Abstract
In the development of the nervous system, one of the critical aspects is the proper navigation of axons to their targets, the problem of axonal guidance. We are using the chick visual system as a model to investigate the role of the lysophospholipids lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) as potential axon guidance cues. We show that both LPA and S1P cause specific, dose-dependent growth cone collapse of retinal neurons in vitro in the chick model system, with slight differences to mouse, but very similar to Xenopus. Because LPA and S1P receptors are GPCRs, we analyzed the intracellular signaling pathways using pharmacological inhibitors in chick retinal neurons. Blocking rho kinase (ROCK) prevented growth cone collapse by LPA and S1P, while blocking PLC or chelating calcium had no effect on growth cone collapse. Inhibiting Gi/o with pertussis toxin resulted in a partial reduction of growth cone collapse, both with LPA and S1P. Inhibition of p38 blocked growth cone collapse mediated by LPA but not S1P. Thus, in addition to the involvement of the G12/13-ROCK pathway, LPA and S1P induced collapse of chick retinal growth cones has a partial requirement for Gi/o.
Keywords: retinal ganglion cells, axon guidance, chicken, lysophosphatidic acid, lpar
Introduction
One important aspect of development in the nervous system is the problem of axon guidance. The visual system is an excellent system for investigation of axonal guidance. There is a well-studied stereotypical pathway from the retina to targets in the brain, which includes several intermediate guidance points as well as final mapping to the target, with the formation of a topographic map. Furthermore, there is only one type of output neuron from the retina, the retinal ganglion cells (RGCs), whose axons navigate relatively long distances to their final target in the brain.
The molecular mechanism of axon guidance involves cues along the pathway that interact with receptors on the navigating axonal growth cone. There have been many protein guidance cues, and their corresponding receptors, that have been demonstrated to play important roles in the visual system, including Ephs and ephrins [see 1], netrin [2,3], slits [4–7], and semaphorins [8,9]. However, to date, these major axon guidance cues identified have been proteins. We are investigating a novel set of biologically active molecules, signaling lysophospholipids, specifically lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P), as potential axon guidance cues. Similar to well-documented, inhibitory guidance cues, LPA and S1P have been shown to cause growth cone collapse in culture in a variety of neuronal cell lines [10–15] and some primary neurons [16,17, 18], including mouse and Xenopus retinal axons [19–22]. Furthermore, there is evidence in Xenopus that S1P may be involved in correct pathfinding into the tectum [22]. Thus, we have investigated their role in the chick visual system.
The lysophospholipids LPA and S1P mediate extracellular signaling by binding to cell surface receptors of the G-protein coupled receptor (GPCR) family [23,24]. There are at least five high-affinity LPA receptors, LPA1 to LPA5, and five S1P receptors, S1P1 to S1P5 [25,26], with additional reports of other potential receptors [27–30]. Ligand binding to these receptors has been shown to activate classic intracellular signaling through G-protein pathways, including Gi/o, Gq, G12/13, and possibly Gs.
In this study, we examine the responses of retinal growth cones to LPA and S1P and the intracellular signaling pathways mediating these responses in the model system of the embryonic chick. The chick has several advantages, including being well-studied with large, early developing eyes and having all stages of embryonic development easily accessible. We compare the responses in chick to previous reports in mouse and Xenopus.
Materials and Methods
Chick retinal culture
Retinas were dissected from embryonic day 6 (E6) chicken embryos (Hamburger and Hamilton stages 28–29) and cut into explants. Explants were cultured in Ham’s F12 media (Hyclone) supplemented with N2 and B27 (Life Technologies), 1 mM sodium pyruvate, 0.5 mg/ml ascorbic acid, 10 ng/ml CNTF (PeproTech), 50 ng/ml BDNF (PeproTech) and penicillin-streptomycin in a 37°C, 5% CO2 incubator. After overnight incubation on 0.1 mg/ml poly-D-lysine (PDL) and 10 µg/ml laminin coated dishes, cultures were treated as described below.
Growth cone collapse assay
Retinal explants were cultured overnight as describe above in Nunc Lab-Tek 4-well chambered coverglass dishes. Lysophosphatidic acid (LPA, 18:1 oleoyl; Avanti Polar Lipids) lyophilized aliquots were dissolved in water and then a 1 mM stock in PBS with 10% fatty acid-free BSA (Sigma) was made, which was diluted with media (above) to form final concentrations. Sphingosine-1-phosphate (S1P, BioMol/Enzo Life Sciences) lyophilized aliquots were dissolved in water containing 4 mg/ml fatty acid-free BSA (Sigma) to form a 100 µM stock, which was diluted with media to form final concentrations. Controls were made by diluting the carrier solution without lysophospholipids into media at similar dilutions. For treatment, 1/3 volume of culture media was removed from each well and 1/3 volume of media containing control or lysophospholipids was added to each well, incubated for 10 minutes in a 37°C, 5% CO2 incubator, then fixed by carefully underlaying cultures with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. After fixation, cultures were permeabilized with 0.1% Triton X-100 in PBS and stained with AlexaFluor 488 phalloidin (Life Technologies) and examined on an Olympus CKX41 fluorescence microscope. To quantify growth cone collapse, isolated growth cones not touching other neurites or growth cones, but attached to neurites emanating from the explant, were categorized as “normal” or “collapsed” and counted, and percent collapsed calculated. A growth cone was categorized as “collapsed” if there was no lamellipodia and there was a single, or no more than 3, extensions that were parallel to the axon; these extensions appeared to be retraction fibers based on morphology (thinner at tip), although we could not rule out they were filopodia. (If a growth cone could not be unambiguously determined to be collapsed or not, it was excluded from the analysis; less than 5% of all growth cones in any experiment or condition were omitted by this criteria. To confirm lack of bias, a subset of data was independently verified by another investigator (EB). Statistical analysis was performed using Fishers Exact Test (GraphPad Prism).
For testing specificity, other lysophospholipids were obtained from Avanti Polar Lipids and used as described for LPA above: 14:0 (stearoyl) lysophosphatidic acid, 18:0 (myristoyl) lysophosphatidic acid, 18:1 dioleoylglycerol pyrophosphate (DGPP), C18:1 Lyso PAF (1-O-oleyl-sn-glycero-3-phosphocholine), and egg lysophosphatidylcholine (LPC).
Time-lapse imaging
Retinal explants were prepared as above and grown overnight on MatTek Glass Bottom Dishes (PDL and laminin coated). Dishes were placed in a stage top incubator chamber (Warner Instruments) at 37°C with CO2 and images were taken every 30 seconds with a Nikon D7000 DSLR camera using Micro-Manager (http://www.micro-manager.org) with ImageJ and NKRemote (Breeze Systems) software and a 20× objective with phase optics on a Nikon Diaphot microscope. Growth cones were imaged for 30–40 minutes to determine baseline growth, then control medium was added to the dish and growth cones imaged for another 30–40 minutes. Then, medium containing lysophospholipids was added to give the final concentration and growth cones imaged for another 30 minutes or until no growth cones were visible in the field, whichever occurred first.
Inhibition of cell signaling pathways
Analysis of inhibition of cell signaling pathways was performed as a growth cone collapse assay as described above except that a 6× final concentration of pharmacological inhibitor (diluted from stock into media) was added prior to addition of 6× final concentration of LPA or S1P (and remained present during lysophospholipid addition). Controls included carrier for inhibitor along with LPA and S1P and also inhibitor without LPA or S1P. Inhibitors were used at the final concentrations of: Y-27632: 10 µM, H1152: 100 nM, U-73122: 1 µM, BAPTA: 50 µM, SB 203580: 20 µM, PD 98059: 20 µM, pertussis toxin: 200 ng/ml. All inhibitors were added 10 minutes prior to LPA or S1P addition except for pertussis toxin, which was added 16–20 hours prior to allow sufficient time for pertussis toxin, an enzyme, to cross the cell membrane and then catalytically ADP-ribosylate Gi [31–34]. All inhibitors were obtained from Calbiochem except pertussis toxin (List Biological Laboratories). Note that data are from at least 3 independent experiments (except 2 for BAPTA with LPA) and at least 125 growth cones analyzed for each treatment condition. (Data on the number of experiments and the number of growth cones analyzed is presented in Supplementary Table 1.) Note that the pertussis toxin data was collected by two investigators (JF and CW), and a subset of the growth cone counts was verified independently by a third investigator (EB). Statistical analysis by Fisher’s Exact Test was performed comparing inhibitor plus lysophospholipid to inhibitor without lysophospholipid and comparing treatment with inhibitor to treatment without inhibitor.
RT-PCR expression analysis
Total RNA from E6 chicken brain and retina was isolated with Trizol (Invitrogen) according to the manufacturer’s protocol. RT-PCR was performed using Qiagen’s One-Step RT-PCR kit as follows: RT for 30 minutes at 50°C, followed by 15 minutes at 95°C, then 35 cycles of PCR at 94°C-30 seconds, 55°C-1 minute, 72°C-1 minute, and a final extension at 72°C for 10 minutes. Products were electrophoresed on a 1.5% agarose gel containing ethidium bromide and imaged on a UV transilluminator system (BioRad). The primers used for RTPCR are given in Table 1.
Table 1.
Primer sequences used for RT-PCR of chicken RNA.
| Target | Forward primer | Reverse primer |
|---|---|---|
| lpar1 | CTATAGTGACTCCTACCTGGTC | AGGATCTGCTTGAAGGTAGC |
| lpar2 | TCAAGACGGTCACCATCATCC | GGGGTATATTTGGGGTTCC |
| lpar3 | CACTAGTGGATCTATAAGC | TCATAACAGAGTTCAGCAG |
| lpar4 | TGGAACTGGAGAGGGCTGAA | GGTGTGCTTCCGAAGGATGTC |
| lpar5 | GAAACGATCAGACAACAAGC | ACGGCCAGATTGAACATGTAG |
| p2y5 | TGACTGCGGTCAGGACTATG | GTAACCGAGGCTGAAACCAA |
| GAPDH | CCTCTCTGGCAAAGTCCAAG | TGGCTGTCACCATTGAAGTC |
Results
LPA and S1P cause growth cone collapse of embryonic chick retinal neurons
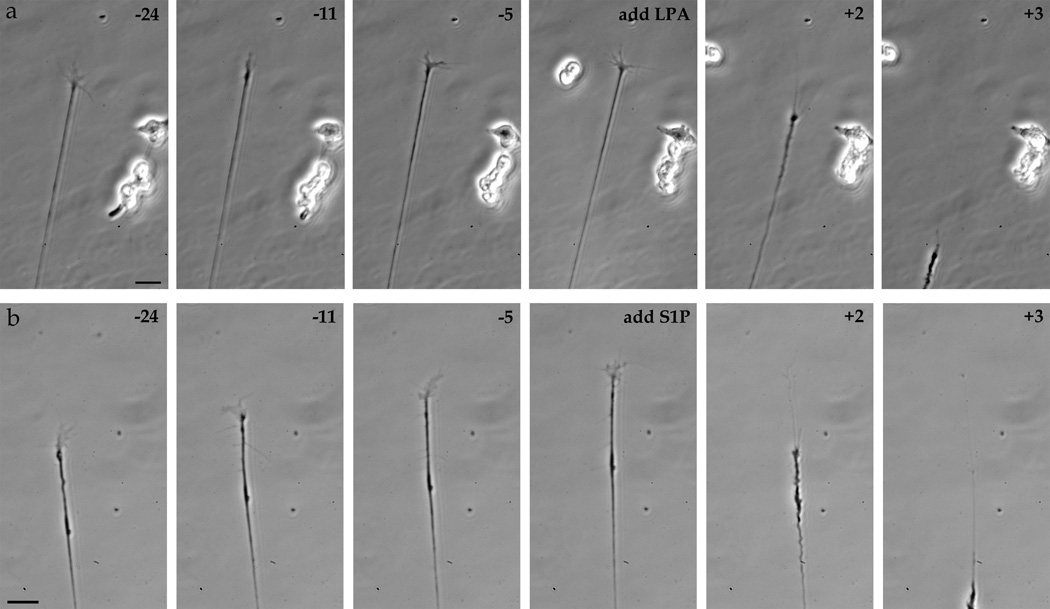
We first established the response of embryonic chicken retinal growth cones to the signaling lysophospholipids LPA and S1P. Treatment with LPA added uniformly to the media resulted in a rapid growth cone collapse, generally followed by neurite retraction (Fig. 1a). This growth cone collapse occurred on average 2.4 minutes (±0.86; n=18) after addition of 1 µM LPA or an average of 4.5 minutes (±5.3; n=37) after addition of 100 nM LPA. Similarly, on embryonic chick retinal growth cones, S1P treatment also produced a rapid growth cone collapse, again usually followed with neurite retraction (Fig. 1b). This response to S1P occurred on average 2.2 minutes (±0.60; n=21) after 1 µM S1P addition.
Figure 1.
Time-lapse imaging of chick retinal growth cones. Time-lapse imaging of growth cones from E6 chick retinal explants before (negative numbers) and after (positive numbers) addition of 1 µM LPA (a) or 1 µM S1P (b). time in minutes. Scale bars, 20 µm.
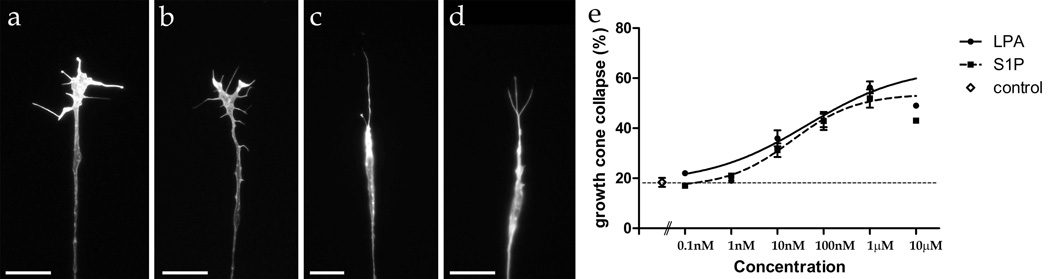
This growth cone collapse response was analyzed quantitatively in a growth cone collapse assay, measuring the number of growth cones showing a collapse morphology (Fig. 2c–d) compared to a normal growth morphology (Fig. 2a–b). Under culture conditions with just media addition, approximately 20% of embryonic chick retinal growth cones showed a collapsed morphology (control, Fig. 2e). However, addition of LPA caused a significant increase in collapsed growth cones, about 60% at 1 µM (Fig. 2e). This growth cone collapse response was dose-dependent, with virtually no growth cone collapse at less than 10 nM; it was statistically significant (p<0.0001) at 10 nM LPA and above. Growth cone collapse began to plateau around 1 µM LPA (Fig. 2e). S1P also showed a similar dose-dependent growth cone collapse on embryonic chick retinal growth cones, with statistical significant growth cone collapse at 10 nM and above (p<0.001; Fig. 2e).
Figure 2.
Growth cone collapse after LPA and S1P treatment. (a–b) Examples of retinal growth cones showing normal morphology under control conditions. (c–d) Examples of retinal growth cones showing collapsed morphology after treatment with 1 µM LPA (c) or 1 µM S1P (d). Scale bars, 20 µm. (e) Dose response of growth cone collapse (%) with different concentrations of LPA and S1P (from 0.1 nM to 10 µM) compared to control (no lipid) on a log scale. Error bars are SEM. The thin dashed line indicates the level of the control for reference.
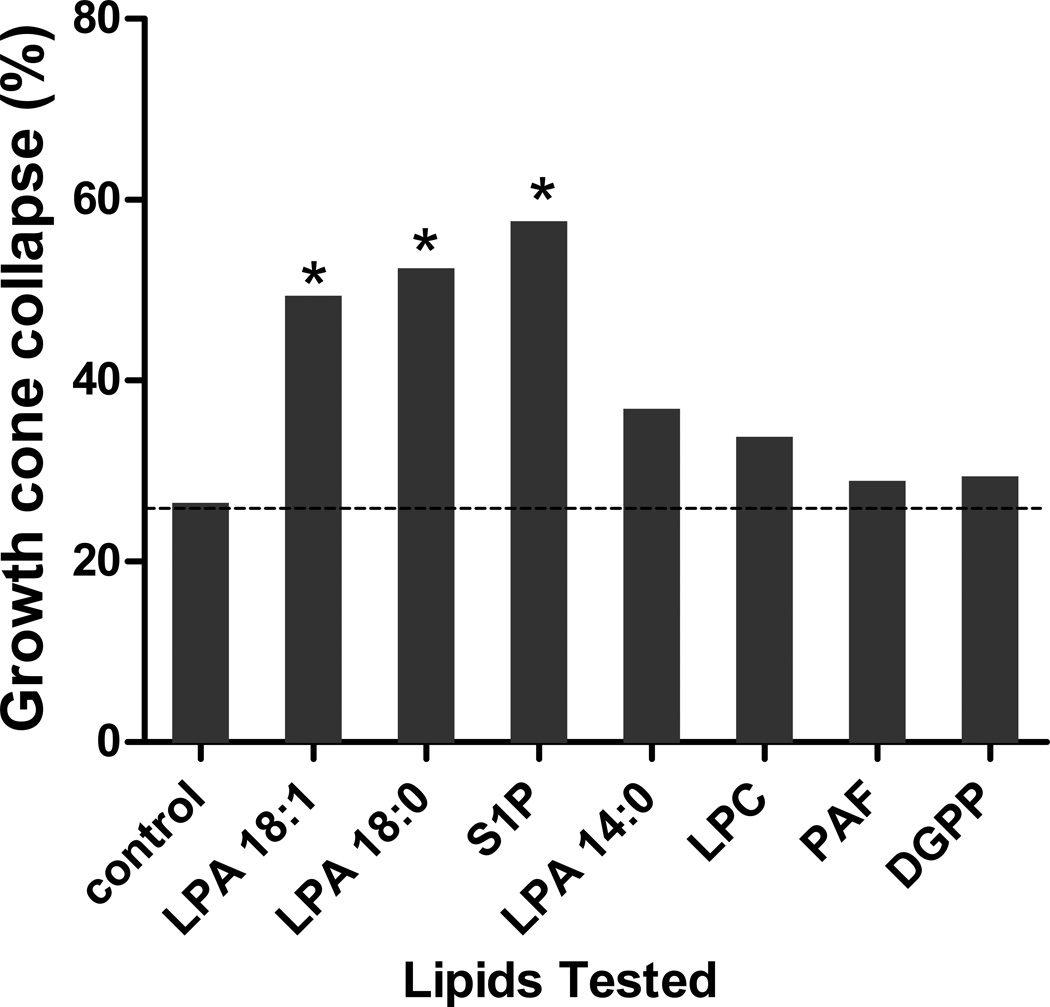
To confirm that the growth cone collapse response to LPA and S1P was specific and not a generalized lipid response, we examined the effect of other lipids (Fig. 3). The longer chain length LPAs, both 18:1 and the saturated 18:0, produced growth cone collapse. The shorter chain length LPA, 14:0, showed reduced growth cone collapse, indicating chain length specificity. Again, S1P caused growth cone collapse. However, the other lipids tested, LPC, PAF, and DGPP, showed no or minimal growth cone collapse over control. Thus, LPA and S1P cause a dose-dependent, specific growth cone collapse of embryonic chick retinal neurons.
Figure 3.
Growth cone collapse response induced by different lipids. Chick retinal growth cone collapse induced by 1 µM concentrations of various lipids compared to control. * indicates p<0.05 compared to control (Fishers exact test). The lipids tested were 18:1 LPA, 18:0 LPA, S1P, 14:0 LPA, lysophosphatidyl choline (LPC), Lyso PAF (PAF), and dioleoylglycerol pyrophosphate (DGPP). The dashed line indicates the level of the control for reference.
GPCR signaling pathways activated in growth cone collapse
The lysophospholipids LPA and S1P have been shown to act by binding to extracellular receptors. There are several LPA and S1P receptors, which are GPCRs and signal through canonical G-protein pathways. Indeed, in different systems in various situations, LPA and S1P receptors have been shown to be able to signal through each of the four canonical pathways, especially G12/13, Gq, and Gi/o. To identify which of these pathways are utilized to produce growth cone collapse of embryonic chicken retinal neurons, we used established pharmacological inhibitors to block these pathways and then assayed for LPA or S1P induced growth cone collapse.
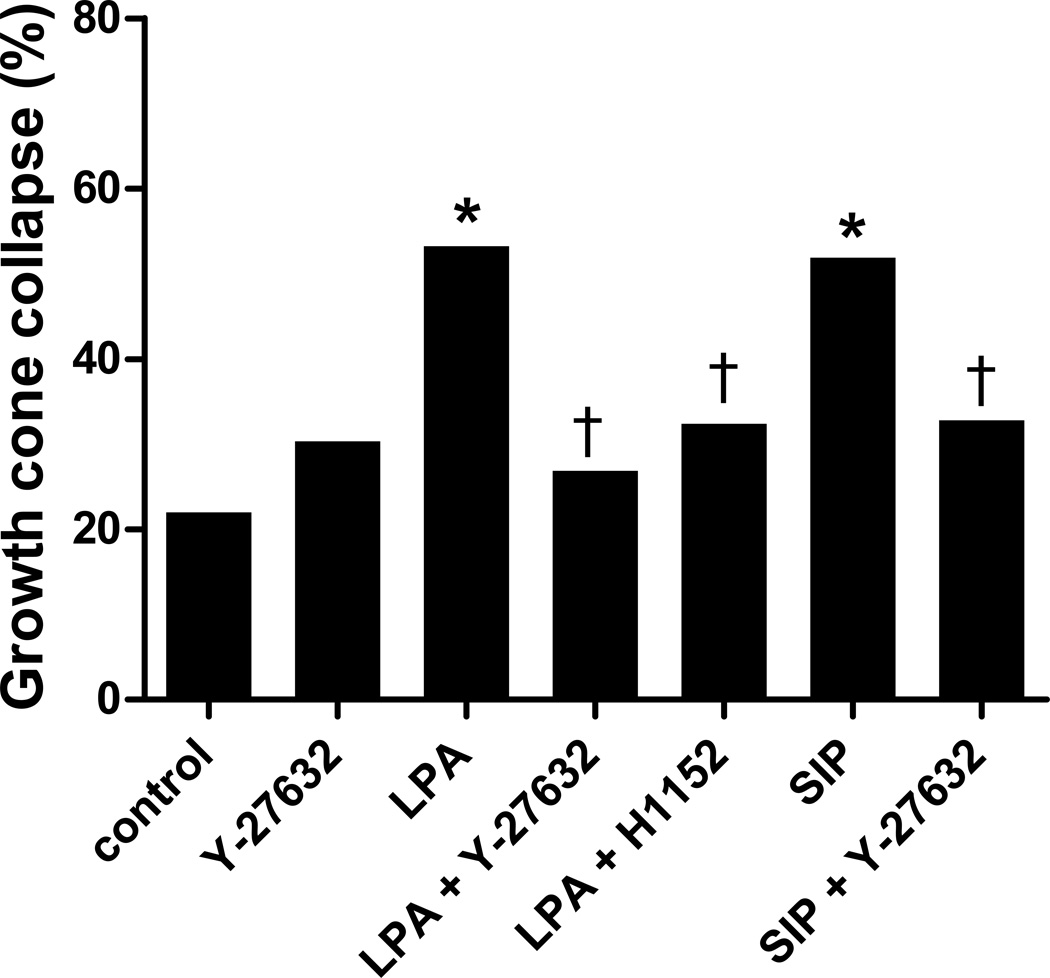
Previous studies in cell lines [11,35] have shown that LPA causes growth cone collapse through the G12/13 pathway and the downstream effectors rho and rho kinase (ROCK). Thus, we verified the involvement of the G12/13 pathway in embryonic chicken retinal neurons using two different ROCK inhibitors, Y-27632 and H1152, both with similar results (Fig. 4). Treatment with control media, or the ROCK inhibitors alone, showed a baseline growth cone collapse. Again, treatment with LPA or S1P produced significant growth cone collapse above this baseline (p<0.0001). However, addition of Y-27632 or H1152 prevented this LPA or S1P induced growth cone collapse, with collapse rates not significantly different than control (p=0.3–0.8). Thus, the G12/13-rho-ROCK pathway is required for mediating chick retinal growth cone collapse by LPA and S1P.
Figure 4.
Growth cone collapse blocked by ROCK inhibitors. Retinal growth cone collapse induced by 1 µM LPA or S1P is prevented by the presence of Y-27632 or H1152 compared to control (without inhibitor) or Y-27632 alone. Statistical significance: * indicates p<0.0001 compared to control; † indicates p<0.0001 compared to treatment with LPA or S1P without inhibitor.
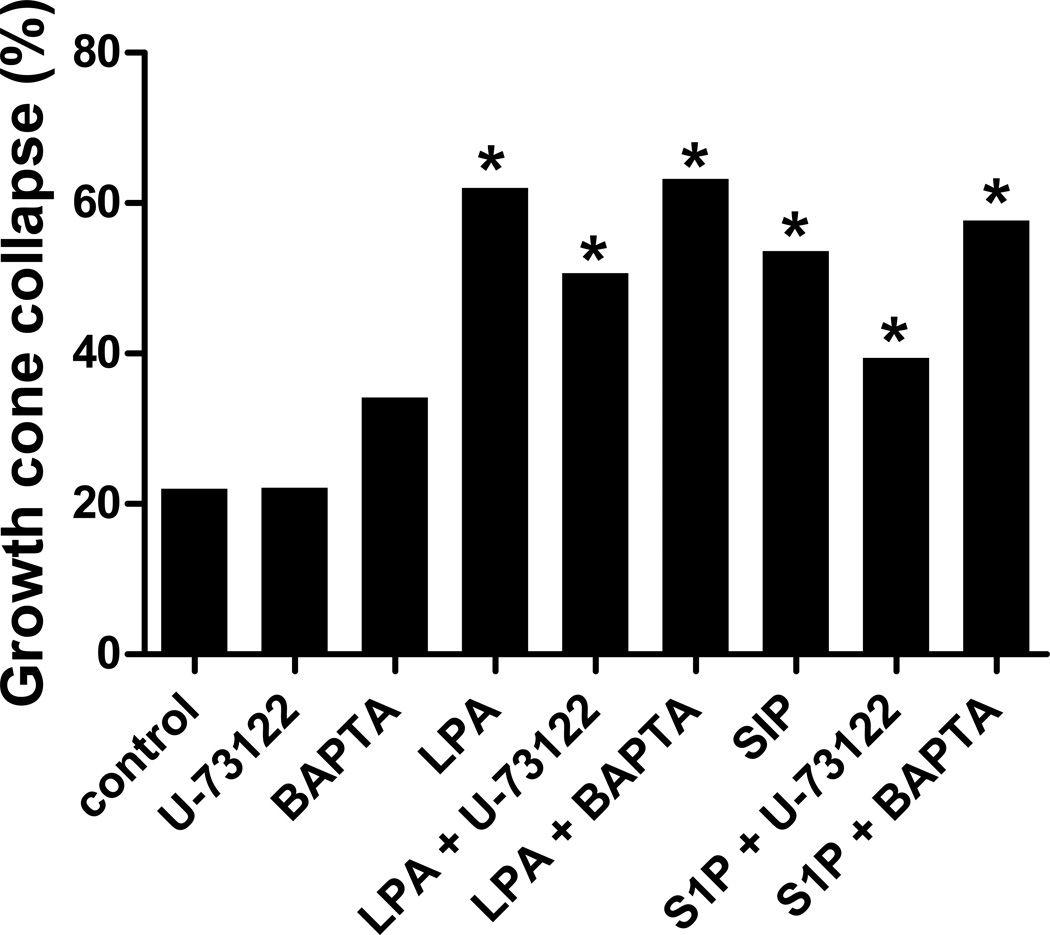
Another signaling pathway activated by GPCRs is Gq, which activates phospholipase C (PLC) and calcium release. We probed the Gq pathway by blocking PLC or chelating calcium. When we blocked PLC with the inhibitor U-73122, there was still significant growth cone collapse upon addition of LPA or S1P (p<0.0001 versus control; Fig. 5). Furthermore, treatment with BAPTA to chelate calcium before addition of LPA or S1P also did not change the significant growth cone collapse (p<0.0001), which was similar to LPA or S1P alone. Thus, blocking PLC or calcium release downstream of Gq had no effect on LPA or S1P induced growth cone collapse, suggesting that the Gq pathway is not required for LPA or S1P mediated growth cone collapse of embryonic chick retinal neurons.
Figure 5.
Growth cone collapse after blocking Gq pathway. Retinal growth cone collapse induced by 1 µM LPA or S1P in the presence of PLC inhibitor U-73122 or calcium chelation by BAPTA compared to control (without inhibitor) or inhibitors alone. Statistical significance: * indicates p<0.0001 compared to control (with or without inhibitor).
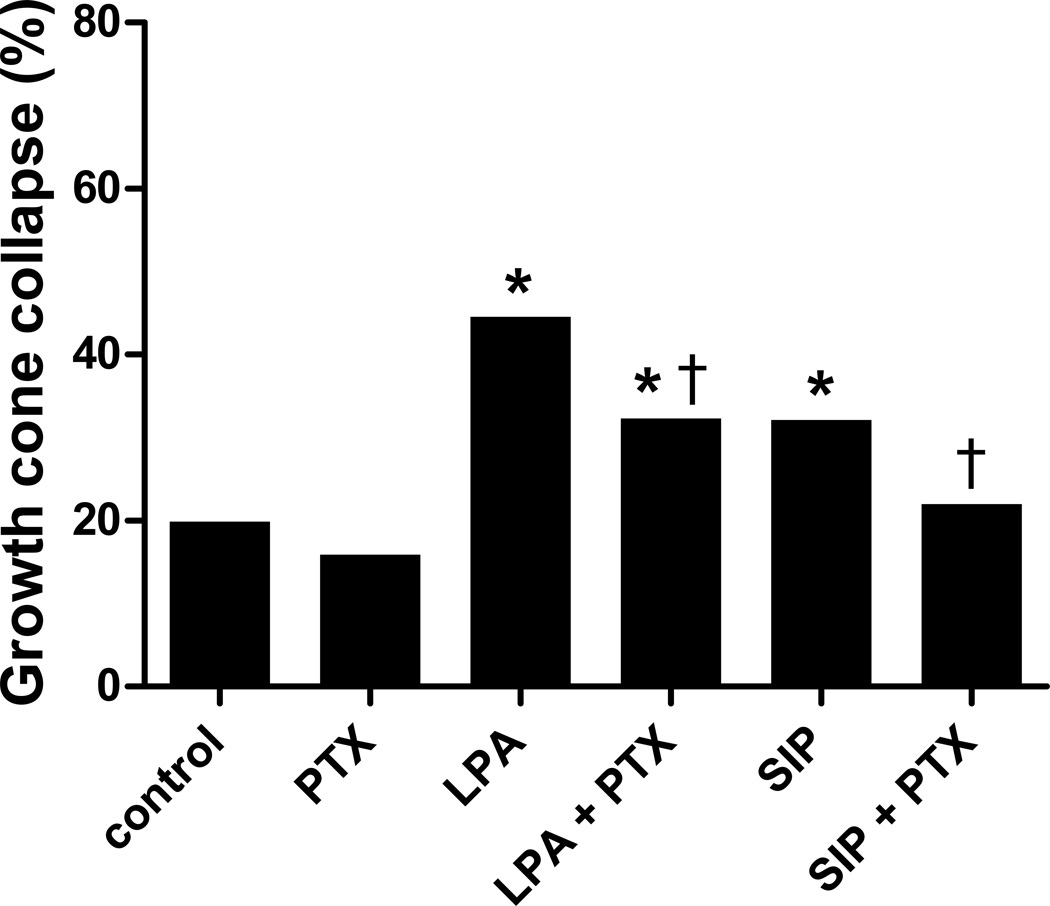
GPCRs can also signal through Gi/o, which includes activating rac for cytoskeletal regulation. Pertussis toxin (PTX) is an irreversible inhibitor of Gi/o, ADP-ribosylating Gi/o [31–34]. Pretreatment with PTX prior to LPA or S1P treatment significantly reduced the growth cone collapse induced by these lysophospholipids (p<0.0001; Fig. 6). However, there was still some growth cone collapse seen, significantly different than control (for LPA: p<0.001). Thus, in the presence of pertussis toxin, LPA and S1P can still induce some growth cone collapse of embryonic chick retinal neurites, but it is reduced compared to no inhibitor. This result suggests that there is a partial role for the Gi/o signaling pathway in LPA and S1P mediated growth cone collapse of embryonic chick retinal neurons.
Figure 6.
Growth cone collapse blocking Gi/o by pertussis toxin. Retinal growth cone collapse induced by 1 µM LPA or S1P in the presence of pertussis toxin (PTX) compared to control (without inhibitor) or PTX alone. Statistical significance: * indicates p<0.0001 compared to control; † indicates p<0.0005 compared to treatment with LPA or S1P without inhibitor.
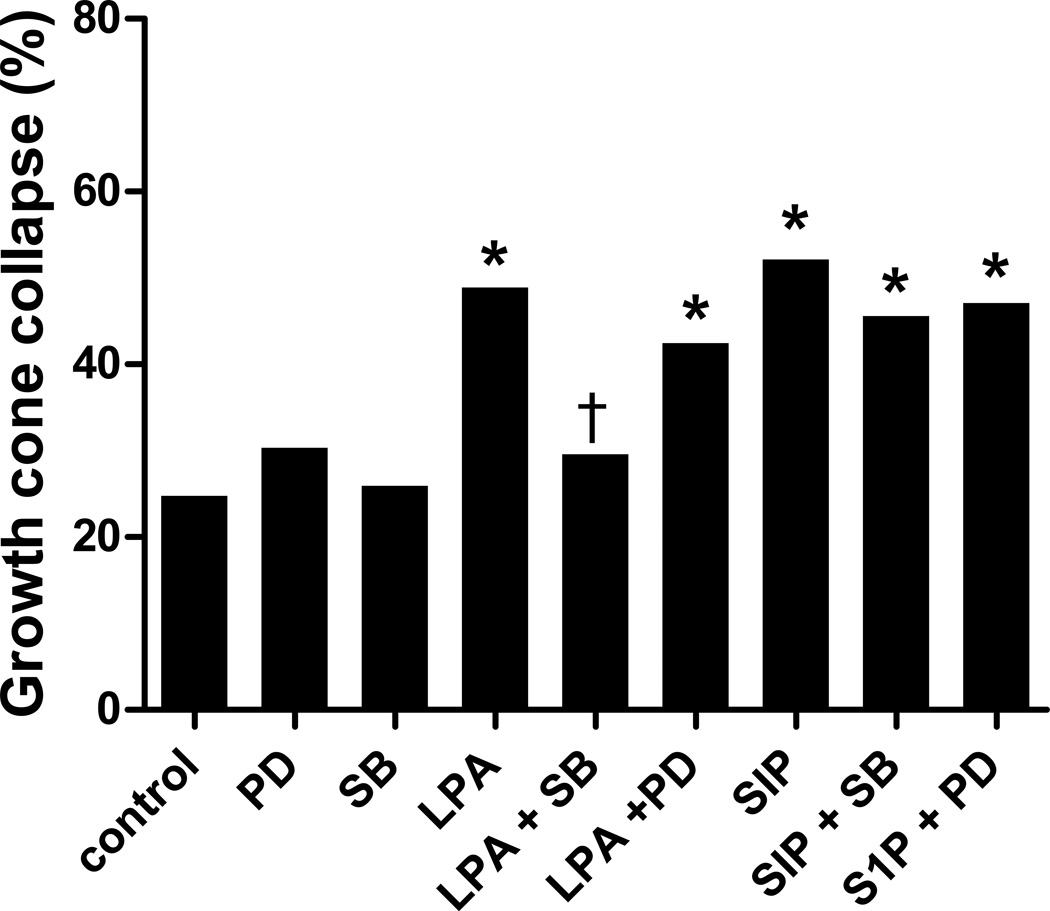
In the Xenopus visual system, Campbell and Holt [21] found a role for p38, but not p42/44, MAPK in LPA-induced growth cone collapse. To investigate the role of MAPKs in embryonic chick retinal growth cone collapse, we analyzed the effect of the p38 and p42/44 inhibitors that they used, SB 203580 and PD 98059 respectively [21]. Similar to the Xenopus system, pretreatment with SB 203580 prevented LPA-induced growth cone collapse (Fig. 7), suggesting a requirement for p38 activity. Blocking p42/44 activity with PD 98059, however, had no effect on LPA-induced growth cone collapse, suggesting that chick also does not require p42/44 activity, similar to Xenopus. However, when examining growth cone collapse induced by S1P, a different response was observed. Neither pretreatment with SB 203580 nor PD 98059 blocked S1P-induced growth cone collapse (Fig. 7), suggesting that neither p38 nor p42/44 activity are required for S1P mediated growth cone collapse of embryonic chick retinal neurons.
Figure 7.
Growth cone collapse with MAPK inhibitors. Retinal growth cone collapse induced by 1 µM LPA or S1P in the presence of p38 inhibitor SB 203580 (SB) and p42/44 inhibitor PD 98059 (PD) compared to control (without inhibitor) or inhibitors alone. Statistical significance: * indicates p<0.0001 compared to control; † indicates p<0.0001 compared to treatment with LPA without inhibitor.
LPA receptor gene expression
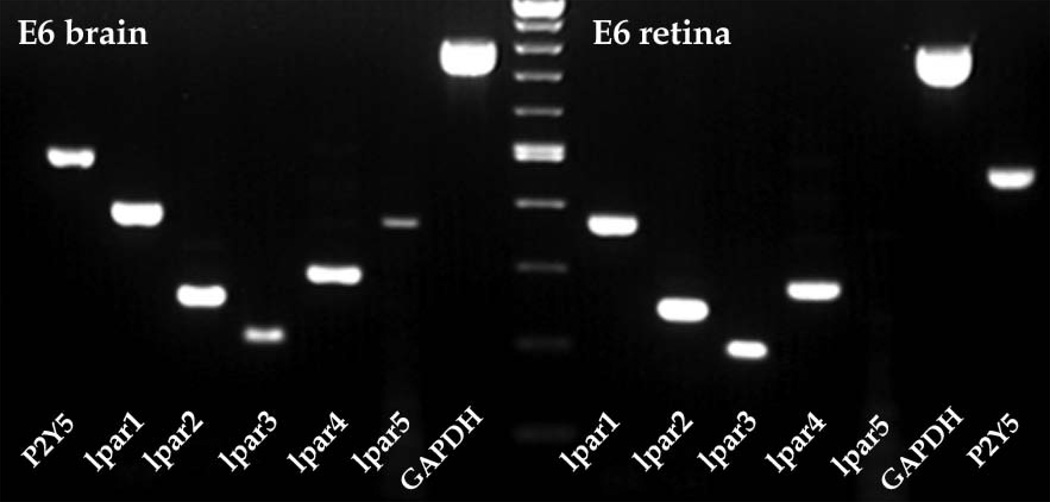
In addition, we analyzed the LPA receptor gene expression in developing chick retina by RT-PCR. Expression for lpar1, lpar2, lpar3 and lpar4 was detected in E6 retina, but lpar5 mRNA was not detected in retina, as shown in sample gel in Figure 8. In addition, the low affinity LPA receptor P2Y5, also referred to as lpar6 [28], was expressed in chick retina. All genes were detected in E6 chick brain samples (Fig. 8), which was used as an expression control. Similar results were seen from chick RNA samples from E5 to E12 (data not shown).
Figure 8.
Lpar gene expression in E6 chick retina by RT-PCR. RT-PCR expression of LPA receptor genes lpar1 to lpar5 as well as P2Y5 (lpar6) and GAPDH control from embryonic day 6 (E6) chick retina (right) compared to a brain control (left). Note the expression of all lpar genes except lpar5 in retina compared to expression of all genes in brain. Controls without reverse transcription (RT-) had no bands after PCR (data not shown). The center lane is a molecular weight marker.
Discussion
We have established that LPA and S1P cause a specific, dose-dependent growth cone collapse of embryonic chicken retinal neurites in vitro. Furthermore, we have analyzed the intracellular signaling pathways mediating this growth cone collapse. Chelation of calcium with BAPTA or inhibition of PLC with U-73122 did not prevent LPA or S1P mediated growth cone collapse, leading us to conclude that the Gq intracellular signaling pathway is not used by LPA and S1P to cause the cytoskeletal rearrangements that are involved in growth cone collapse. However, blocking rho kinase (ROCK) with either Y-27632 or H1152 downstream of G12/13 and rho did prevent growth cone collapse both by LPA and S1P. This is not surprising as rho and ROCK have been shown to mediate cytoskeletal retraction [36,37], including growth cone collapse [17,38–40]. Furthermore, in neuroblastoma cells as well as PC12 cell lines, blocking rho or ROCK inhibited LPA as well as S1P mediated neurite retraction [11,12,41]. Thus, as in other systems, the G12/13-rho-ROCK pathway is required for LPA and S1P induced growth cone collapse of chick retinal axons.
Interestingly, blocking the Gi/o pathway with pertussis toxin treatment reduced, but did not eliminate, growth cone collapse induced by LPA and S1P. Thus, there may be a role for the Gi/o pathway in modulating growth cone collapse, possibly in some growth cones but not in others. This result could be consistent with other investigations, as it has been reported that LPA-induced neurite retraction in PC12 cells is independent of pertussis toxin treatment [12]. However, other reports have shown that pertussis toxin treatment does block growth cone collapse [42–47]. Exactly why pertussis toxin blocks growth cone collapse in some retinal growth cones but not others requires further investigation. It is well known that cAMP levels can influence whether a particular axon guidance molecule is attractive or repulsive [48–50], so there may be heterogeneity of baseline cAMP levels in our cultures such that blocking Gi/o before LPA or S1P treatment would have different effects. Furthermore, the Gi/o pathway is known to activate rac, which has been shown to be involved in cytoskeletal protrusion and growth cone extension as well as neurite growth [13,51]. Thus, blocking Gi/o could influence rac activation, which could be involved in mediating the cytoskeletal rearrangements we see as growth cone collapse. However, if LPA and S1P normally activate Gi/o and rac, this activation would likely not lead to growth cone collapse, but rather extension; thus when Gi/o is blocked, one might expect more, not less, growth cone collapse. However, as cytoskeletal dynamics are critical for growth cone extension, blocking Gi/o and influencing the levels of rac activation could cause other effects on growth cone motility. Interestingly, other reports have suggested an interplay between rac and rho that is blocked by pertussis toxin [46,47]. Furthermore, recent reports suggest that growth cone collapse can be modulated by a non-canonical Gi/o pathway. The chemokine SDF-1 reduced, but did not block, slit-2 induced growth cone collapse of chick retinal neurons through the receptor CXCR4, thus acting as a modulator of the growth cone collapse response [52]. Signaling by SDF-1 led to increased cAMP levels [52] and required Gαi, Gαq, and Gβγ [53]. Pertussis toxin blocked the SDF-1 modulation, but by a non-canonical pathway as SDF-1 signaling led to an increase in cAMP, not a decrease as in the canonical Gαi signaling [52,53]. Overall, this partial involvement of Gi/o or reduction on LPA and S1P mediated growth cone collapse is interesting and warrants further study.
We note that the results we obtained on embryonic chick retinal axons are very similar to basic previous reports on Xenopus [20–22]. Our baseline growth cone collapse of chick retinal neurons of about 20% is similar to that reported in Xenopus, and a maximal growth cone collapse of about 60% is similar to the 50–60% reported for LPA and S1P in Xenopus [22]. We also examined the requirement for p42/p44 and p38 signaling in the LPA and S1P mediated growth cone collapse compared to Xenopus. In Xenopus, Campbell and Holt [21] showed that LPA induced growth cone collapse of retinal growth cones could be blocked by p38 inhibitors but not by p42/p44 inhibitors. In our experiments on chick retinal neurites, the p38 inhibitor SB 203580 also blocked growth cone collapse by LPA, but not the p42/44 inhibitor PD 98059, similar to Xenopus. Thus, this pathway for LPA induced growth cone collapse appears to be conserved between chick and frog. Interestingly, neither the p38 inhibitor SB 203580 nor the p42/44 inhibitor PD 98059 blocked growth cone collapse of chick retinal neurites induced by S1P. Thus, it appears that S1P induced growth cone collapse proceeds by a slightly different mechanism, although it still has other similarities to LPA induced growth cone collapse, such as the requirement for ROCK.
In comparison to the mouse system, we note some differences between this current study on chick and our previous study on mouse [19]. First, in chick, both LPA and S1P cause retinal growth cone collapse, while in mouse, S1P does not cause retinal growth cone collapse, only LPA. Second, in chick, the percentage of retinal growth cone collapse did not reach 100% as it did in mouse. Time-lapse imaging analysis of chick retinal neurites suggests that there are only a very small percentage of growth cones that do not respond to LPA (data not shown). However, in chick there appears to be a relatively rapid desensitization to LPA, as growth cones reemerge and neurites begin to extend again (within 20–30 minutes). Because of this regrowth, we have used a 10 minute treatment for LPA and S1P in our growth cone collapse assay. This 10 minute treatment allowed us to see the majority of collapsed growth cones, although some growth cones may have re-extended while others may not have collapsed yet, resulting in a lower, but still significant, growth cone collapse response. Thus, there are some interesting species differences between chick and mouse.
Lysophospholipids and axon guidance
What are the in vivo roles for LPA and S1P for retinal axons? Could they be involved in axon guidance? This work and others has demonstrated that the lysophospholipids LPA and S1P cause growth cone collapse in a variety of cell types, including RGCs [16,19,22]. Growth cone collapse is a hallmark of an inhibitory axon guidance cue. Indeed, one of the original growth cone collapsing factors, collapsin, now known as Semaphorin III [8,9,54–58], was identified based on its growth cone collapsing function and subsequently shown to be involved in axon guidance in vivo [56–58]. Several other bona fide inhibitory axon guidance cues have been shown to possess growth cone collapse activity, including slit [5,7,59], and the ephrins [1,60–68]. The in vitro growth cone collapse assay obviously differs from the in vivo situation in that the collapsing agent is added uniformly around the growth cone. It is thought that in vivo the collapsing agent would be directional, causing the growth cone to “collapse”, or be inhibited, on one side and then turn in the opposite direction [69]. Thus, growth cone collapse activity can imply inhibitory axon guidance function, suggesting that LPA and S1P may act as axon guidance cues; however, true axon guidance activity would need to be confirmed in vivo to establish this role.
What could be the possible roles for LPA and S1P in the visual system? Retinal ganglion cell axonal growth cones navigate a defined pathway with multiple guidance decisions made along the way [70,71] as well as specificity at the target for formation of a topographic map. The potential role of LPA and/or S1P in this pathway is currently not known. In Xenopus, S1P or a sphinogosine kinase inhibitor affected the growth of RGC axons into the tectum [22], suggesting that S1P may be involved in mediating retinal ganglion cell axon entry into the tectum. Furthermore, autotaxin, a major enzyme responsible for LPA production, is expressed strongly in caudal diencephalon [72,73], suggesting that LPA could be responsible for preventing retinal ganglion cell axon growth beyond the tectum. There is also the possibility that LPA and/or S1P may be involved in topographic mapping of RGC axons to the tectum, although preliminary evidence suggests that this may not be the case. Analysis of our chick retinal explants taken from all regions of the retina did not show a bimodal distribution of response to LPA or S1P (data not shown). If LPA and/or S1P was involved in topographic mapping, one might expect some explants, from one part of the retina, to show growth cone collapse while other explants, from a different retinal region, not to show growth cone collapse, and this was not apparent from our data. Furthermore, a preliminary analysis of responses from retinal explants from different quadrants to LPA and S1P did not show any major differences in growth cone collapse. For instance, there was no significant difference in growth cone collapse from nasal explants compared with temporal explants to either LPA or S1P. However, these results are preliminary and further analysis is warranted.
Another aspect of LPA and S1P as potential axon guidance molecules is the requirement of receptors on the responding cells. LPA and S1P bind to and activate a set of GPCRs. There are at least five receptors for LPA [25,26], with others likely, including P2Y5 [27,28], and at least five receptors for S1P [25,26]. Using RT-PCR, we have shown that LPA receptor genes lpar1, lpar2, lpar3, lpar4 and p2y5, but not lpar5, are expressed in developing chick retina. Furthermore, the S1P receptor genes s1p1r and s1p3r are also expressed in developing chick retina (unpublished). Thus, LPA and S1P receptors are being expressed in the appropriate tissue to act as retinal axon guidance factors, although definitive expression in retinal ganglion cells and their growth cones requires further analysis.
In summary, we have shown that the lysophospholipids LPA and S1P are inhibitory for embryonic chick retinal growth cones in vitro, causing growth cone collapse. This growth cone collapse is mediated by classic G12/13-rho-ROCK signaling. In addition, there is an involvement of Gi/o, although this is a partial requirement. These responses are similar to previous reports in mouse and Xenopus, but with some minor differences. This growth cone collapse is suggestive of a potential axon guidance role of lysophospholipids in the visual system.
Supplementary Material
Acknowledgements
We thank the Tyson Farms Complex of Monroe, NC for providing chicken eggs. This work was supported by grants from Winthrop University and from NIH Grant Number P20 RR-16461 from the National Center for Research Resources for support of the program entitled “South Carolina IDeA Networks of Biomedical Research Excellence” (SC-INBRE).
References
- 1.Feldheim DA, O'Leary DD. Visual map development: bidirectional signaling, bifunctional guidance molecules, and competition. Cold Spring Harb Perspect Biol. 2010;2:a001768. doi: 10.1101/cshperspect.a001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 3.Deiner MS, Sretavan DW. Altered midline axon pathways and ectopic neurons in the developing hypothalamus of netrin-1- and DCC-deficient mice. J Neurosci. 1999;19:9900–9912. doi: 10.1523/JNEUROSCI.19-22-09900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erskine L, Williams SE, Brose K, Kidd T, Rachel RA, Goodman CS, Tessier-Lavigne M, Mason CA. Retinal ganglion cell axon guidance in the mouse optic chiasm: expression and function of robos and slits. J Neurosci. 2000;20:4975–4982. doi: 10.1523/JNEUROSCI.20-13-04975.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niclou SP, Jia L, Raper JA. Slit2 is a repellent for retinal ganglion cell axons. J Neurosci. 2000;20:4962–4974. doi: 10.1523/JNEUROSCI.20-13-04962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ringstedt T, Braisted JE, Brose K, Kidd T, Goodman C, Tessier-Lavigne M, O'Leary DDM. Slit inhibition of retinal axon growth and its role in retinal axon pathfinding and innervation patterns in the diencephalon. J Neurosci. 2000;20:4983–4991. doi: 10.1523/JNEUROSCI.20-13-04983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, Tessier-Lavigne M. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron. 2002;33:219–232. doi: 10.1016/s0896-6273(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 8.Oster SF, Bodeker MO, He F, Sretavan DW. Invariant Sema5A inhibition serves an ensheathing function during optic nerve development. Development. 2003;130:775–784. doi: 10.1242/dev.00299. [DOI] [PubMed] [Google Scholar]
- 9.Campbell DS, Regan AG, Lopez JS, Tannahill D, Harris WA, Holt CE. Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J Neurosci. 2001;21:8538–8547. doi: 10.1523/JNEUROSCI.21-21-08538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jalink K, Eichholtz T, Postma FR, van Corven EJ, Moolenaar WH. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 1993;4:247–255. [PubMed] [Google Scholar]
- 11.Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tigyi G, Fischer DJ, Sebok A, Marshall F, Dyer DL, Miledi R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: neurite- protective effects of cyclic AMP signaling. J Neurochem. 1996;66:549–558. doi: 10.1046/j.1471-4159.1996.66020549.x. [DOI] [PubMed] [Google Scholar]
- 13.Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato K, Tomura H, Igarashi Y, Ui M, Okajima F. Exogenous sphingosine 1-phosphate induces neurite retraction possibly through a cell surface receptor in PC12 cells. Biochem Biophys Res Commun. 1997;240:329–334. doi: 10.1006/bbrc.1997.7666. [DOI] [PubMed] [Google Scholar]
- 15.MacLennan AJ, Devlin BK, Marks L, Gaskin AA, Neitzel KL, Lee N. Antisense studies in PC12 cells suggest a role for H218, a sphingosine 1-phosphate receptor, in growth-factor-induced cell-cell interaction and neurite outgrowth. Dev Neurosci. 2000;22:283–295. doi: 10.1159/000017452. [DOI] [PubMed] [Google Scholar]
- 16.Saito S. Effects of lysophosphatidic acid on primary cultured chick neurons. Neurosci Lett. 1997;229:73–76. doi: 10.1016/s0304-3940(97)00397-2. [DOI] [PubMed] [Google Scholar]
- 17.Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, Narumiya S. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron. 2000;26:431–441. doi: 10.1016/s0896-6273(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhang XF, Schaefer AW, Burnette DT, Schoonderwoert VT, Forscher P. Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron. 2003;40:931–944. doi: 10.1016/s0896-6273(03)00754-2. [DOI] [PubMed] [Google Scholar]
- 19.Birgbauer E, Chun J. Lysophospholipid receptors LPA1–3 are not required for the inhibitory effects of LPA on mouse retinal growth cones. Eye and Brain. 2010;2010:1–13. doi: 10.2147/EB.S7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 21.Campbell DS, Holt CE. Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron. 2003;37:939–952. doi: 10.1016/s0896-6273(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 22.Strochlic L, Dwivedy A, van Horck FP, Falk J, Holt CE. A role for S1P signalling in axon guidance in the Xenopus visual system. Development. 2008;135:333–342. doi: 10.1242/dev.009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anliker B, Chun J. Lysophospholipid G protein-coupled receptors. J Biol Chem. 2004;279:20555–20558. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- 24.Birgbauer E, Chun J. New developments in the biological functions of lysophospholipids. Cell Mol Life Sci. 2006;63:2695–2701. doi: 10.1007/s00018-006-6155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 26.Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2010;62:579–587. doi: 10.1124/pr.110.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasternack SM, von Kugelgen I, Aboud KA, Lee YA, Ruschendorf F, Voss K, Hillmer AM, Molderings GJ, Franz T, Ramirez A, Nurnberg P, Nothen MM, Betz RC. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet. 2008;40:329–334. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- 28.Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, Tajima Y, Taguchi R, Shimizu T, Ishii S. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J Biol Chem. 2009;284:17731–17741. doi: 10.1074/jbc.M808506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2007;363:861–866. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 30.Murakami M, Shiraishi A, Tabata K, Fujita N. Identification of the orphan GPCR, P2Y(10) receptor as the sphingosine-1-phosphate and lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2008;371:707–712. doi: 10.1016/j.bbrc.2008.04.145. [DOI] [PubMed] [Google Scholar]
- 31.Katada T, Ui M. Slow interaction of islet-activating protein with pancreatic islets during primary culture to cause reversal of alpha-adrenergic inhibition of insulin secretion. J Biol Chem. 1980;255:9580–9588. [PubMed] [Google Scholar]
- 32.Katada T, Amano T, Ui M. Modulation by islet-activating protein of adenylate cyclase activity in C6 glioma cells. J Biol Chem. 1982;257:3739–3746. [PubMed] [Google Scholar]
- 33.Kaslow HR, Burns DL. Pertussis toxin and target eukaryotic cells: binding, entry, and activation. Faseb J. 1992;6:2684–2690. doi: 10.1096/fasebj.6.9.1612292. [DOI] [PubMed] [Google Scholar]
- 34.Yanagida K, Ishii S, Hamano F, Noguchi K, Shimizu T. LPA4/p2y9/GPR23 mediates rho-dependent morphological changes in a rat neuronal cell line. J Biol Chem. 2007;282:5814–5824. doi: 10.1074/jbc.M610767200. [DOI] [PubMed] [Google Scholar]
- 35.Tigyi G, Fischer DJ, Sebok A, Yang C, Dyer DL, Miledi R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and Rho. J Neurochem. 1996;66:537–548. doi: 10.1046/j.1471-4159.1996.66020537.x. [DOI] [PubMed] [Google Scholar]
- 36.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 37.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn TB, Brown MD, Wilcox CL, Raper JA, Bamburg JR. Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of rac1. J Neurosci. 1999;19:1965–1975. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 40.Jin Z, Strittmatter SM. Rac1 mediates collapsin-1-induced growth cone collapse. J Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Postma FR, Jalink K, Hengeveld T, Moolenaar WH. Sphingosine-1-phosphate rapidly induces Rhodependent neurite retraction: action through a specific cell surface receptor. Embo J. 1996;15:2388–2392. [PMC free article] [PubMed] [Google Scholar]
- 42.Bates CA, Meyer RL. Heterotrimeric G protein activation rapidly inhibits outgrowth of optic axons from adult and embryonic mouse, and goldfish retinal explants. Brain Res. 1996;714:65–75. doi: 10.1016/0006-8993(95)01468-3. [DOI] [PubMed] [Google Scholar]
- 43.Clark GD, Zorumski CF, McNeil RS, Happel LT, Ovella T, McGuire S, Bix GJ, Swann JW. Neuronal platelet-activating factor receptor signal transduction involves a pertussis toxin-sensitive G-protein. Neurochem Res. 2000;25:603–611. doi: 10.1023/a:1007598617374. [DOI] [PubMed] [Google Scholar]
- 44.Goshima Y, Kawakami T, Hori H, Sugiyama Y, Takasawa S, Hashimoto Y, Kagoshima-Maezono M, Takenaka T, Misu Y, Strittmatter SM. A novel action of collapsin: collapsin-1 increases antero- and retrograde axoplasmic transport independently of growth cone collapse. J Neurobiol. 1997;33:316–328. doi: 10.1002/(sici)1097-4695(199709)33:3<316::aid-neu9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 45.Nakayama T, Goshima Y, Misu Y, Kato T. Role of cdk5 and tau phosphorylation in heterotrimeric G protein-mediated retinal growth cone collapse. J Neurobiol. 1999;41:326–339. [PubMed] [Google Scholar]
- 46.Sato K, Horiuchi Y, Jin Y, Malchinkhuu E, Komachi M, Kondo T, Okajima F. Unmasking of LPA1 receptor-mediated migration response to lysophosphatidic acid by interleukin-1beta-induced attenuation of Rho signaling pathways in rat astrocytes. J Neurochem. 2011;117:164–174. doi: 10.1111/j.1471-4159.2011.07188.x. [DOI] [PubMed] [Google Scholar]
- 47.Van Leeuwen FN, Olivo C, Grivell S, Giepmans BN, Collard JG, Moolenaar WH. Rac activation by lysophosphatidic acid LPA1 receptors through the guanine nucleotide exchange factor Tiam1. J Biol Chem. 2003;278:400–406. doi: 10.1074/jbc.M210151200. [DOI] [PubMed] [Google Scholar]
- 48.Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo M-m. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 49.Song H, Ming G, He Z, Lehmann M, Tessier-Lavigne M, Poo M-m. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 50.Hopker VH, Shewan D, Tessier-Lavigne M, Poo M-m, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- 51.de Curtis I. Functions of Rac GTPases during neuronal development. Dev Neurosci. 2008;30:47–58. doi: 10.1159/000109851. [DOI] [PubMed] [Google Scholar]
- 52.Chalasani SH, Sabelko KA, Sunshine MJ, Littman DR, Raper JA. A chemokine, SDF-1, reduces the effectiveness of multiple axonal repellents and is required for normal axon pathfinding. J Neurosci. 2003;23:1360–1371. doi: 10.1523/JNEUROSCI.23-04-01360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Twery EN, Raper JA. SDF1-induced antagonism of axonal repulsion requires multiple G-protein coupled signaling components that work in parallel. PLoS One. 2011;6:e18896. doi: 10.1371/journal.pone.0018896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 55.Raper JA, Kapfhammer JP. The enrichment of a neuronal growth cone collapsing activity from embryonic chick brain. Neuron. 1990;2:21–29. doi: 10.1016/0896-6273(90)90440-q. [DOI] [PubMed] [Google Scholar]
- 56.Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- 57.Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, Yagi T. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron. 1997;19:519–530. doi: 10.1016/s0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
- 58.Ulupinar E, Datwani A, Behar O, Fujisawa H, Erzurumlu R. Role of semaphorin III in the developing rodent trigeminal system. Mol Cell Neurosci. 1999;13:281–292. doi: 10.1006/mcne.1999.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- 60.Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 61.Brennan C, Monschau B, Lindberg R, Guthrie B, Drescher U, Bonhoeffer F, Holder N. Two Eph receptor tyrosine kinase ligands control axon growth and may be involved in the creation of the retinotectal map in the zebrafish. Development. 1997;124:655–664. doi: 10.1242/dev.124.3.655. [DOI] [PubMed] [Google Scholar]
- 62.Meima L, Kljavin IJ, Moran P, Shih A, Winslow JW, Caras IW. AL-1-induced growth cone collapse of rat cortical neurons is correlated with REK7 expression and rearrangement of the actin cytoskeleton. Eur J Neurosci. 1997;9:177–188. doi: 10.1111/j.1460-9568.1997.tb01365.x. [DOI] [PubMed] [Google Scholar]
- 63.Meima L, Moran P, Matthews W, Caras IW. Lerk2 (ephrin-B1) is a collapsing factor for a subset of cortical growth cones and acts by a mechanism different from AL-1 (ephrin-A5) Mol Cell Neurosci. 1997;9:314–328. doi: 10.1006/mcne.1997.0621. [DOI] [PubMed] [Google Scholar]
- 64.Davenport RW, Thies E, Zhou R, Nelson PG. Cellular localization of ephrin-A2, ephrin-A5, and other functional guidance cues underlies retinotopic development across species. J Neurosci. 1998;18:975–986. doi: 10.1523/JNEUROSCI.18-03-00975.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wahl S, Barth H, Ciossek T, Aktories K, Mueller BK. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J Cell Biol. 2000;149:263–270. doi: 10.1083/jcb.149.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frisén J, Yates PA, McLaughlin T, Friedman GC, O'Leary DDM, Barbacid M. Ephrin-A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron. 1998;20:235–243. doi: 10.1016/s0896-6273(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 67.Feldheim DA, Kim YI, Bergemann AD, Frisen J, Barbacid M, Flanagan JG. Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron. 2000;25:563–574. doi: 10.1016/s0896-6273(00)81060-0. [DOI] [PubMed] [Google Scholar]
- 68.Pfeiffenberger C, Yamada J, Feldheim DA. Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J Neurosci. 2006;26:12873–12884. doi: 10.1523/JNEUROSCI.3595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan J, Raper JA. Localized collapsing cues can steer growth cones without inducing their full collapse. Neuron. 1995;14:263–274. doi: 10.1016/0896-6273(95)90284-8. [DOI] [PubMed] [Google Scholar]
- 70.Oster SF, Deiner M, Birgbauer E, Sretavan DW. Ganglion cell axon pathfinding in the retina and optic nerve. Semin Cell Dev Biol. 2004;15:125–136. doi: 10.1016/j.semcdb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 71.Erskine L, Herrera E. The retinal ganglion cell axon's journey: insights into molecular mechanisms of axon guidance. Dev Biol. 2007;308:1–14. doi: 10.1016/j.ydbio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 72.Ohuchi H, Fukui H, Matsuyo A, Tomonari S, Tanaka M, Arai H, Noji S, Aoki J. Autotaxin controls caudal diencephalon-mesencephalon development in the chick. Dev Dyn. 2010;239:2647–2658. doi: 10.1002/dvdy.22403. [DOI] [PubMed] [Google Scholar]
- 73.Ohuchi H, Hayashibara Y, Matsuda H, Onoi M, Mitsumori M, Tanaka M, Aoki J, Arai H, Noji S. Diversified expression patterns of autotaxin, a gene for phospholipid-generating enzyme during mouse and chicken development. Dev Dyn. 2007;236:1134–1143. doi: 10.1002/dvdy.21119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.