Abstract
Background : The emergence and propagation of different phylogenetic groups of antimicrobial-resistant E. coli have become a worldwide health concern in human and veterinary medicine. Therefore, the evaluation of the phylogenetic distribution of antibiotic-resistant E. coli is important for therapeutic and economic purposes. The aims of this study were to determine phylogenetic groups and patterns of antibiotic resistance of E. coli strains isolated from human urinary tract infection and avian colibacillosis. Methods: A total of 50 E. coli isolates (25 from human urinary tract infection and 25 from avian colibacillosis) were characterized by culture and assigned as different phylogenetic groups (A, B1, B2, and D) by triplex PCR assay. Kirby-Bauer disk diffusion method was used to assess the susceptibility of all isolates to ten antibiotics. Results: Results showed that the majority of the human and poultry isolates belonged to phylogenetic groups A and B2 and phylogenetic group B1 of the avian pathogenic strain isolates were the most drug-resistant isolates. Most of the isolates were resistant to at least five antibiotics, and multiple drug resistance was observed in 98% of E. coli isolates. A high degree of resistance was seen against penicillin and erythromycin. Conclusion: According to the results of this study, multidrug-resistance among isolates and high relation between phylogenetic groups and resistance in both human and poultry isolates were observed.
Key Words: Escherichia coli, Avian colibacillosis, Phylogenetic grouping
INTRODUCTION
E. coli is well known for its capacity to cause a variety of infections. In addition to gastrointestinal illness typically manifested as diarrhea, E. coli also causes a variety of diseases outside the intestinal tracts of humans and animals, which include urinary tract infections, meningitis, sepsis, abdominal infections, osteomyelitis, cellulitis, wound infections, and colibacillosis [1, 2].
Colibacillosis is one of the most frequently reported diseases in the poultry industry. This disease is economically relevant to poultry producers, because it causes high mortality and poor egg quality in broilers and laying hen flocks, respectively. Especially on rural farms, E. coli infections seriously affect production and bird survival, since biosecurity and hygiene are frequently unheeded. The disease can be controlled using antimicrobials for therapy and prophylaxis [3]. On rural farms, increase in antimicrobial resistance in developing countries has become a major concern due to frequent use of antibiotics, which promotes multiple drug resistance (MDR) in urinary pathogenic E. coli (UPEC) in both veterinary and human medicine [4, 5]. E. coli is responsible for up to 90% of all community-acquired and almost 50% of nosocomial urinary tract infections. β-lactam and quinolone antimicrobials are the most frequently prescribed drugs for treatment in clinical settings [5]. Transfer of antimicrobial-resistant strains of E. coli to the food chain from poultry source is a well-recognized phenomenon. The avian pathogenic strains (APEC), which cause cellulitis, septicemia and colibacillosis in poultry, may link to extra-intestinal pathogenic E. coli strains in humans. These extra-intestinal pathogenic E. coli possess some virulence factors that enable them to cause disease outside the intestinal tract. Therefore, the resistant APEC may transfer antimicrobial-resistant strains to human via the food chain and can have implications for treatment of urinary tract and other extra-intestinal infections. This matter may have effect on treatment of salmonellosis and other enteric infections as well. This occurrence of any changes in the resistance profile of avian strains of E. coli should be mentioned and evaluated [2].
Four main phylogenetic groups have been shown in E. coli, including phylogenetic groups of A, B1, B2, and D. Phylogenetic grouping can carried out by multilocus enzyme electrophoresis, ribotyping or patterns of the strains in the E. coli reference collection, but these reference techniques are complex and time-consuming and also require a collection of typed strains [6, 7]. Clermont et al. [6] described a rapid technique for determining the phylogenetic groups of E. coli strains based on PCR detection of the chuA and yjaA genes and DNA fragment TspE4.C2. The virulent extra-intestinal strains belong mainly to group B2 and, to a lesser extent, to group D, whereas most commensal strains belong to groups A and B1 [6]. Thus, according to the importance of different E. coli phylogenetic groups and the role of its antibiotic resistance pattern, the purposes of this study were as follow: 1) to determine different phylogenetic groups of isolated E. coli; 2) to determine antibiotic resistance profile of isolated E. coli, and 3) to determine any correlation between different phylogenetic groups and antibiotic resistance of isolated E. coli.
MATERIALS AND METHODS
Sample collection. From January to November 2012, a total of 235 samples including 91 urine samples from hospitalized patients and 144 samples from poultry carcasses suspected to colibacillosis were collected.
Isolation of Escherichia coli. Samples were cultured on McConkey agar (Merck, Germany) and eosin methylen blue agar agar plates (Merck, Germany) and incubated at 37°C for 24 hours. Suspected E. coli colonies were identified by standard methods based on colonial appearance, and bacterial morphology, followed by biochemical characteristics. Pure colonies, which were urease negative, indole positive, oxidase negative, citrate negative, motility positive, methyl red positive, Voges-Proskauer negative, and lactose fermentation positive on triple sugar iron agar were identified as E. coli [2].
Antibiotic susceptibility testing. The antibacterial susceptibility testing of all E. coli isolates was performed using the Kirby-Bauer disk diffusion method. A volume of 100 µl of an overnight growth of each E. coli isolate on Mueller-Hinton broth with 0.5 McFarland standard turbidity was streaked on Mueller-Hinton agar plates. The routinely used 10 antibiotic discs, all from HiMedia® (India), including penicillin, ampicillin, amoxicillin, cefixime, cephalexin, ciprofloxacin, nalidixic acid, erythromycin, tetra-cycline and gentamicin were placed on the surface of the inoculated plates. The plates were incubated at 37°C for 24 hours. The zones of inhibition were measured and compared with standard chart and with E. coli ATCC 25922 and Staphylococcus aureus ATCC 29213 as antibiotic controls. Isolates with intermediate resistance were defined as susceptible, and the isolates were considered as multidrug resistant if they were resistant to at least three classes of antibiotics [4, 8, 9].
DNA extraction. Two colonies of pure isolated bacteria were placed into a tube containing 100 µl of double distilled water. Tubes were heated at 100ºC for 10 minutes, and then the cells were pelleted by centrifugation. The supernatant containing DNA was taken out and stored at -20ºC [2].
Multiplex PCR reaction for isolates. All E. coli isolates tested by multiplex PCR have been described previously [6]. As shown in Table 1, three sets of primers were used in this study, including ChuA, YjaA, and TspE4C2, which generate 279 bp, 211 bp, and 152 bp fragments, respectively. Multiplex PCR reaction was performed in a 25 µl reaction mixture, containing PCR buffer (10 mM Tris-HCl, 50 mM KCl, and 1.5 mM MgCl2, pH 8.7), dNTP (200 μM), each primer (0.4 μM), Taq DNA polymerase (1U), and template DNA (2 µl). PCR reaction was performed in a DNA thermocycler (Model CP2-003; Corbett, Sydney, Australia) as follows: Initial denaturation at 94ºC for 4 min, 30 cycles of denaturation at 94ºC for 5 s, annealing at 59ºC for 10 s, elongation at 72ºC for 30 s and a final extension step of at 72ºC for 5 min, followed by a hold at 4ºC. PCR products were electrophoresed on 1.5% agarose gel containing ethidiumbromide at 80 V for 1 h.
Table 1.
Primer characteristics used in this study
| Primer | Target gene | Primer length (bp) | Sequence |
Amplified
fragment size (bp) |
References |
|---|---|---|---|---|---|
| ChuA.1 | ChuA | 20 | 5´-GACGAACCAACGGTCAGGAT-3´ | 279 | [6] |
| ChuA.2 | 20 | 5´-TGCCGCCAGTACCAAAGACA-3´ | |||
| YjaA.1 | YjaA | 20 | 5´-TGAAGTGTCAGGAGACGCTG-3´ | 211 | [6] |
| YjaA.2 | 21 | 5´-ATGGAGAATGCGTTCCTCAAC-3´ | |||
| TspE4C2.1 | TspE4C2 | 20 | 5´-GAGTAATGTCGGGGCATTCA-3´ | 152 | [6] |
| TspE4C2.2 | 20 | 5´-CGCGCCAACAAAGTATTACG-3´ |
Determination of different phylogenetic groups. Isolates were assigned to one of four groups (A, B1, B2, or D) based on their possession of two genes (chuA and yjaA) and a DNA fragment (TSPE4.C2) (Table 2).
Table 2.
Assignation of different phylogenetic groups
Not present (group does not possess gene),
Variable (possession of gene is variable),
Present (group possesses gene)
RESULTS
Identification of Escherichia coli. Out of 91 urine samples from hospitalized patients, 27.47% (n = 25) E. coli and out of 144 poultry carcasses samples suspected to colibacillosis, 17.36% (n = 25) E. coli were isolated using culture and biochemical tests.
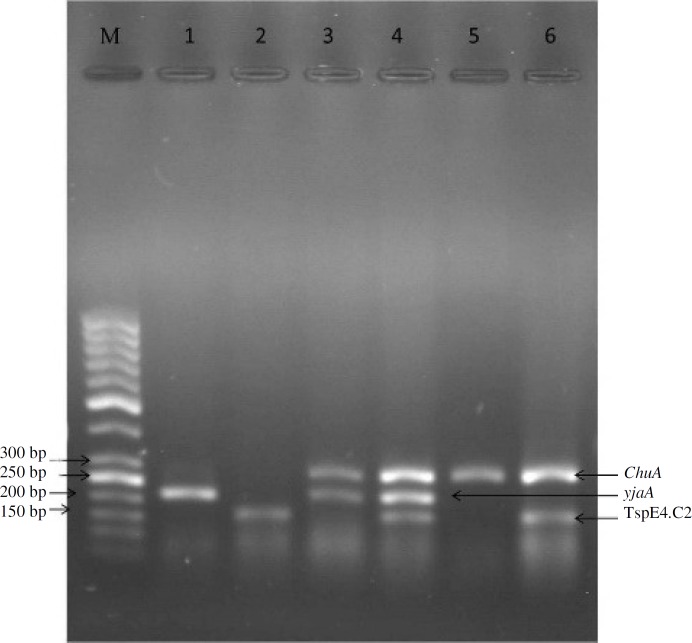
Phylogenetic grouping of isolates using multiplex PCR. a) In UPEC isolates, multiplex PCR (triplex PCR) analysis (Fig. 1) of the 25 isolates revealed that the distribution of different phylogenetic groups among UPEC isolates for groups A, B2, and D were 8 (32%),
Fig. 1.
Triplex PCR of isolates. Lane M, (ladder 50 bp, Fermentas); lane 1, group A; lane 2, group B1; lanes 3 and 4, group B2, and lanes 5 and 6, group D
10 (40%), and 7 (28%), respectively. However, phylogenetic group B1 were not detected in UPEC isolates. b) In avian pathogenic strain isolates, the distribution of different phylogenetic groups using multiplex PCR among 25 APEC isolates for groups A, B1, B2, and D were 9 (36%), 4 (16%), 7 (28%), and 5 (20%), respectively (Table 3). c) Among all E. coli isolates, the majority belonged to phylogenetic groups A and B2.
Table 3.
Number of different phylogenetic groups between UPEC and APEC
| Group |
A
No. (%) |
B1
No. (%) |
B2
No. (%) |
D
No. (%) |
Total |
|---|---|---|---|---|---|
| Isolates | |||||
| UPEC | 8 (32) | 0 (0) | 10 (40) | 7 (28) | 25 |
| APEC | 9 (36) | 4 (16) | 7 (28) | 5 (20) | 25 |
| Total | 17 (34) | 4 (8) | 17 (34) | 12 (24) | 50 |
UPEC, urinary pathogenic E. coli; APEC, avian pathogenic strains
Pattern of antibiotic resistance. From 50 tested E. coli isolates, all of them (100%) were resistant to penicillin and erythromycin, followed by 49 (98%) to nalidixic acid, 47 (94%) to cephalexin, 43 (86%) to amoxicillin, 42 (84%) to ampicillin, 37 (74%) to ciprofloxacin, 32 (64%) to tetracycline, 27 (54%) to cefixime and 18 (36%) to gentamicin. The results showed that the most effective antibiotic against UPEC isolates was ciprofloxacin (48%) and against APEC isolates was gentamicin (96%). Forty nine (98%) of the MDR isolates were resistant to ≥ 5 antimicrobial medicines. The pattern of drug resistance in different phylogenetic groups of UPEC and APEC is shown in Table 4. In phylogenetic group A, all APEC and UPEC isolates were highly resistant to penicillin, cephalexin, nalidixic acid and tetracycline but not too much to gentamicin. Also, the APEC isolates were much more sensitive to gentamicin as compared to the UPEC isolates. In phylogenetic group B1, APEC isolates were resistant to almost all of the antimicrobial medicines.
Table 4.
Number (%) of resistant and susceptible isolates against different antibiotics among different phylogenetic groups
| Group |
A
|
|
B
1
|
|
|
B
2
|
|
D
|
Total (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | UPEC | APEC | UPEC | APEC | UPEC | APEC | UPEC | APEC | ||||||||
| Number | 8 | 9 | 0 | 4 | 10 | 7 | 7 | 5 | 50 | |||||||
| Gentamicin | R | 5 | 0 | - | 1 | 7 | 0 | 5 | 0 | 18 (36) | ||||||
| S | 3 | 9 | - | 3 | 3 | 7 | 2 | 5 | 32 (64) | |||||||
| Tetracycline | R | 3 | 6 | - | 4 | 7 | 1 | 7 | 4 | 32 (64) | ||||||
| S | 5 | 3 | - | 0 | 3 | 6 | 0 | 1 | 18 (36) | |||||||
| Erythromycin | R | 8 | 9 | - | 4 | 10 | 7 | 7 | 5 | 50 (100) | ||||||
| S | 0 | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 (0) | |||||||
| Ciprofloxacin | R | 5 | 9 | - | 3 | 5 | 7 | 3 | 5 | 37 (74) | ||||||
| S | 3 | 0 | - | 1 | 5 | 0 | 4 | 0 | 13 (26) | |||||||
| Nalidixic acid | R | 7 | 9 | - | 4 | 10 | 7 | 7 | 5 | 49 (98) | ||||||
| S | 1 | 0 | - | 0 | 0 | 0 | 0 | 0 | 1 (2) | |||||||
| Cefixime | R | 3 | 1 | - | 3 | 8 | 4 | 5 | 3 | 27 (54) | ||||||
| S | 5 | 8 | - | 1 | 2 | 3 | 2 | 2 | 23 (46) | |||||||
| Cephalexin | R | 8 | 8 | - | 4 | 10 | 5 | 7 | 5 | 47 (94) | ||||||
| S | 0 | 1 | - | 0 | 0 | 2 | 0 | 0 | 3 (6) | |||||||
| Amoxicillin | R | 4 | 9 | - | 4 | 8 | 7 | 7 | 4 | 43 (86) | ||||||
| S | 4 | 0 | - | 0 | 2 | 0 | 0 | 1 | 7 (14) | |||||||
| Ampicillin | R | 4 | 8 | - | 4 | 9 | 5 | 7 | 5 | 42 (84) | ||||||
| S | 4 | 1 | - | 0 | 1 | 2 | 0 | 0 | 8 (16) | |||||||
| Penicillin | R | 8 | 9 | - | 4 | 10 | 7 | 7 | 5 | 50 (100) | ||||||
| S | 0 | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 (0) | |||||||
| MDR | 7 | 9 | - | 4 | 10 | 7 | 7 | 5 | 49 (98) | |||||||
R, resistant; S, susceptible; UPEC, urinary pathogenic E. coli; APEC, avian pathogenic strains; MDR, multiple drug resistance
In phylogenetic group B2, all isolates of both APEC and UPEC were resistant to penicillin, erythromycin, and nalidixic acid, and almost all of the isolates were resistant to ampicillin, amoxicillin, and cephalexin. There was a different pattern of sensitivity to gentamicin and tetracycline in the isolates of different sources (UPEC and APEC). In phylogenetic group D, all isolates in both UPEC and APEC were resistant to penicillin, ampicillin, cephalexin, nalidixic acid, and erythromycin, and almost all of them were resistant to amoxicillin, cefixime and tetracycline. Furthermore, 10 (40%) of UPEC isolates belonging to group B2 and 9 (36%) of APEC isolates belonging to group A were the main phylogenetic groups of MDR isolates.
DISCUSSION
The emergence, propagation, accumulation, and maintenance of strains of antimicrobial-resistant pathogenic bacteria have become a worldwide health concern in human and veterinary medicine. The intensive therapeutic uses and misuses of antimicrobial agents in humans and companion animals as well as their therapeutic, prophylactic, and subtherapeutic uses for growth promotion in food animals have substantially increased selective pressures on both pathogenic and commensal bacteria, thus favoring the propagation, accumulation, and maintenance of antimicrobial-resistant bacteria [10].
In the present study, identification of E. coli was conducted using standard culture and biochemical tests from hamun urine and avian colibacillosis samples, followed by multiplex PCR to assign each isolate to a certain phylogenetic group (A, B1, B2, and D). According to the recent phylogenetic studies on E. coli [1, 4, 11, 12], extra-intestinal pathogenic E. coli strains are mostly derived from the B2 phylogenetic group and, to a lesser extent, from group D, which are in line with the results of present study. It has been shown that the most commensal E. coli strain belongs to group A [13], and the majority of APEC isolates in this study belongs to group A.
There is a hypothesis that the urovirulent E. coli clones, present in the human intestine, come from fecal-oral route, and poultry is a candidate vehicle that transmits E. coli from poultry to human [1, 14]. The results obtained from phylogenetic typing in the present study, were not enough to accept or reject this theory. Indeed, further studies based on serogrouping, plasmid-related genes genotyping, and virulence gene genotyping will clarify this hypothesis [15]. In the present study, none of the UPEC isolates belonged to phylogenetic group B1, contrary to some of the previous studies [5, 11, 16]. This controversy could probably be due to the bacterial characteristics in different geographic regions, antibiotics usage or host genetic factors, and the number of isolated E. coli in present study.
The present investigation was conducted to achieve resistance profile of clinical isolates from our local area against commonly prescribed antibiotics. The in vitro antibiotic susceptibility pattern of the isolates showed high resistance to commonly used antibiotics such as penicillin, erythromycin, nalidixic acid, cephalexin, amoxicillin, ampicillin and ciprofloxacin. Our findings are in agreement with those of previous studies [2, 4, 8, 17, 18]. This high degree of resistance could be explained by the fact that these drugs are easily available without physicians' prescriptions from pharmacy in developing countries. In our study, 100% of the isolates were resistant against at least five antibiotics, which makes them a serious health problem, and 98% of them were MDR, which is close to the prevalence (≥70%) reported in Europe, India, and USA [10, 19-21].
The relation between phylogenetic background and antibiotic resistance showed that all UPEC isolates of group A were both resistant to penicillin and cephalexin. In contrast, all APEC isolates in group A were resistant to penicillin, erythromycin, ciprofloxacin, nalidixic acid, cephalexin, amoxicillin, and ampicillin. These results revealed that treatment of the diseases associated with APEC isolates in group A is much more difficult than UPEC isolates. In group B1 of APEC isolates, all of them were resistant to tetracycline, erythromycin, nalidixic acid, cefixime, cephalexin, penicillin, amoxicillin, and ampicillin. Because of extensive use of antibiotics to promote weight gain and for prophylaxis purposes, this high level of resistance was expected in commensal organisms (group A and B1 of APEC isolates) [22].
In group B2 of UPEC isolates, all of them were resistant to erythromycin, nalidixic acid, cephalexin, ampicillin and penicillin, whereas group B2 of APEC isolates were resistant to penicillin, erythromycin, ciprofloxacin, nalidixic acid, and amoxicillin. Among group D of UPEC isolates, all of them were resistant to tetracycline, erythromycin, nalidixic acid, cephalexin, penicillin, amoxicillin and ampicillin. However, in group D of APEC isolates, all of them were resistant to erythromycin, ciprofloxacin, nalidixic acid, cephalexin, amoxicillin, ampicillin, and penicillin. This fact could be due to the high level of prevalence [15, 23, 24], virulence [1, 2, 15, 25], resistance [2, 22], and plasmid-mediated resistance gene transfer in these isolates (groups B2 and D).
The results of this study, contrary to the previous reports [2, 4], revealed that phylogenetic group B1 of the APEC isolates, group D (UPEC and APEC), group A of APEC isolates, group B2 (UPEC and APEC), and group A of UPEC isolates are the most drug-resistant isolates. Evidence suggests that there is a relation between the overuse of antimicrobials, antimicrobial residues in poultry production, and the increasing emergence of resistant bacteria [26-28]. Therefore, careful choice of antibiotics based on the surveillance programs is necessary to avoid treatment failures and to prevent transmission of antimicrobial residues from poultry production to human food chain.
ACKNOWLEDGEMENTS
We would like to acknowledge all the staff of the Microbiology Department of Veterinary Faculty Urmia University for their technical support during the processing and analysis of the samples.
References
- 1.Xia X, Meng J, Zhao S, Bodeis-Jones S, Gaines SA, Ayers SL, et al. Identification and antimicrobial resistance of extraintestinal pathogenic Escherichia coli from retail meats. J Food Prot. 2011 Jan;74(1):38–44. doi: 10.4315/0362-028X.JFP-10-251. [DOI] [PubMed] [Google Scholar]
- 2.Obeng AS, Rickard H, Ndi O, Sexton M, Barton M. Antibiotic resistance, phylogenetic grouping and virulence potential of Escherichia coli isolated from the faeces of intensively farmed and free range poultry. Vet Microbiol. 2012 Jan;154(3-4):305–15. doi: 10.1016/j.vetmic.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Shtylla T, Circella E, Madio A, Paola GD, Çabeli P, Kumbe I, et al. Multiple antimicrobial resistance among avian Escherichia coli strains in Albania. ItalJAnimSci. 2009;8:771–4. [Google Scholar]
- 4.Saira B, Yasra S, Aamir A, Mashkoor M, Muhammad AS, Ayesha T, et al. multiple drug resistance patterns in various phylogenetic groups of Uropathogenic E.coli isolated from faisalabad region of Pakistan. Braz J Microbiol. 2011 Oct;42(4):1278–83. doi: 10.1590/S1517-83822011000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao X, Cavaco LM, Lv Y, Li Y, Zheng B, Wang P, et al. Molecular characterization and antimicrobial susceptibility testing of Escherichia coli isolates from patients with urinary tract infections in 20 Chinese hospitals. J Clin Microbiol. 2011 Jul;49(7):2496–501. doi: 10.1128/JCM.02503-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000 Oct;66(10):4555–8. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leopold SR, Sawyer SA, Whittam TS, Tarr PI. Obscured phylogeny and possible recombinational dormancy in Escherichia coli. BMC Evol Biol. 2011 Jun;11:183. doi: 10.1186/1471-2148-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukh AS, Schonheyder HC, Emmersen JM, Sogaard M, Bastholm S, Roslev P. Escherichia coli phylogenetic groups are associated with site of infection and level of antibiotic resistance in community-acquired bacteraemia: a 10 year population-based study in Denmark. J Antimicrob Chemother. 2009 Jul;64(1):163–8. doi: 10.1093/jac/dkp156. [DOI] [PubMed] [Google Scholar]
- 9.Blanco J, Mora A, Mamani R, Lopez C, Blanco M, Dahbi G, et al. National survey of Escherichia coli causing extraintestinal infections reveals the spread of drug-resistant clonal groups O25b:H4-B2-ST131, O15:H1-D-ST393 and CGA-D-ST69 with high virulence gene content in Spain. J Antimicrob Chemother. 2011 Sep;66(9):2011–21. doi: 10.1093/jac/dkr235. [DOI] [PubMed] [Google Scholar]
- 10.Alali WQ, Scott HM, Harvey RB, Norby B, Lawhorn DB, Pillai SD. Longitudinal study of antimicrobial resistance among Escherichia coli isolates from integrated multisite cohorts of humans and swine. Appl Environ Microbiol. Jun 2008;74(12):3672–81. doi: 10.1128/AEM.02624-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skjot-Rasmussen L, Ejrnaes K, Lundgren B, Hammerum AM, Frimodt-Moller N. Virulence factors and phylogenetic grouping of Escherichia coli isolates from patients with bacteraemia of urinary tract origin relate to sex and hospital- vs. community-acquired origin. Int JMed Microbiol. 2012 Jul;302(3):129–34. doi: 10.1016/j.ijmm.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Usein CR, Grigore LA, Georgescu RM, Bãltoiu MC, Condei M, Teleman MD. Phylogenetic background and extraintestinal virulence genotypes of Escherichia coli vaginal strains isolated from adult women. Rev Romana Med Lab. 2011;19(1):37–45. [Google Scholar]
- 13.Kariyawasam S, Scaccianoce JA, Nolan LK. Common and specific genomic sequences of avian and human extraintestinal pathogenic Escherichia coli as determined by genomic subtractive hybridization. BMC Microbiol. 2007 Aug;7:81. doi: 10.1186/1471-2180-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewers C, Li G, Wilking H, Kiessling S, Alt K, Antao EM, et al. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: how closely related are they? Int J Med Microbiol. 2007;297(3):163–76. doi: 10.1016/j.ijmm.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Siek KE, Giddings CW, Doetkott C, Johnson TJ, Fakhr MK, Nolan LK. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology. 2005 Jun;151(Pt 6):2097–110. doi: 10.1099/mic.0.27499-0. [DOI] [PubMed] [Google Scholar]
- 16.Mushtaq S, Irfan S, Sarma JB, Doumith M, Pike R, Pitout J, et al. Phylogenetic diversity of Escherichia coli strains producing NDM-type carbapenemases. J Antimicrob Chemother. 2011 Sep;66(9):2002–5. doi: 10.1093/jac/dkr226. [DOI] [PubMed] [Google Scholar]
- 17.Molina-López J, Aparicio-Ozores G, Ribas-Aparicio RM, Gavilanes-Parra S, Chávez-Berrocal ME, Hernández-Castro R, et al. Drug resistance, serotypes, and phylogenetic groups among uropathogenic Escherichia coli including O25-ST131 in Mexico city. J Infect Dev Ctries. 2011 Dec;5(12):840–9. doi: 10.3855/jidc.1703. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira FA, Paludo KS, Arend LN, Farah SM, Pedrosa FO, Souza EM, et al. Virulence characteristics and antimicrobial susceptibility of uropathogenic Escherichia coli strains. Genet Mol Res. 2011 Oct;10(4):4114–25. doi: 10.4238/2011.October.31.5. [DOI] [PubMed] [Google Scholar]
- 19.Hussain A, Ewers C, Nandanwar N, Guenther S, Jadhav S, Wieler LH, et al. Multiresistant uropathogenic Escherichia coli from a region in India where urinary tract infections are endemic: genotypic and phenotypic characteristics of sequence type 131 isolates of the CTX-M-15 extended-spectrum-β-lactamase-producing lineage. Antimicrob Agents Chemother. 2012;56(12):6358–65. doi: 10.1128/AAC.01099-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahm DF, Thornsberry C, Mayfield DC, Jones ME, Karlowsky JA. Multidrug-resistant urinary tract isolates of Escherichia coli: prevalence and patient demographics in the United States in 2000. Antimicrob Agents Chemother. 2001 May;45(5):1402–6. doi: 10.1128/AAC.45.5.1402-1406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SH, Byun JH, Choi SM, Lee DG, Kim SH, Kwon JC, et al. Molecular epidemiology of extended-spectrum beta-lactamase-producing Escherichia coli in the community and hospital in Korea: emergence of ST131 producing CTX-M-15. BMC Infect Dis. 2012;12:149. doi: 10.1186/1471-2334-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gundogdu A, Long YB, Katouli M. Prevalence and pathogenesis of extended-spectrum beta-lactamase producing Escherichia coli causing urinary tract infection in hospitalized patients. Eur J Clin Microbiol Infect Dis. 2012 Nov;31(11):3107–16. doi: 10.1007/s10096-012-1672-0. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura-Sato K, Yoshida R, Shibayama K, Ohta M. Virulence genes, quinolone and fluoroquinolone resistance, and phylogenetic background of uropathogenic Escherichia coli strains isolated in Japan. Jpn J Infect Dis. 2010 Mar;63(2):113–5. [PubMed] [Google Scholar]
- 24.Cooke NM, Smith SG, Kelleher M, Rogers TR. Major differences exist in frequencies of virulence factors and multidrug resistance between community and nosocomial Escherichia coli bloodstream isolates. J Clin Microbiol. 2010 Apr;48(4):1099–104. doi: 10.1128/JCM.02017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schubert S, Norenberg D, Clermont O, Magistro G, Wieser A, Romann E, et al. Prevalence and phylogenetic history of the TcpC virulence determinant in Escherichia coli. Int JMed Microbiol. 2010 Nov;300(7):429–34. doi: 10.1016/j.ijmm.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Shuford JA, Patel R. Antimicrobial growth promoter use in livestock- implications for human health. Rev Med Microbiol. 2005;16(1):17–24. [Google Scholar]
- 27.Yu HS, Lee JC, Kang HY, Ro DW, Chung JY, Jeong YS, et al. Changes in gene cassettes of class 1 integrons among Escherichia coli isolates from urine specimens collected in Korea during the last two decades. J Clin Microbiol. 2003 Dec;41(12):5429–33. doi: 10.1128/JCM.41.12.5429-5433.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebrahimzadeh MA, Mahdavee MR, Vahedi M. Antibiotic resistance in E. coli isolated from urine: a 2-year study on isolates from patients with urinary tract infections in Iran. Cell Tissue Res. 2005;5(2):445–8. [Google Scholar]