Scholar]
- Manirarora JN, Kosiewicz MM, Parnell SA, Alard P. APC activation restores functional CD4(+)CD25(+) regulatory T cells in NOD mice that can prevent diabetes development. PLoS One. 2008;3(11):e3739. doi: 10.1371/journal.pone.0003739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D, Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- Mariño E, Silveira PA, Stolp J, Grey ST. B cell-directed therapies in type 1 diabetes. Trends Immunol. 2011;32:287–294. doi: 10.1016/j.it.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Zaccone P, Di Marco R, Di Mauro M, Magro G, Grasso S, et al. The effects of a nonimmunogenic form of murine soluble interferon-gamma receptor on the development of autoimmune diabetes in the NOD mouse. Endocrinology. 1996;137:5567–5575. doi: 10.1210/endo.137.12.8940385. [DOI] [PubMed] [Google Scholar]
- Nolan-Sorden NL, Lesiak K, Bayard B, Torrence PF, Silverman RH. Photochemical crosslinking in oligonucleotide-protein complexes between a bromine-substituted 2-5A analog and 2-5A-dependent RNase by ultraviolet lamp or laser. Anal Biochem. 1990;184:298–304. doi: 10.1016/0003-2697(90)90684-2. 37. [DOI] [PubMed] [Google Scholar]
- Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmi C, Lleo A, Zuin M, Podda M, Rossaro L, Gershwin ME. Interferon alpha and its contribution to autoimmunity. Curr Opin Investig Drugs. 2006;7:451–456. [PubMed] [Google Scholar]
- Silveira PA, Grey ST. B cells in the spotlight: innocent bystanders or major players in the pathogenesis of type 1 diabetes. Trends Endocrinol Metab. 2006;17:128–135. doi: 10.1016/j.tem.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Silverman RH. 2-5A dependent RNase L: A regulated endoribonuclease in the IFN system. In: D'alessio G, Riordan JF, editors. Ribonucleases:structure and function. Academic Press Inc.; New York: 1996. pp. 517–547. [Google Scholar]
- Silverman RH, Zhou A, Auerbach MB, Kish D, Gorbachev A, Fairchild RL. Skin allograft rejection is suppressed in mice lacking the antiviral enzyme, 2’,5’-oligoadenylate-dependent RNase L. Viral Immunol. 2002;15:77–83. doi: 10.1089/088282402317340242. [DOI] [PubMed] [Google Scholar]
- Stewart TA, Hultgren B, Huang X, Pitts-Meek S, Hully J, MacLachlan NJ. Induction of type I diabetes by interferon-alpha in transgenic mice. Science. 1993;260:1942–1946. doi: 10.1126/science.8100367. [DOI] [PubMed] [Google Scholar]
- Solomon M, Sarvetnick N. The pathogenesis of diabetes in the NOD mouse. Adv Immunol. 2004;84:239–264. doi: 10.1016/S0065-2776(04)84007-0. [DOI] [PubMed] [Google Scholar]
- Wong S, Guerder S, Visintin I, Reich EP, Swenson KE, Flavell RA, et al. Expression of the co-stimulator molecule B7-1 in pancreatic beta-cells accelerates diabetes in the NOD mouse. Diabetes. 1995;44:326–329. doi: 10.2337/diab.44.3.326. [DOI] [PubMed] [Google Scholar]
- Xin Yi, Chun Zeng, Hongli Liu, Xiaoli Chen, Ping Zhang, Boo Seok Yun, Ge Jin, Aimin Zhou. Lack of RNase L attenuates macrophage functions. PLoS One. 2013;48(12):e81269. doi: 10.1371/journal.pone.0081269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JW, Jun HS. Cellular and molecular pathogenic mechanisms of insulin-dependent diabetes mellitus. Ann NY Acad Sci. 2001;928:200–211. doi: 10.1111/j.1749-6632.2001.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Zhou A, Hassel BA, Silverman RH. Expression cloning of 2-5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell. 1993;72:753–765. doi: 10.1016/0092-8674(93)90403-d. [DOI] [PubMed] [Google Scholar]
- Zhou A, Paranjape J, Brown TL, Nie H, Naik S, Dong B, et al. Interferon action and apoptosis are defective in mice devoid of 2’,5’-oligoadenylate-dependent RNase L. EMBO J. 1997;16:6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, Paranjape JM, Hassel BA, Nie H, Shah S, Galinski B, et al. Impact of RNase L overexpression on viral and cellular growth and death. J. Interferon Cytokine Res. 1998;18:953–961. doi: 10.1089/jir.1998.18.953. [DOI] [PubMed] [Google Scholar]