Abstract
Background: Enterotoxigenic Escherichia coli (ETEC) strains are the major causes of diarrheal disease in humans and animals. Colonization factors and enterotoxins are the major virulence factors in ETEC pathogenesis. For the broad-spectrum protection against ETEC, one could focus on colonization factors and non-toxic heat labile as a vaccine candidate. Methods: A fusion protein is composed of a major fimbrial subunit of coli surface antigen 3, and the heat-labile B subunit (LTB) was constructed as a chimeric immunogen. For optimum level expression of protein, the gene was synthesized with codon bias of E. coli. Also, recombinant protein was expressed in E. coli BL21DE3. ELISA and Western tests were carried out for determination of antigen and specificity of antibody raised against recombinant protein in animals. The anti-toxicity and anti-adherence properties of the immune sera against ETEC were also evaluated. Results: Immunological analyses showed the production of high titer of specific antibody in immunized mice. The built-in LTB retains native toxin properties which were approved by GM1 binding assay. Pre-treatment of the ETEC cells with anti-sera significantly decreased their adhesion to Caco-2 cells. Conclusion: The results indicated the efficacy of the recombinant chimeric protein as an effective immunogen inducing strong humoral response. The designated chimer would be an interesting prototype for a vaccine and worthy of further investigation.
Key Words: Recombinant vaccine, Enterotoxigenic Escherichia coli (ETEC), cstH, eltB
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) is mostly prevalent in developing countries, and it causes diarrhea among children [1, 2] and visitors [1-3]. Diarrhea, which is caused by ETEC infection, leads to symptoms ranging from a nearly mild indisposition to a dehydrating condition resembling cholera disease [3]. In acute conditions [4], the ratio of death among children under five years old is 400,000 annually [2]. Colonization factor antigens or adhesions [5] and enterotoxins (heat stable and heat labile) [6] are two major ETEC virulence factors. Colonization factors are categorized as colonization factor antigens (CFA) or coli surface antigens (CS) [5]. The study of human ETEC strains revealed diverse antigenic sorts of CFA [3, 7]. CFA/I, CFA/II, and CFA/IV are predominant ones isolated from 50 to 75% of individuals infected with ETEC strains in different geographic areas throughout the world [8]. CS1, CS2, and CS3 are subsets of CFA/II and CFA/IV composed of CS4, CS5, and CS6 antigens [1, 9]. CFA/I and CS1-CS6 have higher prevalence among a variety of (>25) identified colonization factors [8]. CS3 is the most stated serotype protein in CFA/II family [6]. It is expressed alone and/or accompanied by CS1 and CS2. CS3 is characterized as a form of a flexible, fine, wiry thread with approximately 2 nm in diameter [10]. CS3 gene clusters are made up of cstA to –H genes, where CstH encodes the major fimbrial subunit of a 17.5-kDa precursor.
Two categories of toxins, heat stable and heat labile, are produced by ETEC [11]. Heat stable is a small molecule with poor ability to stimulate immunogenic reaction [7]. Heat labile has been proved as an immunogen in human [8] that stimulates mucosal and systemic immune responses [11]. It consists of two subunits, known as A and B, with B subunit (labile B subunit [LTB]) devoid of toxicity [11, 12], and it is in charge of toxin binding process [11, 13].
Even though the main treatment for ETEC diarrheal illness has been antibiotic therapy, the numbers of available antibiotics have been limited by increasing in anti-microbial resistance [14]. One of the most important prevention methods against ETEC is vaccine development. Although O antigens stimulate antibody responses, their diversification are too high to be used as vaccine [2]. Besides, flagellar and lipopoly-saccharide serogroups variation causes prohibition of the O and H antigens to be objective points in vaccine design [15]. Thus, the majority of vaccine development strategies depend on multivalent approaches, containing colonization factors with a heat-labile portion that provide a vast extent inclusion [8, 16].
Factors such as colonization and heat sensitivity of protective antigens imply that a fusion vaccine consisting of a heat-labile toxoid and CS3, CFA/I, and CS6 would cover more than 85% of ETEC isoltes worldwide. Existence of more widespread CFA in a vaccine formulation affords an encouraging vaccine [2, 10, 14] integrity, of which would be enhanced by adding anti-heat-labile immunity [5].
Until now, a lot of works have been carried out to generate ETEC vaccines, and all have considered heat labile and/or the colonization factors. Injection of purified heat labile and fimbriae in transcutaneous form, oral administration of microencapsulated purified fimbriae, DNA vaccines, killed whole and live attenuated ETEC cells, and expression of heat-labile B by transgenic plants are attempts made in this field [17, 18]. Adhesion-toxin chimeric antigens have the ability to induce anti-toxin and anti-adhesion immunity simultaneously [13] and such a vaccine deserves to be ideal because of conferring protective immunity against ETEC virulence factors [5]. In the present research, we designed a chimeric vaccine containing B subunit of heat labile and the major subunit of CS3 (cstH) to cover immunity against both ETEC virulence factors.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media. E. coli strains TOP10 and BL21DE3 (Invitrogen, Carlsbad, CA) were used to construct recombinant strains. Plasmids pET28a and pET32a (Novagen, USA) were served as vectors for expression of recombinant genes. The constructed E. coli strains were grown in Luria Bertani (LB) medium containing kanamycin (70 μg/ml) and ampicillin (50 μg/ml) (Merck, Germany) at 37°C for protein expression. Stock cultures (positive colonies) were maintained in 20% glycerol at -70°C.
Construction and cloning of cs3 (cstH-eltB): A bioinformatic analysis was carried out to design and optimize the sequence with E. coli codon usage [19]. A suitable linker (EAAAK)4 was incorporated between the eltB and cstH gene sequences. I-TASSER software was used to estimate tertiary structure prediction. The chimeric encoding gene was synthesized by Shine Gene Molecular Biotech, Inc. (Shanghai, China) into pUC57 cloning vector. Specific primers were designed according to the optimized gene on the basis of cloning each subunit individually. The synthetic gene was subcloned into pET28a, and the pET28a vector and pUC18 containing the chimeric gene was digested with EcoRI and HindIII. The linearized pET28a and the insert obtained from digestion of pUC18 were purified using the Bioneer Gel extraction kit (Bioneer, South korea). In total, 50 ng vector and 23 ng insert were mixed with 2 U of T4 DNA ligase (Fermentas, Lithuania) and T4 ligation buffer (300 mM Tris-HCl, 100 mM MgCl2, 100 mM DTT, and 10 mM ATP) in a 20-μl reaction mixture and incubated at 12oC overnight. The transformants were screened with restriction enzyme analysis and then confirmed by DNA sequencing. DNA fragments encoding eltB (312 bp) and cstH (438 bp) were amplified by PCR using synthetic gene as template and were cloned into pET28a and pET32a, respectively.
Expression and purification of recombinant proteins. The genes were expressed in E. coli BL21 (DE3). The transformants were grown in LB broth supplemented with 30 µg/ml concentration of kanamycin. The chimeric protein expression was induced with 1 mM IPTG upon OD600 of 0.5. The protein was analyzed by SDS-PAGE, and protein was purified by nickel-nitrilotriacetic acid (Qiagen, USA) resin under denaturing condition. The cell lysate was centrifuged twice at 14,000 ×g for 20 min, and supernatant was loaded in a nickel-nitrilotriacetic acid affinity column. The protein was eluted by pH gradient according to the manufacturer’s instructions. The eluted proteins were refolded in a step wise dialysis under descending concentrations of urea (from the 8 to 2 M) with a final dialysis in the absence of urea.
Western blot. The purified protein extracts were separated on 12% SDS-PAGE and transferred to a nitrocellulose membrane (Sigma, USA). The membrane was blocked with 5% skimmed milk at 4°C overnight and then incubated with mice anti-His tag antibody (Abcam, UK) at 1:10,000 dilution in PBS-T (PBS + 0.05% Tween 20) at 37°C for 1 h. The proteins were detected using 3, 3'-diaminobenzidine as a substrate.
Animal immunization. A group of 10 female BALB/C mice were given four doses of each protein. First round immunization included 20 μg recombinant protein with complete Freund’s adjuvant injected subcutaneously and intraperitoneally. The second and third doses were given as boosters of 15 and 10 μg protein, respectively using incomplete Freund’s adjuvant. The last dose with 10 μg protein was administered without adjuvant. Blood samples were collected two weeks after each booster dose. The sera were maintained at -70°C. Purified chimeric protein (CS3) was used intramuscularly to immunize adult rabbits. A volume of 200,150,100, and 50 μg recombinant protein was homogenized in an equal amount of adjuvant injected on days 0, 14, 28, and 42, respectively. Blood samples were collected after each injection.
Serum analysis. The level of antibodies against LTB, CS3, and CStH were determined by ELISA and compared with each other. Polystyrene 96-well plates (Thermo Scientific, USA) coated with 5 μg antigen in a coating buffer (64 mM Na2CO3, 136 mM NaHCO3, NaN3 pH 9.8) at 4°C overnight. The plates were washed with PBST after each step, and the non-specific sites were blocked with 5% skimmed milk powder in PBST at 37°C for 1 h. Immune and non-immune sera were serially diluted from 1:100 to 1:6400 in PBST, added to the ELISA plates and incubated at 37°C for 30 min. Horseradish peroxidase (HRP)-goat anti-mouse IgG (1:2,000 in PBST) (Abcam, UK) was added and then incubated at 37°C for 30 min. The development step was then performed using O-phenylenediaminedihydrochloride as a chromogen.
GM1 ELISA. To assess the ability of recombinant anti-CS3 antibody in neutralizing native toxin and inhibiting its binding to GM1 receptor, the GM1-ELISA test was conducted. The heat-labile toxin was purified as described previously [20]. ELISA strips were coated by 0.5 μg GM1. After an overnight incubation at 4°C, skimmed milk in PBS (1:1, v/v) was added at 37°C for 1 h as a blocking step. Serum collected from immunized mice were serially diluted and treated with heat-labile toxin at 37°C for 1 h and then added to the wells. The plates were incubated at 37°C for 30 min and followed by washing and addition of 1:2000 dilution of HRP-goat anti-rabbit IgG (Sigma). The color development was performed using O-phenylenediaminedihydrochloride as a chromogen.
Rabbit ileal loop assay. Bacteria were grown at 37°C for 72 h in brain heart infusion broth containing 2% casamino acids supplemented with polymyxin B (100 mg/ml). The cells were centrifuged at 12,000 ×g for 10 min, and the supernatants were filter sterilized to obtain cell-free supernatants. A white rabbit was kept at suitable condition and fasted for 24 h prior to use. Under local anesthesia, the small intestine was flushed with 10 ml PBS. A segment of small intestine was divided into three loops, each about 10 cm in length with 3 cm hiatuses. Intestinal loops were inoculated with cell-free supernatants from ETEC, ETEC + anti-LTB. The loops inoculated with PBS served as negative control. After injection of the loops, the abdomen was closed. The animal was sacrificed 18 h later by injection of pentobarbital into their veins. The loops were cut out, and the volume of fluid in each segment was measured. The lengths of the empty segments were determined, and the volumes per length ratios (ml/cm) were recorded [21]. Research was conducted in compliance with the Animal Welfare Act and regulations related to experiments involving animals.
Caco-2 cell adherence inhibition assay. Overnight culture of ETEC in CFA broth was prepared and inoculated to fresh media. Cells were harvested at exponential phase, washed three times in PBS buffer and set the optical density to 0.5 at 600 nm. Caco-2 cells, which were grown in a culture flask containing RPMI with 10% FBS, were trypsinized and transferred to a sterile 6-well cell culture plate. In order to establish cellular monolayer, 8 × 104 trypsinized cells were added to each well and incubated for 24-72 h. Bacterial cells (300 μl) pretreated with 40 μl immunized rabbit anti-sera were added to test wells. The plates were incubated at room temperature for 1.5 h to allow possible attachment. The cells were trypsinized and spread on LB agar plates. After 18 h incubation, the numbers of colonies in test and control plates were counted and the number of neutralized bacteria by antibody was calculated [22, 23].
Statistical analysis. Statistical analyses were carried out by SPSS 12.0 and student t-test. Significant difference was demonstrated by P<0.05.
RESULTS
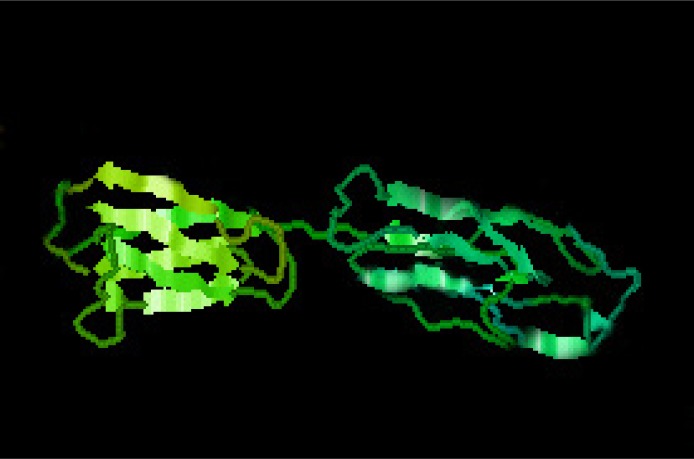
Design and construction of chimeric gene. A synthetic chimeric sequence encoding the cstH and eltB genes was designed using E. coli codon bias. To optimize the synthetic gene, negative cis acting motifs and repeated sequences were avoided. Both the wild type and the synthetic chimera were analyzed for their codon bias and GC content. The overall GC content was improved from 38.96% to 48.75% upon codon optimization, which increased the overall stability of mRNA. ΔG of the best predicted structure was -147.5 kcal/mol. The nucleotides at the starting of the 5′ did not have a long stable hairpin or pseudoknot, whereas in the native mRNA, the ΔG was -112 kcal/mol. The chimeric gene showed a codon adaptation index of 0.96 compared to that of the wild-type gene, which was only 0.72. Ab initio modeling of the synthetic sequence was exploited to produce three dimensional models of the chimeric protein. The result of tertiary structure of the chimeric protein construction using I-TASSER showed a protein with two main domains linked together with a linker (Fig. 1).
Fig.1.
Modeled structure of chimeric protein by I-TASSER software
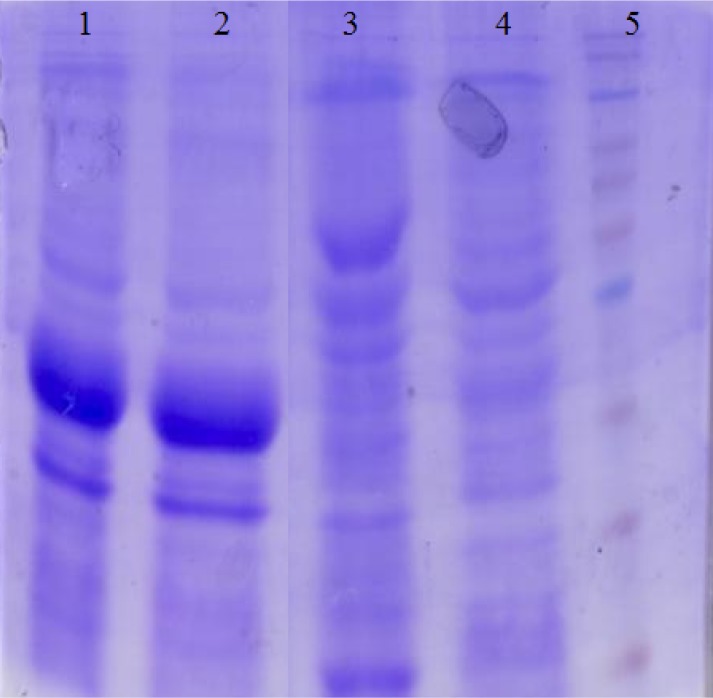
Expression and purification of recombinant protein. The synthetic chimeric gene was expressed in E. coli (BL21DE3) with the N-terminal 6×-His tag and analyzed by SDS-PAGE (Fig. 2). The SDS-PAGE analysis showed the presence of a 33-KD recombinant chimeric protein. Purification of the recombinant chimeric protein was carried out under denaturing conditions, and SDS-PAGE analysis revealed the presence of the protein as a major band (Fig. 3). The expression of recombinant chimeric protein was confirmed by Western blotting using anti-His tag antibodies.
Fig. 2.
Expression of recombinant proteins analyzed by SDS-PAGE. Lanes 1 and 2, total proteins of E. coli BL21DE3/ pET28a-CstH:LTB induced with IPTG; lanes 3 and 4, un-induced E. coli BL21DE3/pET28a-CstH:LTB gene as control, and lane 5, protein molecular marker
Fig. 3.
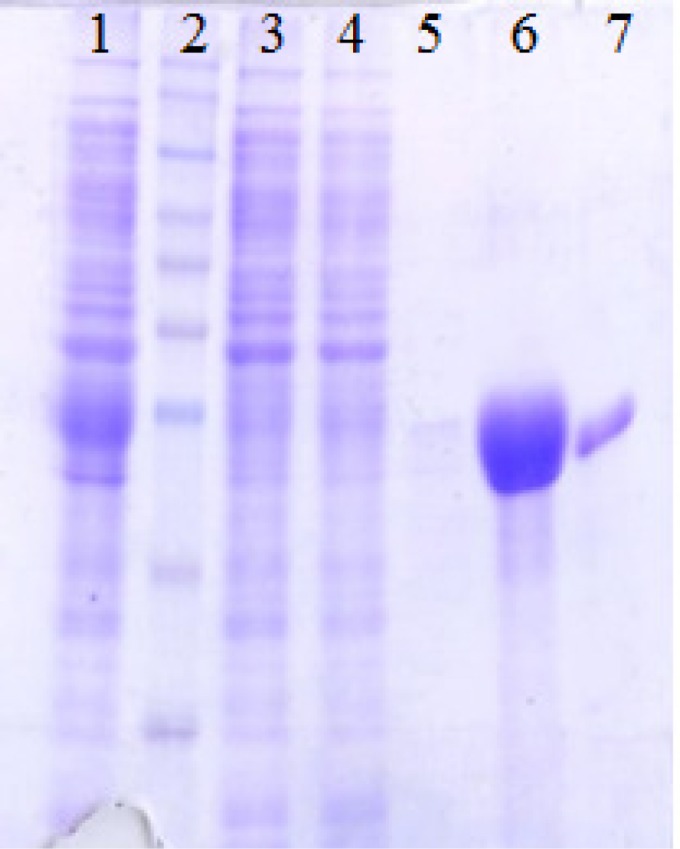
Purification of recombinant proteins with nickel-nitrilotriacetic acid column. Lane 1, expressed protein before purification; lane 2, protein molecular marker; lane 3, flow-through; lanes 4 and 5, wash column with C and D buffer (8M Urea, 20 mM NaH2PO4, 500 mM NaCl); lane 6, purified protein after elution with Elution buffer (8M Urea, 20 mM NaH2PO4, 500 mM NaCl), and lane 7, wash column MES (2-N-morpholine-ethanesulfonic acid) buffer
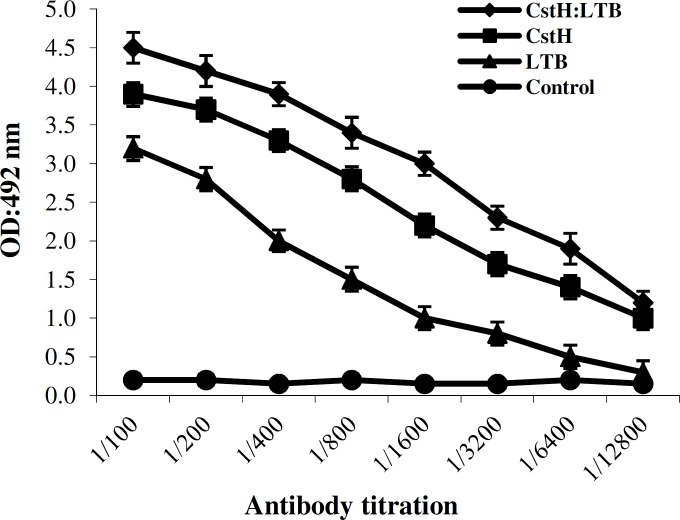
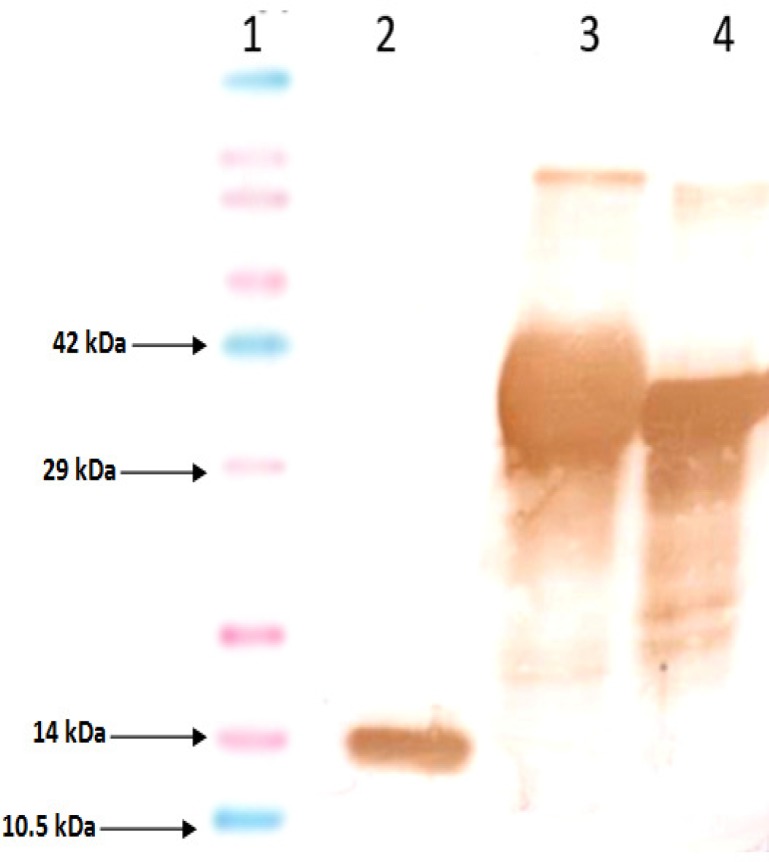
Immunization responses. The antigenicity of the recombinant proteins was determined by means of injecting CstH:LTB, CstH, and LTB into the mice and the rabbit subcutaneously and intraperitoneally. The humoral immune responses of the immunized mice were measured by ELISA technique. A high titer of antibody was produced in immunized animals. Remarkable titers of antibodies were noted after the second booster, which was further increased at subsequent booster doses (Fig. 4). The presence of LTB as an adjuvant resulted in elevated immune responses in comparison to the single antigen administration. The ability of anti-CstH:LTB antibody to recognize single proteins and also fusion one was verified by Western blot analysis (Fig. 5).
Fig. 4.
Evaluation of antibody production in animals receiving protein CstH:LTB, CstH, and LTB. Animals were injected with recombinant proteins using complete and incomplete Freund’s adjuvants. Immunizations were performed four times within eight weeks. Non-immunized mice sera were used as control (P<0.05).
Fig. 5.
Western blot analysis of protein with CstH:LTB anti-serum. Lane 1, recombinant CstH:LTB protein reacted with CstH:LTB anti-serum; lane 2, CstH protein responded to CstH:LTB antibody; lane 3, recombinant LTB objected to Western blot with CstH:LTB anti-serum, and lane 4, protein molecular marker. Western blot analysis of protein with CstH:LTB anti-serum. lane 1, protein molecular marker; lane 2, recombinant LTB objected to Western blot with CstH:LTB anti-serum; lane 3, CstH protein responded to CstH:LTB antibody and Lane 4, recombinant CstH:LTB protein reacted with CstH:LTB anti-serum
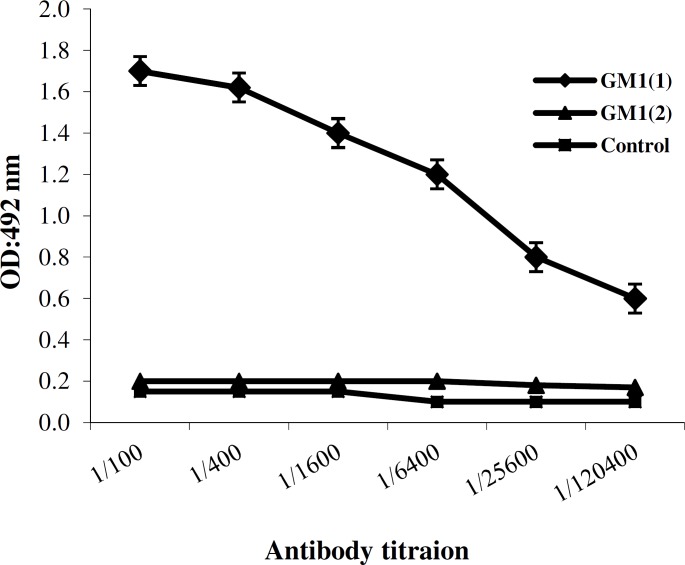
Activity inhibition assay of toxin. Biological activity and toxin neutralizing efficacy of the CstH:LTB-specific antibodies were tested by GM1-binding inhibition assay. Incubation of toxin with CstH:LTB antibody resulted in toxin neutralization and its prevention to bind to GM1 (Fig. 6). The sera from control mice or PBS did not show such effect. Induction of fluid accumulation by toxin was studied in rabbit ileal loops. The volume of secretion for each ileal loop was measured after excision. The fluid accumulation was not observed in rabbit ileal loops 18 h post infection with ETEC treated with the immunized serum. The volume per length ratios of ileal loops (ml/cm) of the rabbit injected with ETEC + anti-chimeric protein, ETEC, and PBS were recorded 0.81, 2.23, and 0.51, respectively.
Fig. 6.
Determination of inhibition of the binding of heat labile to GM1 using GM1 ELISA assay. Each graph shows the mean OD ± SD in three independent experiments. GM1(1), GM1 binding to the native toxin as positive control; GM1(2), neutralized toxin by CstH:LTB anti-serum lost GM1 binding ability, and control, no GM1 coated in control strips
Binding inhibition of ETEC to Caco-2 cells. ETEC cells pretreated with serum from the non-immunized rabbit were densely distributed on the Caco-2 cells, whereas ETEC-treated cells with rabbit anti-serum blocked their binding to Caco-2-cells. Immunized rabbit antibody inhibited 52% of bacterial cells to attach to Caco-2 cells compared to the ETEC cells treated with non-immunized rabbit serum.
DISCUSSION
ETEC attachment to the enteric epithelial cells with the help of adhesions and induction of excessive fluid secretion by enterotoxins lead to diarrhea. Hence, obstructing adherence of bacteria and toxin activation through receptor binding are the main strategies for prevention and control of ETEC disease [15]. Trial vaccines including whole-cell E. coli strains, which express a toxin and an adhesion antigen or joining both antigens as subunit vaccines, would confer anti-adhesion and anti-toxin immunity to the receiving hosts [18]. Administration of CS3 antigen combined with heat-labile toxoid in a purified form results in antibody development against both CS3 and LT antigens in human volunteers [24]. In this study, we fused cstH and eltB coding sequences with a suitable (EAAAK)4 linker, and (EAAAK)4 sequences were introduced among different domains for more flexibility and efficient separation. Our successful experience of using four repeated EAAAK sequences in chimeric gene has shown that it could lead to logically acceptable results [19, 22].
The fusion has been synthesized because of codon optimization applied according to E. coli codon bias. The cstH subunit was selected in chimera construction because of being the major fimbrial subunit, and it seems to be the most important antigen in CS3 operon to elicit antibody. Likewise, LTB selection depended on its non-toxic nature and also its immune stimulatory and adjuvant activity, which triggers B-cell differentiation and antibody production [25]. Several studies have been approved LTB as a potent adjuvant in chemical or genetic integration or in simultaneous administration with other antigens [11, 26-29]. Consequently, heat labile can function both as an antigen and an adjuvant [26]. In consistence with these references [27-30], our findings also indicated that the chimer protein consisting LTB and CstH had better immune responses than that of CstH alone.
Anti-His tag antibody utilization in Western blotting approved CstH:LTB integrity. Additional approval was added by the demonstration of the antigenic determinants of both CstH and LTB using anti-CstH:LTB antibody.
Recombinant strains expressing CstH:LTB were capable of inducing anti-CS3 and anti-heat-labile immunity, and immunization was particularly revealed more effective antibody responses than immunization with each antigen alone [10]. The main purpose in using booster doses after firs injection was to accomplish excellent immune responses and activate the B cell immune cascade by decreasing antigen doses [27]. After immunization with the chimeric protein, animals developed high titers of antibodies capable of reducing fimbrial attachment to Caco-2 cells and also neutralized toxin as shown in GM1 ELISA and ileal loop assay.
In conclusion, the recombinant fusion protein induced anti-adhesion antibodies against ETEC. The protein could also neutralize native toxin and inhibit its binding to GM1 receptor. The designated chimer is protective against ETEC.
ACKNOWLEDGEMENTS
This work was supported by Basic Sciences Research Center of Shahed University (Tehran). We are grateful to Miss Shakiba Alipoor for her technical assistance.
References
- 1.Byrd W, Mog SR, Cassels FJ. Pathogenicity and immune response measured in mice following intranasal challenge with enterotoxigenic Escherichia coli strains H10407 and B7A. Infect Immun. 2003 Jan;71(1):13–21. doi: 10.1128/IAI.71.1.13-21.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nazarian S, Gargari SL, Rasooli I, Hasannia S, Pirooznia N. A PLGA-encapsulated chimeric protein protects against adherence and toxicity of enterotoxigenic Escherichia coli. Microbiol Res. 2014 Feb-Mar;169(2-3):205–12. doi: 10.1016/j.micres.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Nazarian S, Gargari SL, Rasooli I, Alerasol M, Bagheri S, Alipoor SD. Prevalent Phenotypic and Genotypic Profile of Enterotoxigenic Escherichiacoli among Iranian Children. Jpn J Infect Dis. 2014 Mar;67(2):78–85. doi: 10.7883/yoken.67.78. [DOI] [PubMed] [Google Scholar]
- 4.Mansouri M, Mousavy SJ, Ehsaei Z, Nazarian S, Zali MR, Moazzeni SM. The codon-optimization of cfaE gene and evaluating its high expression capacity and conserved immunogenicity in Escherichia coli. Biologicals. 2013 May;41(3):169–75. doi: 10.1016/j.biologicals.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 5.You J, Xu Y, He M, McAllister TA, Thacker PA, Li X, et al. Protection of mice against enterotoxigenic E. coli by immunization with a polyvalent enterotoxin comprising a combination of LTB, STa, and STb. Appl Microbiol Biotechnol. 2011;89(6):1885–93. doi: 10.1007/s00253-010-2991-7. [DOI] [PubMed] [Google Scholar]
- 6.Gao RK, Zhang ZS, Li SQ, Huang CF. Construction of a novel display vector deriving from CS3 fimbriae of human enterotoxigenic Escherichia coli. Yi Chuan Xue Bao. 2001;28(10):971–9. [PubMed] [Google Scholar]
- 7.Sommer U, Petersen J, Pfeiffer M, Schrotz-King P, Morsczeck C. Comparison of surface proteomes of enterotoxigenic Escherichia coli (ETEC) and commensal Escherichia coli strains. J Microbiol Methods. 2010 Oct;83(1):13–9. doi: 10.1016/j.mimet.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Isidean SD, Riddle MS, Savarino SJ, Porter CK. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine. 2011 Aug 26;29(37):6167–78. doi: 10.1016/j.vaccine.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 9.Cassels F, Wolf M. Colonization factors of diarrheagenic E coli and their intestinal receptors. J Ind Microbiol. 1995 Sep;15(3):214–26. doi: 10.1007/BF01569828. [DOI] [PubMed] [Google Scholar]
- 10.Altboum Z, Barry EM, Losonsky G, Galen JE, Levine MM. Attenuated Shigella flexneri 2a DguaBA Strain CVD 1204 Expressing Enterotoxigenic Escherichia coli (ETEC) CS2 and CS3 Fimbriae as a Live Mucosal Vaccine. Infect Immun. 2001 May;69(5):3150–8. doi: 10.1128/IAI.69.5.3150-3158.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fingerut E, Gutter B, Meir R, Eliahoo D, Pitcovski J. Vaccine and adjuvant activity of recombinant subunit B of E. coli enterotoxin produced in yeast. Vaccine. 2005 Sep 7;23(38):4685–96. doi: 10.1016/j.vaccine.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 12.Rosales-Mendoza S, Soria-Guerra RE, Moreno-Fierros L, Govea-Alonso DO, Herrera-Díaz A, Korban SS, et al. Immunogenicity of nuclear-encoded LTB: ST fusion protein from Escherichia coli expressed in tobacco plants. Plant Cell Rep. 2011 Jun;30(6):1145–52. doi: 10.1007/s00299-011-1023-0. [DOI] [PubMed] [Google Scholar]
- 13.Fleckenstein JM, Hardwidge PR, Munson GP, Rasko DA, Sommerfelt H, Steinsland H. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect. 2010 Feb;12(2):89–98. doi: 10.1016/j.micinf.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones FR, Hall ER, Tribble D, Savarino SJ, Cassels FJ, Porter C, et al. The New World primate, Aotus nancymae, as a model for examining the immuno-genicity of a prototype enterotoxigenic Escherichia coli subunit vaccine. Vaccine. 2006 May 1;24(18):3786–92. doi: 10.1016/j.vaccine.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005 Jul;18(3):465–83. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaastra W, Svennerholm AM. Colonization factors of human enterotoxigenic Escherichia coli (ETEC) Trends Microbiol. 1996 Nov;4(11):444–52. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 17.Favre D, Lüdi S, Stoffel M, Frey J, Horn MP, Dietrich G. Expression of enterotoxigenic Escherichia coli colonization factors in Vibrio cholerae. Vaccine. 2006 May 15;24(20):4354–68. doi: 10.1016/j.vaccine.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C, Zhang W. Escherichia coli K88ac fimbriae expressing heat-labile and heat-stable (STa) toxin epitopes elicit antibodies that neutralize cholera toxin and STa toxin and inhibit adherence of K88ac fimbrial E. coli. Clin Vaccine Immunol. 2010 Dec;17(12):1859–67. doi: 10.1128/CVI.00251-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nazarian S, Mousavi Gargari SL, Rasooli I, Amani J, Bagheri S, Alerasool M. An in silico chimeric multi subunit vaccine targeting virulence factors of enterotoxigenic Escherichia coli (ETEC) with its bacterial inbuilt adjuvant. J Microbiol Methods. 2012 Jul;90(1):36–45. doi: 10.1016/j.mimet.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Evans DJJ, Evans DG, Richardson , SH , Gorbacj SL. Polymyxin B-induced release of low-molecular-weight, heat-labile enterotoxin from Escherichia coli. Infect Immun. 1974 Nov;10(5):1010–17. doi: 10.1128/iai.10.5.1010-1017.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De SN, Chatterje DN. An experimental study of the mechanism of action of Vibrio cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–62. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- 22.Bagheri S, Mousavi Gargari SL, Rasooli I, Nazarian S, Alerasol M. A CssA, CssB and LTB chimeric protein induces protection against Enterotoxigenic Escherichia coli. Braz J Infect Dis. 2014 Jan;18(3):308–14. doi: 10.1016/j.bjid.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JY, Yu J, Henderson D, Langridge WH. Plant-synthesized E. coli CFA/I fimbrial protein protects Caco-2 cells from bacterial attachment. Vaccine. 2004 Nov;23(2):222–31. doi: 10.1016/j.vaccine.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Lapa JA, Sincock SA, Ananthakrishnan M, Porter CK, Cassels FJ, Brinkley C, et al. Randomized clinical trial assessing the safety and immunogenicity of oral microencapsulated enterotoxigenic Escherichia coli surface antigen 6 with or without heat-labile enterotoxin with mutation R192G. Clin Vaccine Immunol. 2008 Aug;15(8):1222–8. doi: 10.1128/CVI.00491-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams N. Immune modulation by the cholera-like enterotoxin B-subunits: from adjuvant to immunotherapeutic. Int J Med Microbiol. 2000 Oct;290(4-5):447–53. doi: 10.1016/S1438-4221(00)80062-4. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Cassels F, Scharton-Kersten T, Hammond SA, Hartman A, Angov E, et al. Transcutaneous immunization using colonization factor and heat-labile enterotoxin induces correlates of protective immunity for enterotoxigenic Escherichia coli. Infect Immun. 2002 Mar;70(3):1056–68. doi: 10.1128/IAI.70.3.1056-1068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weltzin R, Guy B, Thomas WD Jr, Giannasca PJ, Monath TP. Parenteral Adjuvant Activities of Escherichia coli Heat-Labile Toxin and Its B Subunit for Immunization of Mice against Gastric Helicobacter pylori Infection. Infect Immun. May 2000;68(5):2775–2782. doi: 10.1128/iai.68.5.2775-2782.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura S, Asanuma H, Tomita T, Komase K, Kawahara K, Danbara H, Hattori N, Watanabe K, Suzuki Y, Nagamine T. Escherichia coli heat-labile enterotoxin B subunits supplemented with a trace amount of the holotoxin as an adjuvant for nasal influenza vaccine. Vaccine. 1994 Sep;12(12):1083–9. doi: 10.1016/0264-410x(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 29.Nawar HF, Arce S, Russell MW, Connell TD. Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities. Infect Immun. 2005 Mar;73(3):1330–42. doi: 10.1128/IAI.73.3.1330-1342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schunk MK, Macallum GE. Applications and optimization of immunization procedures. ILAR J. 2005;46(3):241–57. doi: 10.1093/ilar.46.3.241. [DOI] [PubMed] [Google Scholar]